Abstract
Acute Type A aortic dissection (ATAAD) is a life-threatening condition, leading to rapid fatality if not promptly treated. The risk for Type A aortic dissection is increased in patients with preexisting thoracic aortic aneurysms, but specific triggering events leading to acute aortic dissection are incompletely understood. Our recent research suggests that influenza may be a risk factor for ATAAD by demonstrating a relationship between regional influenza activity and hospital admissions for ATAAD. However, patient level data is needed to establish a causal relationship between influenza infection and ATAAD events. This vignette describes the case of a 27-year-old male who presented with an ATAAD and subsequently tested positive for influenza A.
Keywords
Aortic dissection, Influenza, Vaccination, COVID-19
Introduction
Acute aortic dissections occur as a result of a tear in the inner layer of the aortic wall, allowing blood to fill in between the wall’s inner and middle layers [1]. This condition is life threatening and can be rapidly fatal if not promptly treated. Aortic dissections are classified as either Type A when the tear of the aortic wall occurs in the ascending aorta or arch, or Type B when the dissection occurs in the aorta distal to the left subclavian artery [2]. While the incidence of aortic dissections is relatively low (4.4 per 100,000 person-years) [3], they carry a mortality risk of 57% if not treated surgically, and 18% with surgery [4]. Complications of aortic dissection include malperfusion syndrome, aortic regurgitation, cardiac failure and stroke [5]. Acute Type A aortic dissections (ATAAD) are associated with high morbidity and mortality, with chances of survival greatly improved by early detection and management [6]. Non-modifiable risk factors for ATAAD include a pre-existing thoracic aortic aneurysm, male sex, age greater than 65 and a number of genetic conditions that weaken the structural support within the aortic wall [7,8]. Modifiable risk factors include longstanding hypertension and smoking [1].
We have recently reported on an association between regional influenza activity and hospital admissions for ATAAD. Comparing Michigan specific influenza activity with University of Michigan admission rates for ATAAD, we found a 30% increase in the average number of monthly admissions for ATAAD during high influenza activity months compared to low influenza activity months (Figure 1). Additionally, there was an increased in-hospital mortality for patients undergoing urgent surgical repair during influenza season compared to non-influenza season (10.99 % vs. 5.79 %, p=0.024) [9]. While a causal link between influenza and ATAAD has not been established, the mechanism for cardiac complications of influenza is thought to be mediated through inflammatory injury [10]. The following case report describes a 27-year-old male presenting with ATAAD, with a respiratory panel positive for Influenza A.
Figure 1: Association between regional influenza activity and admissions for acute Type A aortic dissection (reproduced with permission from Ashur et al. [7]). (a) Influenza patterns and average admissions per month for acute Type A aortic dissection for all 455 patients admitted between 10/2009 and 4/2019. (b) Data stratified by influenza monthly activity determined by monthly rates of influenza-like illness, state lab confirmed influenza hospitalization and University of Michigan lab confirmed cases of influenza. Data both unadjusted and adjusted for temperature. (c) Data stratified by month between winter season (yellow, average temperature <35 Fahrenheit) and non-winter season (blue, average temperature >35 Fahrenheit).
Case Presentation
A 27-year-old African-American male with a past medical history of refractory hypertension and chronic kidney disease (baseline creatinine ranges from 1.73-2.0 mg/dl) presented to the emergency department with an initial complaint of sudden onset severe chest and back pain. A computed tomography (CT) scan was performed immediately and showed an aortic dissection extending from the aortic arch into the abdominal aorta. High sensitivity troponin was elevated to 37 pg/ml, and an electrocardiogram (EKG) showed sinus rhythm, left ventricular hypertrophy and repolarization abnormalities. The patient was hypertensive and was started on intravenous esmolol and nicardipine, and then transferred to a tertiary medical center for escalation of care.
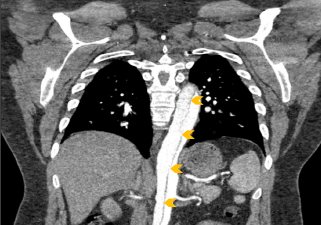
Upon arrival the patient’s blood pressure was 154/79 mmHg. He continued to experience chest and back pain, but was otherwise alert and oriented. His exam was grossly normal, and he reported no numbness or weakness in his extremities. The pre-transfer CT scan was reviewed and a Type A aortic dissection with an intramural hematoma of the ascending aorta and thrombus of the false lumen extending from the aortic arch down to the iliac arteries was confirmed (Figure 2). The patient was taken emergently to the operating room for aortic root repair and aortic valve resuspension with ascending and hemiarch replacement. He was noted to have a normal aortic valve with a primary tear above the sinotubular junction in the ascending aorta that spared the coronary arteries. The root was not enlarged (approximately 3-3.5 cm), and the arch vessels were not dissected. The total cross clamp time was 171 minutes and hypothermia circulatory arrest time was 17 minutes with a lowest temperature of 28 degrees Celsius. The patient was weaned off cardiopulmonary bypass easily and the expected coagulopathy was treated with cryoprecipitate, fresh frozen plasma and platelets. The pericardium was closed partially and two chest tubes were placed. The sternum was closed and the patient was transferred in stable condition and intubated to the surgical intensive care unit (ICU). Approximately 4 hours later he was noted to have decreased bilateral lower extremity blood flow and was taken to the interventional radiology operating room for infrarenal aortic fenestration and aorto-illiac stenting.
