Abstract
Introduction: Dendritic cells (DC) present antigens for an immune reaction. Subtypes of DC such as plasmocytic (pDC), monocytoid (mDC), immature (imDC), classical (cDC), follicular (fDC), and interdigitating (intDC) and Langerhans cells (LHC) either aggravate or limit an immune reaction. Not much is known about the role of LHC and DCs in respiratory bronchiolitis-interstitial lung disease (RB-ILD), and desquamative interstitial pneumonia (DIP) and Langerhans cell histiocytosis (LHCH).
Material and Methods: 20 cases of RB-ILD, 7 cases of DIP, and 14 cases of LHCH were investigated. All patients were excessive cigarette smokers. Gender distribution showed a higher incidence of males. Immunohistochemistry was performed using antibodies for S100 protein, HLA-DR, CD11b, CD11c, CD14, CD15, CD33, CD35, CD64, CD123, CD303, and CD207.
Results and Conclusion: LHC were enriched in LHCH, and increased in RB-ILD, whereas absent in DIP. cDC were more frequent in LHCH compared to RB-ILD and DIP, whereas mDC were equally found in all three diseases. intDC and imDC were increased in LHCH compared to RB-ILD and DIP. Accumulation of imDC and pDC could be a sign of impaired maturation. Tertiary lymph follicles seen in RB-ILD and LHCH, but absent in DIP, were populated by fDC, other DCs were absent, again pointing to an impaired immune reaction. Follicle centers were seen in RB-ILD but absent in LHCH. pDC were equally seen in LHCH and RB-ILD, but absent in DIP. RB-ILD despite similarities with DIP is a different entity with different subsets of DCs.
Keywords
Subtypes of dendritic cells, Smoking-associated lung diseases, Langerhans cell histiocytosis, Respiratory bronchiolitis-interstitial lung disease, Desquamative interstitial pneumonia
Introduction
Dendritic cells (DC) are antigen presenting cells found in every organ system. Different subtypes are known, which exert different functions. They are known as conventional or classical DC (cDC), monocytoid DC (mDC), interdigitating DC (intDC), follicular DC (fDC), plasmocytoid DC (pDC), and Langerhans cells (LHC) [1-3]. They all express some common markers, such as S100 protein and HLA-DR [4], but can also express more specific markers, which helps in sorting them in disease states. Conventional ones can be stained in tissues by CD1c, CD11c, and CD33 [5], monocytoid by CD11b and CD64 [6], interdigitating by CD14 and CD83 [7], follicular by CD21 and CD35 [8], plasmocytoid by CD123 (a-IL3R), CD303, CD304, and FasL [9,10], and LHC by CD1a and Langerin [11]. Some of the surface molecules are less specific compared to other, for example CD1c, CD11c and CD11b, but combining these with the more specific ones assists in sorting. Also immature DCs (imDC) exist, which are thought to be responsible for tolerance signaling during development, but these imDCs can also be found as resting cells in all organs [12]. imDCs can promote regulatory Tcells (Treg) and induce T cell anergy. They will stain for common dendritic cell markers, but may also express CD1c and CD303. Some authors separate Langerhans cells and other histiocytes from dendritic cells, which in our opinion does not make sense, as these function primarily as antigen presenting cells similar to dendritic cells. Macrophages can present antigens, but their predominant function is phagocytosis and maintenance of homeostasis within the peripheral lung. In this study we exclude macrophages and granulocytes, although they also perform duties in antigen processing and presentation.
Smoking related lung diseases are well known in the lung, comprising respiratory bronchiolitis-interstitial lung disease (RB-ILD), desquamative interstitial pneumonia (DIP), smoking-related interstitial fibrosis (SRIF), and Langerhans cell histiocytosis (LHCH) [13-21]. Chronic Obstructive Pulmonary Disease (COPD) is another common smoking-induced disease, characterized by chronic airway inflammation and emphysema. It will not be included in this study, because it is composed of different subtypes, affecting large or small airways. In addition, there are changes of inflammatory cells during the course of the disease, which makes a selection of uniform cases difficult. Tobacco smoking is known to produce incomplete combusted plant proteins, which can act as antigens [22-24].
LHCH is characterized by a nodular and diffuse accumulation of LH cells, usually starting around small bronchi and bronchioles. In early stages numerous eosinophils accompany the LHC, which vanish in late stages (depending on the amount of secreted IL4 and IL5). Other dendritic cells are scarce. RBILD is characterized by an accumulation of macrophages in bronchioles, almost occluding the lumina and extending into the centroacinar region of the peripheral lung. DIP is characterized by an accumulation of macrophages in the peripheral lung, occluding alveoli, not much extending into terminal bronchioles. DIP often present with ground glass opacities, which is suspect of early cancer. Nothing is known about dendritic cells in RBILD and DIP.
Dendritic cells can be divided into subtypes by their expression of surface molecules and also by their function. Some dendritic cells such as LHC preferentially reside in the skin and along the bronchial tree, whereas other dendritic cells are migrating to the tissues, when there is a need for antigen take-up and processing. Two lineages are known, a myeloid and a lymphoid one [25]. Usually, these dendritic cells migrate as immature cells (monocytoid, interdigitating, follicular), but during contact with antigens they mature into either classic or plasmocytoid DC. Follicular DC mature when reaching tertiary lymphoid structures or lymph nodes [26-28].
