Abstract
We investigated the function and in vivo role of dopamine receptors (BmDopRs) in the silkworm Bombyx mori. During our studies, we discovered that BmDopRs play a crucial role in regulating feeding behavior in silkworm larvae. Here, we review recent findings [1] on the importance of dopamine (DA) and its receptors, particularly the BmDopR3 subtype, in regulating feeding behavior in Bombyx mori. Future discoveries of natural ingredients and compounds affecting BmDopR3 could significantly impact sericulture and pest control, including the development of artificial diets and feeding behavior regulators.
Keywords
Dopamine, G protein-coupled receptors (GPCRs), Silkworm, Feeding behavior
BmDopRs and Silkworm Feeding Behavior
Of the five BmDopRs mentioned above, BmDopR4 and BmEcDopR have a weak response to DA; therefore, little is known about beyond what is shown in Figure 2. However, the intracellular signaling of BmDopR1–3 and their pharmacological properties (agonist and antagonist drug ranking) are elucidated. For example, flupentixol and chlorpromazine, known as the mammalian DA receptor antagonists, affect BmDopRs in the order BmDopR2>BmDopR1>>BmDopR3 [1,4]. With no prior knowledge of BmDopR-mediated effects on the physiology and behavior of silkworms, we injected these compounds (2 μL of 10 mM) into fifth instar silkworm larvae (20 nmol?larva-1) as a trial. The results demonstrated a clear feeding suppression effect. Subsequently, we developed an interest in the relationship between silkworm feeding behavior and BmDopRs, injecting various compounds into silkworms to assess their effects. One such compound was bromocriptine (Bro; Figure 1), and its injection results had a significant impact on our research as described below.