Figure 2: Coronal chest CT angiography demonstrating acute aortic dissection (arrows) extending from aortic root (off plane) to iliac arteries (off screen).
His post-operative course was complicated by acute kidney injury requiring hemodialysis. On post-operative day 2, he developed atrial flutter with 2:1 conduction and ST elevation. His dynamic EKG changes are shown in Figure 3. Cardiology was consulted and although myocardial injury was present [high-sensitivity troponin 2710 and 2659 pg/ml on repeat (<19 is normal), CK-MB 33 and 20 ng/ml on repeat; and CK MB index 0. 7%], he was medically treated with intravenous metoprolol for atrial flutter. Invasive angiography was not performed due to low suspicion for acute coronary syndrome and risk of contrast in the setting of his acute kidney injury. Echocardiography the same day showed severe left ventricular hypertrophy and normal systolic function with an ejection fraction of 55%. There were no regional wall motion abnormalities, and grade 2 diastolic dysfunction was present. The patient continued to have signs of diffuse spinal cord and cerebral ischemic strokes with significant lower extremity weakness. On post-operative day 4, he became febrile with increased respiratory secretions; an infectious work up was performed and the respiratory sample was positive for Influenza A by real time PCR, and quantitative respiratory culture grew Hemophilus influenzae with >10.000 cfu/ml. He completed a 5-day course of Tamiflu in addition to a course of intravenous antibiotic with piperacillin-tazobactam. He was extubated on post-operative day 7.
Figure 3: Hospitalization Electrocardiograms (EKGs).
The remaining ICU, surgical care was uncomplicated and he continued to improve. He was discharged to inpatient rehabilitation and participated in a 4-week program on the Physical Medicine Spinal Cord Injury Service. He was seen in nephrology ambulatory clinic 3.5 months post-surgery, where his renal function showed significant improvement, with stable chronic kidney disease stage III no longer requiring hemodialysis. Work up for causes of secondary hypertension remained unrevealing and his blood pressure was well controlled on multiple medications. He was seen in the cardiovascular genetic clinic and he was recommended to have genetic testing for aortic disease. Moreover, all first-degree relatives were invited to participate in a screening evaluation for hereditary thoracic aortic aneurysms and dissections. The complete history of presentation is outlined in Table 1.
|
Day 1 |
Presents with chest and back pain, found to have Type A aortic dissection on CT
|
|
Day 3 |
EKG with new atrial flutter followed by new ST segment elevations, determined too high risk for coronary catheterization |
|
Day 3 |
Initiated on continuous renal replacement therapy |
|
Day 4 |
Increased respiratory secretions and new fever, respiratory panel positive for Influenza A and Hemophilus influenza. Treated with Tamiflu and piperacillin-tazobactam. |
|
Day 7 |
Extubated |
|
Day 10 |
Started on hemodialysis |
|
Day 20 |
Discharged to inpatient rehabilitation on the Physical Medicine Spinal Cord Injury Service.
|
|
Day 53 |
Discharged from inpatient rehabilitation to his home on the same antihypertensive medications as above and continues hemodialysis. |
|
Day 100 |
Seen for follow up in the nephrology clinic. Renal function has improved and he no longer requires hemodialysis. Has stable CKD stage III. Extensive evaluation for secondary hypertension was unrevealing. Blood pressure remains well controlled on same regimen as above with addition of Spironolactone 25 mg. Patient was evaluated in the cardiovascular genetic clinic and an aortic panel for genetic causes for aortic dissection was recommended. |
Discussion
ATAADs carry a high burden of morbidity and mortality. Survival can be improved by early detection and management [6], and potentially through intervention on modifiable risk factors for disease. Patients presenting to the hospital with an aortic dissection are typically unable to provide a history due to their compromised state and need for emergent procedure, which makes identification of a triggering event, such as an influenza infection, difficult if not specifically sought after. Early determination of a concurrent influenza infection can lead to early anti-viral therapy and may help improve post-operative outcomes.