The aim of this study was to analyze the accumulation of different subtypes of dendritic cells in LHCH, RBILD, and DIP, and to evaluate their possible functional role in these diseases. Whereas the accumulation of Langerhans cells in LHCH was interpreted, that these cells were not functional, nothing is known for RBILD and DIP. DIP and RBILD are regarded as identical by some authors, whereas separated by others [13,14,16,19,29-33]. Both are characterized by an accumulation of macrophages in the peripheral lung, in RBILD involving respiratory bronchioles with extension into centrolobular areas, in DIP macrophages fill alveoli and do not involve larger bronchioles. The criteria to separate both are not entirely clear. Therefore, we also aim to clarify this aspect.
Material and Methods
From the Archive of Lung Pathology at the Institute of Pathology, Medical University of Graz, 20 cases of RB-ILD, 7 cases of DIP, and 14 cases of LHCH were selected. From all cases open lung biopsies (VATS) or cryobiopsies were available. In LHCH they were predominantly from the right lung (5 UL right, 9 LL right, 1 ML, 1 UL and LL left, 4 LL left), in DIP they were from both lower lobes (3 LL right and 4 LL left). Biopsies from RBILD were from different lung lobes (5 UL right, 1 ML, 8 UL left, 1 UL and ML, 5 LL left). We did not include cases of smoking-related interstitial fibrosis (SRIF), because most cases showed combinations with other diseases, predominantly with RB-ILD, and only 3 cases showed a SRIF-only pattern. These numbers were too small for an inclusion. Biopsies were taken because of clinical and radiological diagnosis of interstitial lung disease and a diagnosis was requested from pathology. Patients at the time of diagnosis were untreated, especially no corticosteroids were applied.
All patients were excessive cigarette smokers, and most were of younger age compared to lung cancer patients (mean age 51 years). Gender distribution showed a higher incidence of male patients.
From formalin-fixed paraffin-embedded blocks 4 µm thick serial sections were cut, the first and last stained by H&E to control that the pathological lesions were found throughout. Immunohistochemistry was performed on these tissue sections using antibodies for S100 protein, HLA-DR, CD11b (ITGAM), CD11c (BDCA1, ITGAX), CD14, CD15, CD33 (Siglec3), CD35, CD64, CD123 (a-IL3R), CD303 (CLEC4C/BDCA2), and CD207 (Langerin). Antibody dilutions were tested beforehand on control blocks. In most instances the recommended dilution given by the companies could be used. Incubations were done as recommended by the companies on the Omnis platform (DAKO). Further details for immunohistochemistry are listed in Table 1. The infiltration of DC was recorded as either diffuse or focal, with/without nodular accumulation of cells, and with/without lymphocyte aggregates - all within the peripheral lung; a peribronchial/peribronchiolar and pleural infiltration was recorded separately. In few cases a perivascular infiltrate was noted. The amount of positively stained DCs was semiquantitatively scored as negative (0), minimal (scattered positive cells, 1+), medium (2+), or dense (including nodular aggregates, 3+). The study was approved by the Ethics Committee of the Medical University Graz (EK 24-135 ex 11/12). Statistics were done by student´s t-test and Wilcoxon test for unpaired data with equal variance, using the program Kaleidagraph® (Vers.5.0, Synergy Software). As other lung diseases such as emphysema or sarcoidosis would show different patterns of dendritic cells, none could serve as a negative control. Normal lung is usually not available in pathology departments.