Introduction
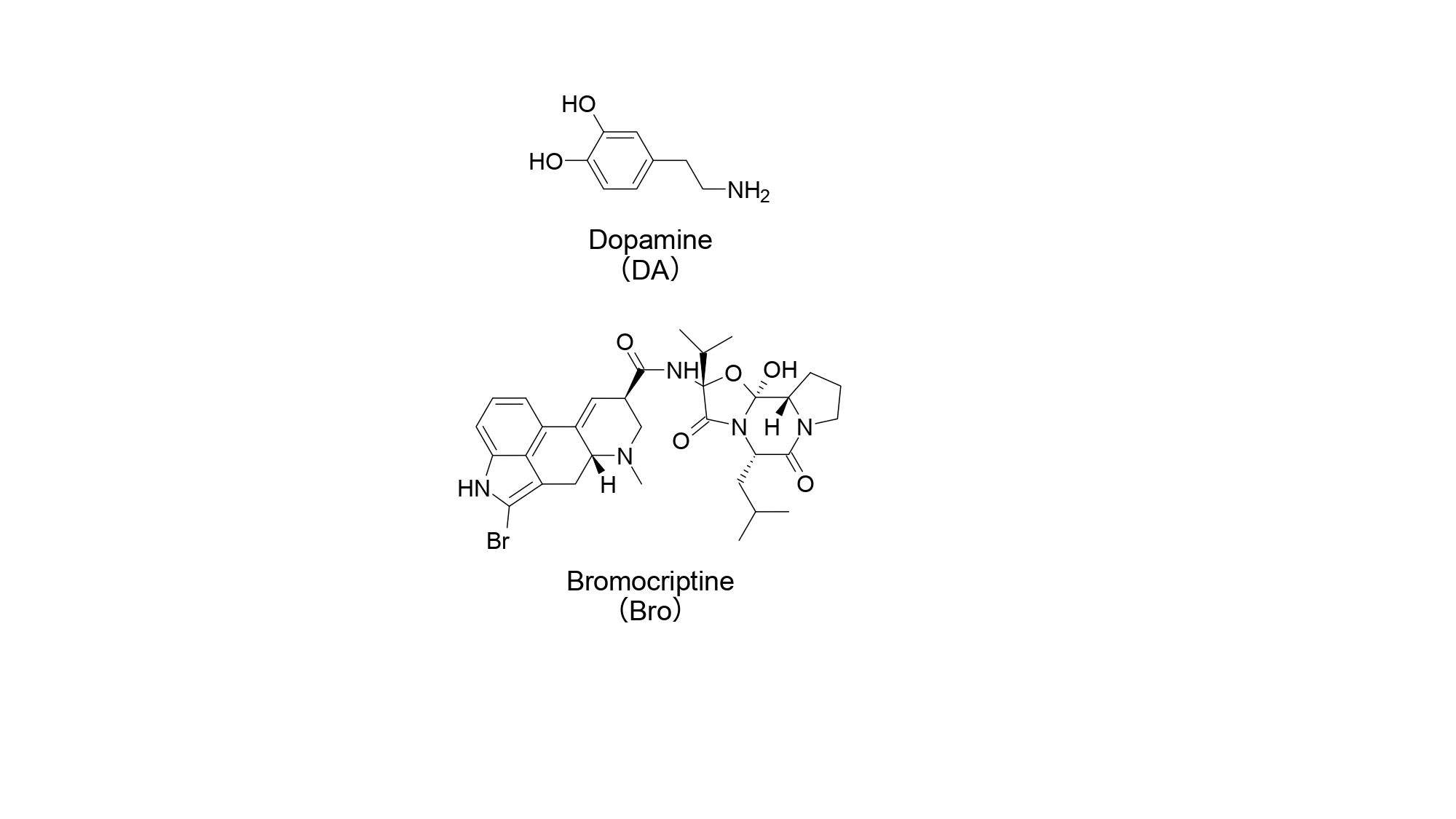
Studies on the biogenic amine DA (Figure 1) often focus on its physiological role in vertebrates, but it is also present in the nervous system of invertebrates. In insects, DA and its receptors, studied in detail in Drosophila melanogaster, have been reported to be deeply involved in various physiological and behavioral processes such as learning, memory, sleep, appetite, and locomotion [2].
Figure 1: Dopamine and bromocriptine. This figure is adapted with modifications from Reference 1. Reprinted with permission from the publisher Elsevier.
Research on biogenic amines in insects has evolved with a focus on pest control, as their receptors are potential targets for insecticides and control agents. Octopamine is a typical example. Octopamine receptors are insect-specific and are known targets for amidine insecticides/miticides such as chlordimeform and amitraz. While DA receptors have also been considered potential targets for pest control [3], there are currently no pesticides on the market that target DA receptors.
To apply insect DA receptor research to pest control, we chose the silkworm Bombyx mori, a model of lepidopteran pest insects, instead of Drosophila. First, we cloned the DA receptors, which had not yet been identified in the silkworm, and conducted functional and pharmacological analyses of the receptors using a cultured cell expression system over many years.