Although an association between ATAAD and influenza has been recently recognized [9], a direct causal mechanism has not been identified. This may be in part due to the difficulty in studying potential triggers for ATAAD given the relatively low incidence of the disease and the rapid fatality that is often attributed to other causes [12], with up to 50% of patients dying before reaching the hospital [13]. A proposed mechanism can be inferred from the well-studied impact of influenza infection on other major cardiovascular events. Specifically, Influenza has been linked with higher rates of myocardial infarction [14] and myocarditis [15], which are thought to be triggered through cytokine mediated vascular inflammation [10] in the setting of a hypercoagulable state [16]. Studies on atherosclerotic apolipoprotein-E deficient mice inoculated with influenza A have demonstrated infiltration of atherosclerotic plaques by inflammatory cells, in addition to the same process of smooth muscle cell proliferation, fibrin deposition, platelet aggregation and thrombosis seen following coronary plaque rupture [17]. Additional mouse models have demonstrated the presence of viable influenza virus, its RNA and its antigens within heart and aortic tissue 7 days after influenza infection. Following an influenza infection, increased aortic expression of chemokines, including MCP-1, RANTES, IP10 and KC have been found, consistent with viral mediated aortitis [18]. Prevention of cardiovascular events through administration of the influenza vaccine has been shown to significantly reduce the risk of major adverse cardiovascular events, with the strongest effect seen in those with active coronary disease [19]. This emphasizes the potential of the influenza vaccine as an emerging preventative tool and underscores the need to educate patients with cardiovascular risk factors on its importance.
During a time of global pandemic caused by the Coronavirus disease of 2019 (COVID-19), it is imperative to understand the role viral syndromes play in precipitating cardiovascular events. To date there have been no reports directly linking COVID-19 to aortic dissection, however several cases of myocarditis, pulmonary emboli, ischemic heart disease and vascular injury have been observed in patients testing positive for COVID-19 [20]. Previous coronavirus and influenza epidemics have suggested a viral trigger for cardiovascular events [21], with more patients dying of cardiovascular events than all other causes in the majority of previous pandemics [22]. The proposed mechanism of cardiovascular injury from COVID-19 is similar to that of influenza, mediated by a cytokine storm and increased concentrations of proinflammatory cytokines. Early reports have demonstrated increased levels of IL1B, IFNγ, IP10, and MCP1, likely leading to activated T-helper-1 cell responses. Additionally, patients requiring ICU admission were noted to have a higher concentration of these cytokines than those not admitted to the ICU, suggesting that the cytokine storm may play a role in disease severity [23].
Despite this increased risk, we found that patients with an ICD 9/10 diagnosis code of thoracic aneurysms or dissections had a significantly lower rate of influenza vaccination (21%) than patients with an ICD 9/10 code for chronic pulmonary disease (26%, p<0.0001), coronary artery disease (26%, p<0.0001), diabetes mellitus (27%, p<0.0001) and hypertension (28%, p<0.0001) [11]. The cause for the lower utilization of influenza vaccine in patients with aortic aneurysm is not entirely clear, but possibly related to the fact that patients with aortic aneurysms are often younger and do not perceive themselves to be at increased risk for cardiovascular complications. Currently, multiple candidate vaccines against COVID-19 are under investigation [24]. If trends in uptake of this vaccine are similar to that of influenza, it will be necessary for medical providers to convey its importance, as influenza vaccination rates remain low in patients with cardiovascular diseases despite evidence of their efficacy in decreasing cardiovascular morbidity and mortality [19]. Recently published findings suggest that a vaccine refusal rate of only 10% could significantly interfere with creation of COVID-19 herd immunity, further highlighting the importance of encouraging vaccine uptake in all patients [25]. Improving influenza and COVID-19 vaccination rates in patients with underlying risk factors for cardiovascular complications is critical in reducing the burden of associated cardiovascular morbidity and mortality with these viral syndromes.
References
2. Harris C, Croce B, Cao C. Type A aortic dissection. Annals of cardiothoracic surgery. 2016 May;5(3):256.
3. DeMartino RR, Sen I, Huang Y, Bower TC, Oderich GS, Pochettino A, et al. Population-Based Assessment of the Incidence of Aortic Dissection, Intramural Hematoma, and Penetrating Ulcer, and Its Associated Mortality From 1995 to 2015. Circulation: Cardiovascular Quality and Outcomes. 2018 Aug;11(8):e004689.
4. Evangelista A, Isselbacher EM, Bossone E, Gleason TG, Eusanio MD, Sechtem U, Ehrlich MP, Trimarchi S, Braverman AC, Myrmel T, Harris KM. Insights from the International Registry of Acute Aortic Dissection: a 20-year experience of collaborative clinical research. Circulation. 2018 Apr 24;137(17):1846-60.