|
Antigen for |
Company |
Clone |
Dilution |
Pretreatment |
Visualization |
DC subtype identified |
|
CD11b/ ITGAM |
Epitomics |
EP45 |
1+100 |
Omnis DAKO, 24min 95oC, high pH |
EnVISION FLEX TRS high pH Kit |
moDC, cDC |
|
CD11c / ITGAX |
Santa Cruz |
B-6 |
1+25 |
Omnis DAKO, 24min 95oC, high pH |
EnVISION FLEX TRS high pH Kit |
cDC1/2, moDC, imDC |
|
CD14 |
ProteinTech |
polyclonal |
1+1000 |
Omnis DAKO, 24min 95oC, low pH |
EnVISION FLEX TRS high pH Kit |
moDC, CDC2 |
|
CD15 |
Dako |
Carb-3 |
Ready to use |
Omnis DAKO, 24min 95oC, low pH |
EnVISION FLEX TRS high pH Kit |
imDC, preDC, monocytes |
|
CD33 / SIGLEC3 |
abcam |
EPR23051-101 |
1+50 |
Omnis DAKO, 24min 95oC, low pH |
EnVISION FLEX TRS high pH Kit |
imDC, cDC1/2 |
|
CD35 |
abcam |
EPR6602 |
1+250 |
Omnis DAKO, 24min 95oC, low pH |
EnVISION FLEX TRS high pH Kit |
follDC |
|
CD64 |
Origene |
3D3 |
1+100 |
Omnis DAKO, 24min 95oC, high pH |
EnVISION FLEX TRS high pH Kit |
moDC |
|
CD123 / IL3RA |
BD Pharmingen
|
7G3 |
1+25 |
Omnis DAKO, 24min 95oC, high pH |
EnVISION FLEX TRS high pH Kit |
imDC plDC |
|
CD207 / Langerin |
Novocastra |
12D6 |
1+100 |
Omnis DAKO, 24min 95oC, low pH |
EnVISION FLEX TRS high pH Kit |
LHC, cDC2 |
|
CD303 / CLEC4C |
R+D Systems |
992258 |
1+50 |
Omnis DAKO, 24min 95oC, low pH |
EnVISION FLEX TRS high pH Kit |
imDC, plDC |
|
S100 protein |
DAKO |
polyclonal |
Ready to use |
Omnis DAKO, 24min 95oC, high pH |
EnVISION FLEX TRS high pH Kit |
all |
|
HLADR |
abcam |
CR3/43 |
1+500 |
Omnis DAKO, 24min 95oC, low pH |
EnVISION FLEX TRS high pH Kit |
all |
Results
Langerhans cell histiocytosis (LHCH)
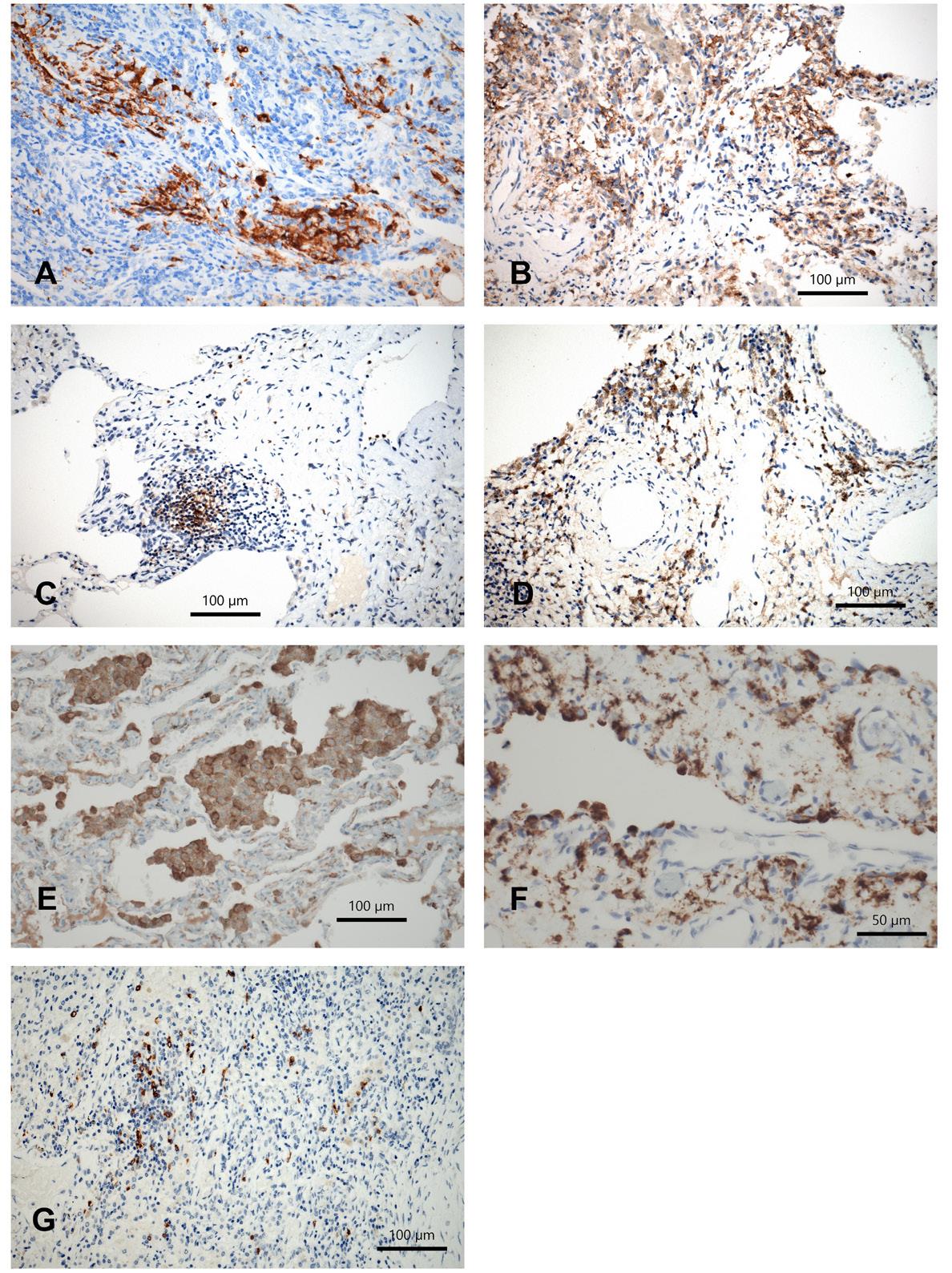
In LHCH langerin-positive cells were seen in all cases, multiple nodular aggregates of LHC were found in 7 whereas small or single aggregates in the other cases (N=14; Figure 1A). Mild interstitial infiltrates by CD11c+cDCs were seen in all cases, in one with dense infiltrates; CD33+cDCs were seen in 8/14 (Figure 1B), the other cases being negative. Infiltrations by mDC were positive in all cases (CD11b+, CD64+); low numbers (1+) of positive cells were stained by CD11b-antibody, whereas they were more frequent by CD64-antibody (8 cases 2+ and 6 cases 1+); in fibrotic areas the infiltration was usually pronounced (3+). fDC were seen in 6 cases (1+), the infiltrations being denser around lymph follicles (2+; Figure 1C). pDC were scarce in LHCH, in 4 cases 1+, in 2 cases 2+. They were more numerous in areas of fibrosis (2-3+).
Figure 1. A) Langerin positive cells in LHCH; B) classical DC in LHCH; C) follicular DC surrounding and within tertiary lymph follicles; D) Langerin positive LHC in RB-ILD; E) classical DC in RB-ILD; F) plasmocytic DC in RB-ILD; G) monocytoid DC in DIP. Immunohistochemistry for different DC subtypes using antibodies for Langerin, CD11c, CD33, CD35, CD123, bars 100 and 50 µm.