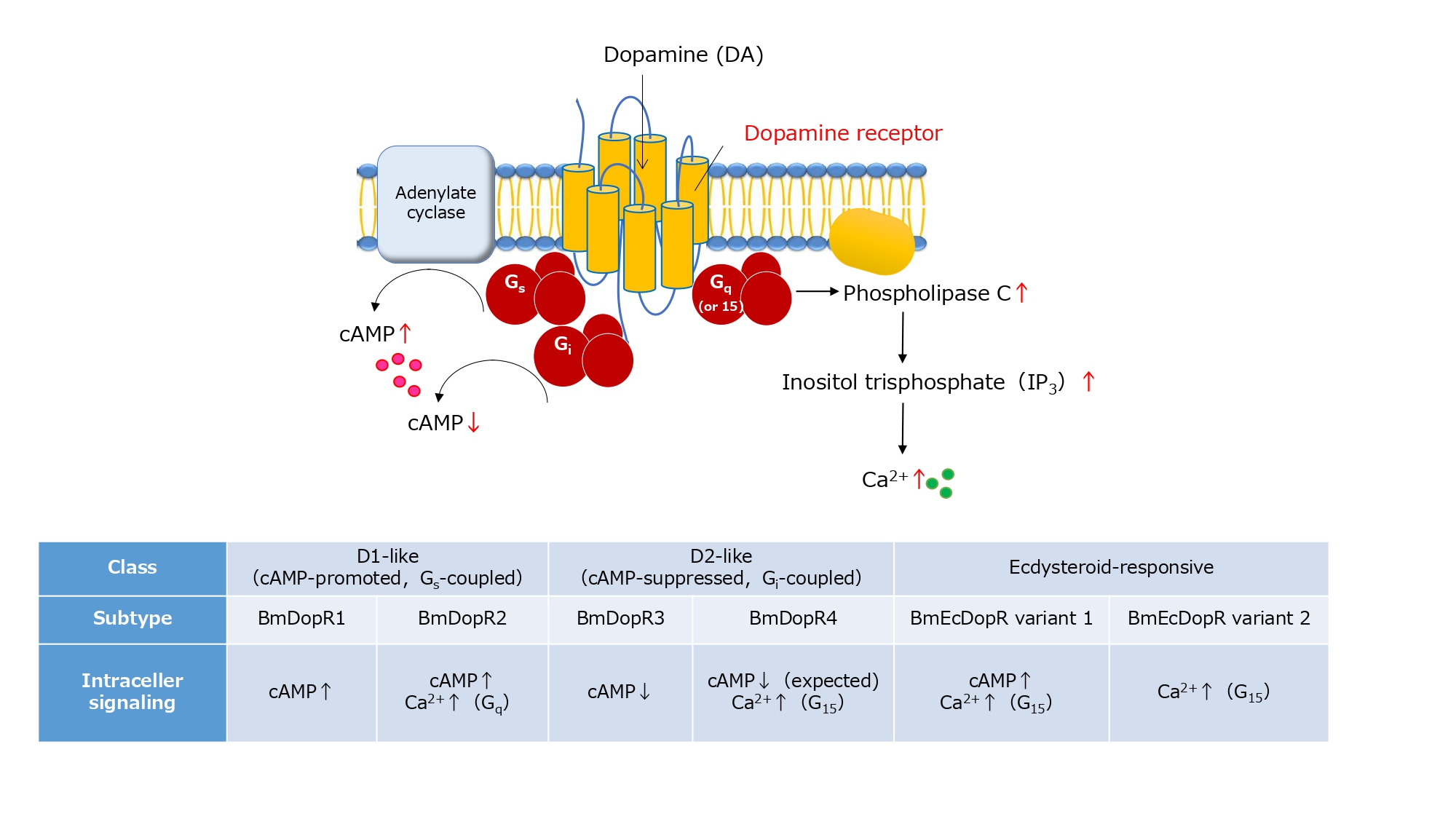
DA receptors are G protein-coupled receptors (GPCRs). We cloned five DA receptors (BmDopR1–4 and BmEcDopR) from silkworms and clarified their intracellular signaling pathways (see References [1,4-6] for details). BmDopR1 and BmDopR2 are D1-like (cAMP-promoted) receptors, BmDopR3 and BmDopR4 are D2-like (cAMP-suppressed) receptors, and BmEcDopR is an ecdysteroid-responsive receptor. The G proteins coupled to each receptor and their downstream intracellular signals are summarized in Figure 2.
Figure 2: Silkworm dopamine receptor signaling. BmDopR1 activates adenylate cyclase via Gs proteins to produce cAMP. BmDopR2 is Gs -coupled and Gq -coupled, leading to an increase in Ca2+ by activating phospholipase C. BmDopR3 is a Gi -coupled receptor and inhibits cAMP production. BmDopR4 is expected to have a similar function to BmDopR3 based on amino acid homology, but the Ca2+ response has been confirmed by aequorin assay with G15α co-expression. BmEcDopR variant 1 showed an increase in cAMP, whereas variant 2 did not (unpublished data). Both variants confirm the Ca2+ response by aequorin assay with G15α co-expression (unpublished data). This figure and caption are adapted from Reference 10 and reprinted with permission from the publisher Hokuryukan.