5. Hagan PG, Nienaber CA, Isselbacher EM, Bruckman D, Karavite DJ, Russman PL, et al. The International Registry of Acute Aortic Dissection (IRAD): new insights into an old disease. Jama. 2000 Feb 16;283(7):897-903.
6. Lee TC, Kon Z, Cheema FH, Grau‐Sepulveda MV, Englum B, Kim S, et al. Contemporary management and outcomes of acute type A aortic dissection: an analysis of the STS adult cardiac surgery database. Journal of cardiac surgery. 2018 Jan;33(1):7-18.
7. Milewicz DM, Ramirez F. Therapies for thoracic aortic aneurysms and acute aortic dissections: old controversies and new opportunities. Arteriosclerosis, Thrombosis, and Vascular Biology. 2019;39:126-136.
8. Bowman MA, Eagle KA, Milewicz DM. Update on Clinical Trials of Losartan with and Without β-Blockers to Block Aneurysm Growth in Patients with Marfan Syndrome: A Review. JAMA cardiology. 2019 Jul 1;4(7):702-7.
9. Ashur C, Norton E, Farhat L, Conlon A, Willer C, Froehlich JB, et al. Higher admission rates and in-hospital mortality for acute type A aortic dissection during Influenza season: a single center experience. Scientific reports. 2020 Mar 13;10(1):1-6.
10. Nguyen JL, Yang W, Ito K, Matte TD, Shaman J, Kinney PL. Seasonal influenza infections and cardiovascular disease mortality. JAMA cardiology. 2016 Jun 1;1(3):274-81.
11. Ashur C, Norton E, Farhat L, Conlon A, Willer C, Froehlich JB, et al. Higher admission rates and in-hospital mortality for acute type A aortic dissection during Influenza season: a single center experience. Sci Rep. 2020 Mar 13;10(1):4723.
12. Auer J, Berent R, Eber B. Aortic dissection: incidence, natural history and impact of surgery. Journal of Clinical and Basic Cardiology. 2000 Jan 1;3(3):151-4.
13. Howard DP, Banerjee A, Fairhead JF, Perkins J, Silver LE, Rothwell PM. Population-based study of incidence and outcome of acute aortic dissection and premorbid risk factor control: 10-year results from the Oxford Vascular Study. Circulation. 2013 May 21;127(20):2031-7.
14. Kwong JC, Schwartz KL, Campitelli MA, Chung H, Crowcroft NS, Karnauchow T, et al. Acute myocardial infarction after laboratory-confirmed influenza infection. New England Journal of Medicine. 2018 Jan 25;378(4):345-53.
15. Ukimura A, Izumi T, Matsumori A. A national survey on myocarditis associated with the 2009 influenza A (H1N1) pandemic in Japan. Circulation Journal. 2010:1008040838.
16. Estabragh ZR, Mamas MA. The cardiovascular manifestations of influenza: a systematic review. International journal of cardiology. 2013 Sep 10;167(6):2397-403.
17. Madjid M, Aboshady I, Awan I, Litovsky S, Casscells SW. Influenza and cardiovascular disease: is there a causal relationship? Texas Heart Institute Journal. 2004;31(1):4.
18. Haidari M, Wyde PR, Litovsky S, Vela D, Ali M, Casscells SW, et al. Influenza virus directly infects, inflames, and resides in the arteries of atherosclerotic and normal mice. Atherosclerosis. 2010 Jan 1;208(1):90-6.
19. Wu HH, Chang YY, Kuo SC, Chen YT. Influenza vaccination and secondary prevention of cardiovascular disease among Taiwanese elders—A propensity score-matched follow-up study. PloS one. 2019;14(7).
20. Driggin E, Madhavan MV, Bikdeli B, Chuich T, Laracy J, Biondi-Zoccai G, et al. Cardiovascular considerations for patients, health care workers, and health systems during the COVID-19 pandemic. Journal of the American College of Cardiology. 2020 May 12;75(18):2352-71.
21. Madjid M, Safavi-Naeini P, Solomon SD, Vardeny O. Potential effects of coronaviruses on the cardiovascular system: a review. JAMA cardiology. 2020 Mar 27.
22. Madjid M, Casscells SW. Of birds and men: cardiologists' role in influenza pandemics. Lancet. 2004 Oct 9;364(9442):1309.
23. Huang C, Wang Y, Li X, Ren L, Zhao J, Hu Y, Zhang L, Fan G, Xu J, Gu X, Cheng Z. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. The lancet. 2020 Feb 15;395(10223):497-506.
24. Chen WH, Strych U, Hotez PJ, Bottazzi ME. The SARS-CoV-2 vaccine pipeline: an overview. Current tropical medicine reports. 2020 Mar 3:1-4.
25. DeRoo SS, Pudalov NJ, Fu LY. Planning for a COVID-19 vaccination program. JAMA. 2020.