Respiratory Bronchiolitis - Interstitial Lung Disease (RB-ILD)
In RB-ILD langerin positive LHC were seen in 14/20 cases (Figure 1D), being negative in 3 cases. Infiltrations were scattered or forming small loose aggregates. In 3 other cases there was only a peribronchial (1+) LHC infiltration sparing the peripheral lung. CD11c+cDC were seen in 15 cases (13 x 1+, 2 x 2+; Figure 1E), whereas by CD33 less cases were stained (9 x 1+, 4 x 2+). CD64+mDC were seen 13 cases (9 x 1+, 4 x 2+), whereas by CD11b all cases were positively stained, but in low numbers (1+), or only scattered cells. There were more lymph follicles in RBLID compared to LHCH; these were absent in DIP. Within these tertiary lymph follicles germinal centers were formed. This resulted in higher numbers of fDC: in 15 cases, half 1+ the other half 2+. In 4 cases there was a predominant staining of fDC in peribronchial areas. pDC were seen in 11 cases (7 x 1+, 4 x 2+; Figure 1F), in 2 cases also a subpleural accumulation was seen (2+); 7 cases were negative.
Desquamative Interstitial Pneumonia (DIP)
In DIP LHC were absent, except one case where few LHC were found peribronchial. There were no CD33+cDCs, whereas CD11c+cDCs were positively stained in 5 cases (1+). mDC were found in 11 and 5 cases, respectively CD11b, CD64 (Figure 1G). They were more numerous in areas of fibrosis (2 cases, 2+). fDC were scarce in DIP (4 cases minimal positive), and pDC were absent.
Cells within the alveolar lumina morphologically looking like alveolar macrophages expressed CD64 and CD14 in RB-ILD and DIP. This has to be interpreted, that mDC and intDC up to one third are among the intraalveolar cells (Figure 2).
Figure 2. RB-ILD, morphological pattern with alveolar macrophages occupying the terminal bronchioles and extension into the centroacinar alveolar periphery. By immunohistochemistry many of these cells qualify for immature or monocytoid dendritic cells. H&E stained section and immunohistochemistry for CD64 highlighting monocytoid DCs. Magnification 200 (H&E) and bar 100 µm.
Comparison of dendritic cells in the three lung diseases
Statistics were calculated for LHCH, RB-ILD, and DIP (Table 2, Figure 3). The infiltration density was compared for each subtype of DC within the peripheral lung. The numbers of LHC were significantly higher in LHCH than in RB-ILD and DIP (p< 0.0001 in both). This was more pronounced in DIP, because no LHC were found in this disease. The number of cDC differed, however, did not reach significance for LHCH versus RB-ILD and DIP (CD11c p 0.075 and p 0.073, respectively); no significant difference for CD33+cDC was seen comparing LHCH and RB-ILD, whereas a significant difference was seen comparing LHCH and DIP (p 0.028). No significant differences were found when comparing all three diseases for mDC (CD11b, CD64). The numbers of imDC were significantly different for LHCH compared to RB-ILD and DIP. The numbers were highest in LHCH, medium in RB-ILD, and lowest in DIP (p 0.025 and p 0.0013 for CD15; p 0.0016 for CD303, respectively). intDC were seen more often in LHCH that RB-ILD and DIP, statistically close to significance (p 0.035 and 0.044, respectively). Finally, also pDC differed between LHCH and DIP, however not reaching significance (p 0.08), whereas pDCs in RB-ILD were seen with similar frequency as in LHCH (Figure 3).
Figure 3. Comparison of numbers of positive dendritic cell populations in the peripheral lung in LHCH, RB-ILD, and DIP (graded semiquantitatively as 0-1-2-3). Statistically significant differences are marked by an asterisk.
|
|
LHCH versus RBILD |
RBILD versus DIP |
LHCH versus DIP |
|
CD207 langerin |
p<0.001 |
p<0.001 |
p<0.001 |
|
CD11c (cDC9) |
p=0.075 |
p=0.46 |
p=0.073 |
|
CD33 (cDC) |
p=0.31 |
p=0.13 |
p=0.028 |
|
CD14 (intDC) |
p=0.035 |
p=0.73 |
p=0.044 |
|
CD15 (imDC) |
p=0.025 |
p=0.031 |
p=0.0013 |
|
CD303 (imDC) |
p=0.38 |
p=0.10 |
p=0.0016 |
|
CD123 (plDC) |
p=0.49 |
p=0.109 |
p=0.080 |
|
CD11b (moDC) |
p=0.86 |
p=0.63 |
p=0.21 |
|
CD64 (moDC) |
p=0.43 |
p=0.68 |
p=0.33 |
|
CD35 (follDC) |
p=0.36 |
p=0.46 |
p=0.16 |
Peribronchiolar infiltrations by LHC were rare in all three diseases, except for scattered cells. cDCs were rare in peribronchial areas in all three diseases; a slightly but statistically not significant increase was seen in LHCH. intDCs numbers were slightly enlarged in DIP and LCHC, whereas almost absent in RB-ILD. mDCs were seen in low numbers in few cases in all three diseases, but not significantly different in each of them. imDCs showed an infiltration different from the periphery: by CD303 the number in peribronchial areas were highest in DIP and lowest in LCHC, reaching statistical significance (p = 0.0016). No numerical differences were found for fDC in all three diseases, whereas the number of pDCs were significantly higher in LHCH compared to RB-ILD and DIP (Figure 4).
Figure 4. Comparison of numbers of positive dendritic cell populations in peribronchial location of the lung in LHCH, RB-ILD, and DIP (graded semiquantitatively as 0-1-2-3). Statistically significant differences are marked by an asterisk.