Bro Injection and Promotion of Feeding
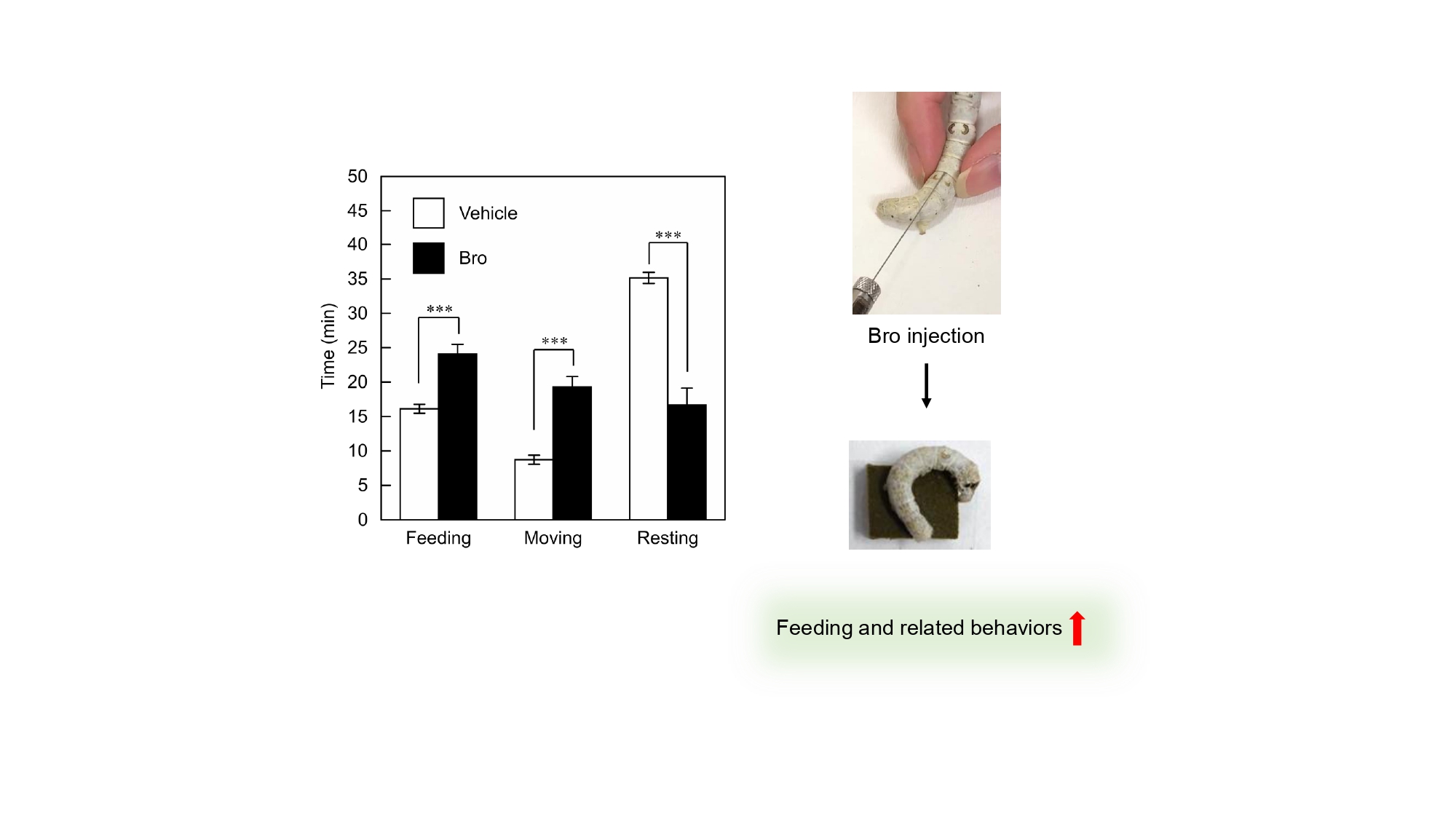
Returning to the pharmacological properties of BmDopR1–3, Bro, a known mammalian D2-like DA receptor agonist, showed almost no agonist activity against BmDopR1 and BmDopR2 [4], but exhibited high agonist activity against BmDopR3 [1]. Injection of the BmDopR3-specific agonist Bro into silkworms may affect their feeding behavior through downstream intracellular signaling via BmDopR3 (i.e., cAMP suppression). Therefore, Bro (2 μL of 0.1 or 1 mM) was injected into the fifth instar silkworm larvae (0.2 or 2 nmol?larva-1). Surprisingly, the food intake increased. Not only that, but the amount of feces also increased. In addition, saliva-like fluid secretions were observed from both sides of the mouthparts. A detailed analysis of Bro-injected silkworm larvae behavior, divided into three states (feeding, moving [other than feeding], and resting), revealed an increase in feeding and moving (especially locomotion), with a corresponding decrease in resting time (Figure 3). The increase in locomotion was unexpected, but it can be hypothesized that Bro injections elicited gait movements associated with feeding behavior, as the larvae moved around for food. Taken together, the receptor pharmacology analysis and the results of the Bro injections suggest that Bro acts as an agonist of BmDopR3 in the silkworm, affecting feeding and a range of related physiological and behavioral processes.
Figure 3: Quantitative analysis of feeding behavior in silkworms. Injected larvae in the glass dishes were fed freely for 1 h. During the 1 h observation period, the three behavioral states of each larva were observed and represented as mean ± SE (n = 20). ***p < 0.001 by unpaired t-test (two-sided). In individuals injected with Bro, both feeding and non-feeding moving time (especially locomotion) increased, while resting time decreased. This figure combines data from Reference 1 and the graphical abstract. Reprinted with permission from the publisher Elsevier.
Involvement of Another D2-like DA Receptor
Inspired by the results of Bro injections, we also attempted injections of another D2-like DA receptor agonist. Upon injecting the compound pergolide into the larvae, an increase in feeding was observed, like Bro. However, unlike Bro, no increase was seen in feeding-related behaviors, such as excretion, saliva-like fluid secretion, and locomotion (unpublished data). The observed differences in the effects of these two compounds led us to speculate that BmDopR3 widely regulates feeding and related behaviors in the silkworm, whereas there is another receptor at which pergolide specifically acts, which is only responsible for the control of feeding promotion. We thought it might be another D2-like DA receptor, BmDopR4 (Figure 2). Currently, we are investigating the agonistic effects of Bro and pergolide on BmDopR3 and BmDopR4. Thus far, these results suggest that Bro has a stronger response with BmDopR3 compared to BmDopR4, whereas pergolide acts preferentially on BmDopR4, supporting our hypothesis described above. Further research is underway to ensure that the difference in injection results between Bro and pergolide can be explained by variations in their agonist actions on BmDopR3 and BmDopR4.
Conclusion
This commentary outlines recent results on the effects of BmDopRs, particularly BmDopR3, on feeding behavior in silkworms. In this study, the BmDopR3-specific compound Bro was used as a powerful tool in identifying the receptor involved in the feeding behavior. However, we acknowledge that the injection experiments are fraught with potential problems, such as off-target effects of the pharmacological agent. Currently, molecular genetics and genome-editing techniques are becoming increasingly prevalent in silkworm research, and future studies should provide direct proof through knockdown or knockout of individual BmDopR subtypes using these techniques, combined with pharmacological approaches.