Discussion
Smoking-associated interstitial lung diseases can be classified into LCHC, RB-ILD, DIP, and COPD. They are characterized by infiltrating monocytoid cells, whereas lymphocytes numerically play a minor role [13,34]. Morphologically macrophages are seen in the alveolar lumina and histiocytic cells in the interstitium. Granulocytes are seen especially in the bronchial mucosa in COPD, but usually their occurrence point to additional complications such as bacterial colonization or superimposed infection. Tobacco smoke is composed of many substances, being toxic, carcinogenic, but also incomplete combusted plant proteins can be detected. Plant proteins and toxins derived from combusted tobacco plants and other sources (for example diesel exhaust) are known to induce an immune reaction [35-37]. This immune reaction is driven by cells of the innate immune system [38,39]. Here we focused on dendritic cells, which have not been extensively evaluated in these diseases. We investigated subtypes of dendritic cells, which are either activating or suppressing an immune reaction. COPD was not included, because it presents with large airway inflammation and emphysema, small airways disease with/without emphysema, and is often associated with lung cancer. In addition, the composition of the cellular infiltration in COPD varies during the time course, making this a heterogeneous disease. Therefore, a selection of cases with a homogenous morphological and timely appearance is complicated [40-44].
As some CD markers can stain different cell types, we chose at least two markers for each dendritic cell type and in addition also used general dendritic cells markers such as S100 protein and HLADR as a prescreening stain for the presence of dendritic cells (Table 1).
Not surprisingly Langerhans cells showed a predominant infiltration in LHCH, but they were also present in substantial numbers in RB-ILD. They were accompanied by classic, immature, interdigitating, and plasmocytoid DCs in both diseases. The accumulation of different subtypes of dendritic cells in the peripheral lung very likely is a sign of functional impairment [45-48]. Normally DCs including LHC should migrate to regional lymph nodes or tertiary lymph follicles, present processed antigens to lymphocytes and mount an immune reaction. Immature DCs when entering the lung should differentiate into mature DCs, either classical or plasmocytoid, depending on the cytokine composition present in the stroma [49-51]. Therefore, it seems migration as well as maturation are inhibited in LHCH and RB-ILD. Two possibilities to explain this inactivation can be offered: Inactivating mutation or posttranslational modification of CCR7 responsible for migration [52], and inhibition of maturation by a failure to secrete CCL5/CCR5 [46]. An inhibition of maturation will result in an impaired immune reaction, consecutively blocking also lymphocyte immigration. The presence of substantial numbers of imDC could explain this situation: imDC by expressing CCR6 and the ligand CCL20 will block migration. In addition, LHC in these diseases do not express CCL5/RANTES and CXCL11, which both would stimulate migration and induce maturation [53]. These functional aspects should be investigated in future studies. Mutations of the BRAF or MAPK genes very likely do not play a major role in migration and maturation of dendritic cells: Not all cases of LHCH carry these mutations, and no such mutations have been reported in RB-ILD [54]. mDC were found in considerable number in LHCH and RB-ILD, which support the assumption that LHC can originate from mDC [55]. This again point to a failure of differentiation into LHC.
In RB-ILD macrophages accumulate in respiratory bronchioles and extending into the centroacinar region, whereas in DIP macrophages occupy the alveolar periphery. In several reports it was questioned, if both entities represent just a variant of the same disease [14]. If that is true, one would expect a similar infiltration pattern by DCs. However, the infiltration of DCs in DIP is different from both other diseases. There are significantly less cDC, pDC, and imDC in DIP, which clearly separates it from LHCH and RB-ILD. Tertiary lymph follicles and consecutively follicular DCs are rarely seen, and LHC are absent in the periphery of DIP but present in peribronchial stroma, pointing to some reaction towards inhaled tobacco smoke antigens. Intraalveolar macrophages are found in large quantities in DIP and RB-ILD [56]. Interestingly, not all these cells are macrophages. The expression of CD64 and CD14 in a substantial number of these cells can be interpreted, that besides macrophages also mDC and intDC accumulate intraalveolar.
CD11c+cDC and CD64+mDC are seen in all three diseases. CD303+imDC are seen in substantial number in DIP but less in RB-ILD, reaching statistical significance only when compared to LHCH. By comparing the composition of DCs in RB-ILD and LHCH is seems that both diseases have much more in common compared to DIP. This might explain, why in some patients both LHCH and RB-ILD can be seen together.
Tertiary lymph follicles with germinal centers are predominantly found in RB-ILD, whereas in LHCH lymphocytic aggregates are usually devoid of germinal centers. Follicular DCs accumulate in these areas in equal amounts in LHCH and RB-ILD. Normally within tertiary lymph follicles different mature subtypes of DC should be present. As no other subtype of DCs is seen in LCHC and RB-ILD, this again point to a failure for mounting a proper immune reaction [57-60]. The absence of follicle centers in LHCH might result that no B cell priming does occur, whereas this seems to happen in RB-ILD.
What are the arguments for functional defects of DCs in these diseases?
DCs are constantly produced in the bone marrow and released into circulation. When they are confronted with antigens either in circulation or within the lung, they will mature. If substantial amounts of imDC are present such as in LHCH and RB-ILD this might be interpreted as impaired maturation. Well known from viral infection, imDC immediately mature to cDC [28]. cDC interact with T cells especially CD8+ lymphocytes, and this protects from infection. It can be speculated, that their higher numbers in LHCH could be a compensatory mechanism for non-functioning cDCs, whereas the accumulation of LHC along the bronchial tree and within alveolar tissue might be due to an impaired migration of LHC. pDC usually limit an immune reaction. In concert with imDC they also suppress effector T cell generation and induce regulatory T cells, thus promoting tolerance and anergy [12,61]. Experimentally pDC protects from emphysema development in smokers [62]. A forced expansion of pDC caused a decrease of macrophages and an increase of the linear intercept – a measurement of emphysema grade (higher numbers correspond to higher numbers of alveoli). Low amounts of interleukin 12 function as a regulating molecule [63]. The higher numbers of pDC in LHCH and RB-ILD (in contrast to DIP) point to such a downregulating mechanism for an immune reaction and might also protect smokers from the development of emphysema in this group of diseases. fDC promote the maintenance of tertiary lymph follicles and provide a long lasting depot for antigens, thus providing B lymphocytes with antigens for antibody production [64]. This seems to function in RB-ILD, whereas less efficient in LHCH. However, there is also a need of other DCs (cDC, LCH) to migrate towards these lymph follicles for establishing a proper immune reaction. Whereas germinal centers are formed in RB-ILD cases, these are rarely seen in LHCH, despite fDC accumulation in tertiary lymph follicles in both diseases. In DIP this does not occur. While mDC were equally seen in all three diseases, their function in promoting TH1 or TH2 reactions is probably not activated [6]. Normally mDC will mature into cDC or pDC and will provoke either a TH1 or a TH2 lymphocyte infiltration, which however, does not occur in any of these diseases. The presence of mDC in these diseases has to be interpreted that they do not differentiate into mature subtypes. This might be caused by a molecular defect of maturation, or imDC inhibit maturation, induce anergy and promote regulatory T cells.
Conclusion
In all three smoking induced lung diseases an impaired function of the innate and adaptive immune system has been found. Maturation of DC is impaired, resulting in the accumulation of immature and monocytoid DC and LHC. Migration of DCs seems to be impaired, as the DCs neither mature nor move to lymph follicles. DIP is different from RB-ILD characterized by a different set of DCs and absence of tertiary lymph follicles. Together with macrophages also dendritic cells are found within alveolar lumina, which was not known before.
Statements and Declarations
No conflict of interest is to be declared by all three authors
No funding was received for the study; expenses for purchase of antibodies and immunohistochemistry were paid from a personal study account to HP.
Authors’ Contribution
HP designed the study, selected and evaluated the cases, wrote the draft; MS and MAE evaluated and tested the antibodies; all authors read and contributed to the final manuscript.
References
2. Richez C, Schaeverbeke T, Dumoulin C, Dehais J, Moreau JF, Blanco P. Myeloid dendritic cells correlate with clinical response whereas plasmacytoid dendritic cells impact autoantibody development in rheumatoid arthritis patients treated with infliximab. Arthritis Res Ther. 2009;11(3):R100.
3. Lommatzsch M, Bratke K, Bier A, Julius P, Kuepper M, Luttmann W, et al. Airway dendritic cell phenotypes in inflammatory diseases of the human lung. Eur Respir J. 2007;30(5):878-86.
4. Zeid NA, Muller HK. S100 positive dendritic cells in human lung tumors associated with cell differentiation and enhanced survival. Pathology. 1993;25(4):338-43.
5. Marzaioli V, Canavan M, Floudas A, Flynn K, Mullan R, Veale DJ, et al. CD209/CD14(+) Dendritic Cells Characterization in Rheumatoid and Psoriatic Arthritis Patients: Activation, Synovial Infiltration, and Therapeutic Targeting. Front Immunol. 2022;12:722349.
6. Donnenberg VS, Donnenberg AD. Identification, rare-event detection and analysis of dendritic cell subsets in broncho-alveolar lavage fluid and peripheral blood by flow cytometry. Front Biosci. 2003;8:s1175-80.
7. Pileri SA, Grogan TM, Harris NL, Banks P, Campo E, Chan JK, et al. Tumours of histiocytes and accessory dendritic cells: an immunohistochemical approach to classification from the International Lymphoma Study Group based on 61 cases. Histopathology. 2002;41(1):1-29.
8. Arellano-Orden E, Calero-Acuña C, Moreno-Mata N, Gómez-Izquierdo L, Sánchez-López V, López-Ramírez C, et al. Cigarette Smoke Decreases the Maturation of Lung Myeloid Dendritic Cells. PLoS One. 2016;11(4):e0152737.
9. Patel VI, Metcalf JP. Identification and characterization of human dendritic cell subsets in the steady state: a review of our current knowledge. J Investig Med. 2016;64(4):833-47.
10. Masten BJ, Olson GK, Tarleton CA, Rund C, Schuyler M, Mehran R, et al. Characterization of myeloid and plasmacytoid dendritic cells in human lung. J Immunol. 2006;177(11):7784-93.
11. Patel VI, Booth JL, Duggan ES, Cate S, White VL, Hutchings D, et al. Transcriptional Classification and Functional Characterization of Human Airway Macrophage and Dendritic Cell Subsets. J Immunol. 2017;198(3):1183-201.
12. Lutz MB, Schuler G. Immature, semi-mature and fully mature dendritic cells: which signals induce tolerance or immunity? Trends Immunol. 2002;23(9):445-9.
13. Yousem SA, Colby TV, Gaensler EA. Respiratory bronchiolitis-associated interstitial lung disease and its relationship to desquamative interstitial pneumonia. Mayo Clin Proc. 1989;64(11):1373-80.
14. Moon J, du Bois RM, Colby TV, Hansell DM, Nicholson AG. Clinical significance of respiratory bronchiolitis on open lung biopsy and its relationship to smoking related interstitial lung disease. Thorax. 1999;54(11):1009-14.