In addition to their traditional use in silk production, the application of silkworms in recombinant technology is gaining momentum [7]. An artificial diet is essential for the stable, year-round large-scale rearing of silkworms. Investigating the mechanisms of feeding behavior in silkworms and identifying compounds or natural ingredients that promote feeding behavior could lead to their inclusion as additives in artificial diets to enhance feeding efficiency. Presently, in addition to artificial diets that utilize l-DOPA as a DA precursor and natural materials containing high concentrations of l-DOPA, we are collaborating with another group to develop a new artificial diet incorporating photosynthetic bacteria [8].
Regarding pest control and insect DA receptors, D2-like DA receptors have been identified as a promising target in studies involving the grain pest, Tribolium castaneum [9]. BmDopR3, being a D2-like DA receptor, also holds potential as a novel agrochemical target. We continue our research on the relationship between silkworm feeding behavior and BmDopRs, aiming to develop feeding behavior regulators as agrochemicals for agronomic applications, including the enhancement of high-performance artificial diets.
Conflicts of Interest
The authors have no interests to acknowledge here in any commercial interests/intellectual property regarding applications.
Funding
This work was partly supported by JSPS KAKENHI Grant Number JP20K06081.
Acknowledgements
We would like to express our gratitude to the editorial team of the Japanese monthly magazine “AGRICULUTURAL BIOTECHNOLOGY” published by Hokuryukan for their permission to submit this commentary article as an English translation with some additions and compilations of Reference 10. Additionally, we thank Editage (https://www.editage.jp) for their English language editing services.
References
2. Karam CS, Jones SK, Javitch JA. Come Fly with Me: An overview of dopamine receptors in Drosophila melanogaster. Basic Clin Pharmacol Toxicol. 2020 Jun;126 Suppl 6(Suppl 6):56-65.
3. Audsley N, Down RE. G protein coupled receptors as targets for next generation pesticides. Insect Biochem Mol Biol. 2015 Dec;67:27-37.
4. Ohta H, Tsuchihara K, Mitsumasu K, Yaginuma T, Ozoe Y, Asaoka K. Comparative pharmacology of two D1-like dopamine receptors cloned from the silkworm Bombyx mori. Insect Biochem Mol Biol. 2009 May-Jun;39(5-6):342-7.
5. Ohta H, Mitsumasu K, Yaginuma T, Tanaka Y, Asaoka K. Functional Characterization of Dopamine and Neuropeptide G Protein-Coupled Receptors from the Silkworm Bombyx mori By Aequorin Bioluminescence-Based Calcium Assay. In: Advances in Agrochemicals: Ion Channels and G Protein-Coupled Receptors (GPCRs) as Targets for Pest Control: Volume 2: GPCRs and Ion Channels. American Chemical Society; 2017. pp. 109-126.
6. Mitsumasu K, Yoshikawa T, Ohta H. Regulation of invertebrate behavior via biogenic amine receptors and agricultural applications. The Cell. 2023 July 20;55(8):91-5. Japanese.
7. Ohta H. The “sericultural revolution” from Kyushu. Seibutsu-kogaku Kaishi. 2019 Oct 25;97(10):634-5. Japanese.
8. Miyasaka H, Uemura K, Ohta H, Koga A. Silkworm growth promoters and methods of promoting silkworm growth. Japanese Patent Application. 2022 Oct 17;2022-166446.
9. Bai H, Zhu F, Shah K, Palli SR. Large-scale RNAi screen of G protein-coupled receptors involved in larval growth, molting and metamorphosis in the red flour beetle. BMC Genomics. 2011 Aug 1;12:388.
10. Mitsumasu K, Yoshikawa T, Ohta H. Dopamine receptors control feeding behavior in the silkworm, Bombyx mori. Agricultural Biotechnology. 2024 Apr 20;8(4);66–70. Japanese.