15. Popper HH. Bronchiolitis, an update. Virchows Arch. 2000;437(5):471-81.
16. Ryu JH, Colby TV, Hartman TE, Vassallo R. Smoking-related interstitial lung diseases: a concise review. Eur Respir J. 2001;17(1):122-32.
17. Katzenstein AL, Mukhopadhyay S, Zanardi C, Dexter E. Clinically occult interstitial fibrosis in smokers: classification and significance of a surprisingly common finding in lobectomy specimens. Hum Pathol. 2010;41(3):316-25.
18. Katzenstein AL. Smoking-related interstitial fibrosis (SRIF), pathogenesis and treatment of usual interstitial pneumonia (UIP), and transbronchial biopsy in UIP. Mod Pathol. 2012;25 Suppl 1:S68-78.
19. Godbert B, Wissler MP, Vignaud JM. Desquamative interstitial pneumonia: an analytic review with an emphasis on aetiology. Eur Respir Rev. 2013;22(128):117-23.
20. Chu T, Jaffe R. The normal Langerhans cell and the LCH cell. Br J Cancer Suppl. 1994;23:S4-10.
21. Yousem SA, Dacic S, Nikiforov YE, Nikiforova M. Pulmonary Langerhans cell histiocytosis: profiling of multifocal tumors using next-generation sequencing identifies concordant occurrence of BRAF V600E mutations. Chest. 2013;143(6):1679-84.
22. Koethe SM, Kuhnmuench JR, Becker CG. Neutrophil priming by cigarette smoke condensate and a tobacco anti-idiotypic antibody. Am J Pathol. 2000;157(5):1735-43.
23. Martínez RD, Moreno A. [Immunoglobulin A and tobacco antigens in patients with pneumopathy]. Rev Alerg. 1992;39(5):106-12.
24. Becker CG, Dubin T, Wiedemann HP. Hypersensitivity to tobacco antigen. Proc Natl Acad Sci U S A. 1976;73(5):1712-6.
25. Steinman RM, Inaba K. Myeloid dendritic cells. J Leukoc Biol. 1999;66(2):205-8.
26. Givi ME, Folkerts G, Wagenaar GT, Redegeld FA, Mortaz E. Cigarette smoke differentially modulates dendritic cell maturation and function in time. Respir Res. 2015;16:131.
27. Lutz MB, Backer RA, Clausen BE. Revisiting Current Concepts on the Tolerogenicity of Steady-State Dendritic Cell Subsets and Their Maturation Stages. J Immunol. 2021;206(8):1681-9.
28. Webb TJ, Sumpter TL, Thiele AT, Swanson KA, Wilkes DS. The phenotype and function of lung dendritic cells. Crit Rev Immunol. 2005;25(6):465-91.
29. Vassallo R, Jensen EA, Colby TV, Ryu JH, Douglas WW, Hartman TE, et al. The overlap between respiratory bronchiolitis and desquamative interstitial pneumonia in pulmonary Langerhans cell histiocytosis: high-resolution CT, histologic, and functional correlations. Chest. 2003;124(4):1199-205.
30. Tazelaar HD, Wright JL, Churg A. Desquamative interstitial pneumonia. Histopathology. 2011;58(4):509-16.
31. Attili AK, Kazerooni EA, Gross BH, Flaherty KR, Myers JL, Martinez FJ. Smoking-related interstitial lung disease: radiologic-clinical-pathologic correlation. Radiographics. 2008;28(5):1383-96; discussion 96-8.
32. Bak SH, Lee HY. Overlaps and uncertainties of smoking-related idiopathic interstitial pneumonias. Int J Chron Obstruct Pulmon Dis. 2017;12:3221-9.
33. Davies G, Wells AU, du Bois RM. Respiratory bronchiolitis associated with interstitial lung disease and desquamative interstitial pneumonia. Clin Chest Med. 2004;25(4):717-26, vi.
34. Colasante A, Poletti V, Rosini S, Ferracini R, Musiani P. Langerhans cells in Langerhans cell histiocytosis and peripheral adenocarcinomas of the lung. Am Rev Respir Dis. 1993;148(3):752-9.
35. Youkeles LH, Grizzanti JN, Liao Z, Chang CJ, Rosenstreich DL. Decreased tobacco-glycoprotein-induced lymphocyte proliferation in vitro in pulmonary eosinophilic granuloma. Am J Respir Crit Care Med. 1995;151(1):145-50.
36. Final Report on Carcinogens Background Document for Formaldehyde. Rep Carcinog Backgr Doc. 2010(10-5981):i-512.
37. Zhang X, Wang L, Zhang H, Guo D, Zhao J, Qiao Z, et al. The effects of cigarette smoke extract on the endothelial production of soluble intercellular adhesion molecule-1 are mediated through macrophages, possibly by inducing TNF-alpha release Neutrophil priming by cigarette smoke condensate and a tobacco anti-idiotypic antibody. Methods Find Exp Clin Pharmacol. 2002;24(5):261-5.
38. DeKruyff RH, Yu S, Kim HY, Umetsu DT. Innate immunity in the lung regulates the development of asthma. Immunol Rev. 2014;260(1):235-48.
39. Bleck B, Tse DB, Jaspers I, Curotto de Lafaille MA, Reibman J. Diesel exhaust particle-exposed human bronchial epithelial cells induce dendritic cell maturation. J Immunol. 2006;176(12):7431-7.
40. Kim DK, Hersh CP, Washko GR, Hokanson JE, Lynch DA, Newell JD, et al. Epidemiology, radiology, and genetics of nicotine dependence in COPD. Respir Res. 2011;12(1):9.
41. Roy MG, Livraghi-Butrico A, Fletcher AA, McElwee MM, Evans SE, Boerner RM, et al. Muc5b is required for airway defence. Nature. 2014;505(7483):412-6.
42. Reid PT, Sallenave JM. Cytokines in the pathogenesis of chronic obstructive pulmonary disease. Curr Pharm Des. 2003;9(1):25-38.
43. Hogg JC, Chu F, Utokaparch S, Woods R, Elliott WM, Buzatu L, et al. The nature of small-airway obstruction in chronic obstructive pulmonary disease. N Engl J Med. 2004;350(26):2645-53.
44. Barnes PJ. Small airways in COPD. N Engl J Med. 2004;350(26):2635-7.
45. Hammad H, de Heer HJ, Soullie T, Hoogsteden HC, Trottein F, Lambrecht BN. Prostaglandin D2 inhibits airway dendritic cell migration and function in steady state conditions by selective activation of the D prostanoid receptor 1. J Immunol. 2003;171(8):3936-40.
46. Grayson MH, Ramos MS, Rohlfing MM, Kitchens R, Wang HD, Gould A, et al. Controls for lung dendritic cell maturation and migration during respiratory viral infection. J Immunol. 2007;179(3):1438-48.
47. Constabel H, Stankov MV, Hartwig C, Tschernig T, Behrens GM. Impaired lung dendritic cell migration and T cell stimulation induced by immunostimulatory oligonucleotides contribute to reduced allergic airway inflammation. J Immunol. 2009;183(5):3443-53.
48. Vassallo R, Walters PR, Lamont J, Kottom TJ, Yi ES, Limper AH. Cigarette smoke promotes dendritic cell accumulation in COPD; a Lung Tissue Research Consortium study. Respir Res. 2010;11(1):45.
49. Tan JK, O'Neill HC. Maturation requirements for dendritic cells in T cell stimulation leading to tolerance versus immunity. J Leukoc Biol. 2005;78(2):319-24.
50. Aiba S. Maturation of dendritic cells induced by cytokines and haptens. Tohoku J Exp Med. 1998;184(3):159-72.
51. Schuurhuis DH, Fu N, Ossendorp F, Melief CJ. Ins and outs of dendritic cells. Int Arch Allergy Immunol. 2006;140(1):53-72.
52. Bratke K, Klug M, Bier A, Julius P, Kuepper M, Virchow JC, et al. Function-associated surface molecules on airway dendritic cells in cigarette smokers. Am J Respir Cell Mol Biol. 2008;38(6):655-60.
53. Annels NE, Da Costa CE, Prins FA, Willemze A, Hogendoorn PC, Egeler RM. Aberrant chemokine receptor expression and chemokine production by Langerhans cells underlies the pathogenesis of Langerhans cell histiocytosis. J Exp Med. 2003;197(10):1385-90.
54. Roden AC, Hu X, Kip S, Parrilla Castellar ER, Rumilla KM, Vrana JA, et al. BRAF V600E expression in Langerhans cell histiocytosis: clinical and immunohistochemical study on 25 pulmonary and 54 extrapulmonary cases. Am J Surg Pathol. 2014;38(4):548-51.
55. Egeler RM, van Halteren AG, Hogendoorn PC, Laman JD, Leenen PJ. Langerhans cell histiocytosis: fascinating dynamics of the dendritic cell-macrophage lineage. Immunol Rev. 2010;234(1):213-32.
56. Popper H, Pongratz M. [Value and indications for bronchoalveolar lavage combined with transbronchial lung biopsy]. Wien Klin Wochenschr. 1987;99(24):848-55.
57. Freeman CM, Curtis JL. Lung Dendritic Cells: Shaping Immune Responses throughout Chronic Obstructive Pulmonary Disease Progression. Am J Respir Cell Mol Biol. 2017;56(2):152-9.
58. Dieu-Nosjean MC, Giraldo NA, Kaplon H, Germain C, Fridman WH, Sautès-Fridman C. Tertiary lymphoid structures, drivers of the anti-tumor responses in human cancers. Immunol Rev. 2016;271(1):260-75.
59. Gago da Graça C, van Baarsen LGM, Mebius RE. Tertiary Lymphoid Structures: Diversity in Their Development, Composition, and Role. J Immunol. 2021;206(2):273-81.
60. Sautes-Fridman C, Petitprez F, Calderaro J, Fridman WH. Tertiary lymphoid structures in the era of cancer immunotherapy. Nat Rev Cancer. 2019;19(6):307-25.
61. Dumitriu IE, Dunbar DR, Howie SE, Sethi T, Gregory CD. Human dendritic cells produce TGF-beta 1 under the influence of lung carcinoma cells and prime the differentiation of CD4+CD25+Foxp3+ regulatory T cells. J Immunol. 2009;182(5):2795-807.
62. Givi ME, Peck MJ, Boon L, Mortaz E. The role of dendritic cells in the pathogenesis of cigarette smoke-induced emphysema in mice. Eur J Pharmacol. 2013;721(1-3):259-66.
63. Maier B, Leader AM, Chen ST, Tung N, Chang C, LeBerichel J, et al. A conserved dendritic-cell regulatory program limits antitumour immunity. Nature. 2020;580(7802):257-62.
64. Fridman WH, Meylan M, Pupier G, Calvez A, Hernandez I, Sautès-Fridman C. Tertiary lymphoid structures and B cells: An intratumoral immunity cycle. Immunity. 2023;56(10):2254-69.