Abstract
Enteric fever is a major public health concern in developing countries. Enteric fever can result from Salmonella typhi and Salmonella paratyphi infections after consuming contaminated food or water. The increase in widespread drug resistance is severely limiting treatment options for this serious disease. This case report covers a 23 year old patient history who got lab acquired infection of Salmonella paratyphi A infection. Initially, he experienced symptoms of fever, and irritability, which progressed to severe loose motions and high-grade fever. Due to the worsening condition, the patient was admitted to the hospital where blood testing was done and the patient was advised to be treated with Azithromycin. With appropriate treatment, the patient showed significant improvement, including resolution of fever and normal bowl movement. Management of Salmonella is often challenging but prompt administration of appropriate antibiotics based on sensitivity testing is crucial for successful management. This case emphasizes the importance of early recognition and effective management of this uncommon yet severe complication of Salmonella infection.
Introduction
Salmonella is a gram-negative facultative anaerobe that belongs to the family Enterobacteriaceae responsible for causing enteric fever in humans. Salmonella enterica serovars Typhi and Paratyphi A, B, and C cause a systemic infection which are named enteric and paratyphoid fever [1]. One of the most prominent identifications includes non-lactose fermenting colonies on blood, chocolate, and MacConkey agar. The production of H2S is an important characteristic of this bacteria. Some bacteria of the Enterobacteriaceae family produce black colonies on XLD agar, and S. typhi is one of them that metabolizes thiosulfate to produce H2S. The cellular properties include an O antigen in the cell wall, and the outer polysaccharide helps to divide bacteria into A-I groups. Two flagellar H antigens synthesize proteins, but only one H antigen is responsible for it at one time. The most important Vi capsular antigen, which has antiphagocytic properties and is used for serotyping, plays an important role as a virulence factor for S. typhi [1]. Symptoms include low and high-grade fever, malaise, constipation, diarrhea, nausea, and abdominal pain; it can also cause pneumonia, meningitis, osteomyelitis, arthritis in severe cases [2]. Enteric fever is reported in 14.8 million people annually in low- and middle-income countries [3]. While non-typhoidal Salmonella estimated cases reported each year are 535,000 with more than 7,700 deaths [4]. According to a global systematic review and meta-analysis, the pooled case-fatality ratio (CFR) in Africa was 17.1%, 14.0% in Asia, 9.9% in Europe, and 9.6% in the Americas [5]. Humans are the only natural hosts, and drinking water and food are the primary vehicles for S. typhi transmission [6]. During their acute illness, infected people can transmit bacteria to others, and 1 to 5% of typhoid fever survivors become carriers [7]. Salmonella typhi has developed antibiotic resistance, leading to a rise in MDR and XDR cases each year.
Case Study
A 23-year-old female student researched pathogenic Salmonella typhi and Paratyphi strains at the institution. The strains were cephalosporin-resistant. After a week of working in the laboratory, the patient began to feel weary and disoriented, with a fever of 99°F. Normal Panadol was used to treat the fever. After a week, the patient fainted, and the doctor recommended regular tests, such as a complete blood count and a blood culture. The results revealed some major problems, including the patient's hemoglobin level dropping to 8, as well as claims of an infection. The fever kept, exceeding 104°F. The patient began feeling dizzy, chills, and sweating at 4-hour intervals and approximately 30 stools every day. The patient couldn't eat anything. After a week, the blood culture report revealed that the patient was infected with Salmonella paratyphi A. It was evident that the patient was working on clinical materials in the laboratory and became infected as a result of poor handling or failure to take adequate precautions. The blood culture revealed non-lactose fermenting colonies on MacConkey agar, which was gram-stained and revealed pink gram-negative colonies. The bacterial type and strains were subsequently identified as Salmonella using the API kit, and serotyping revealed Salmonella paratyphi A. The antibiotic sensitivity tests on Mueller Hinton Agar indicated that the bacterial strains were resistant to third-generation, cephalosporins. The rise in temperature and loose feces were unusually severe signs of Salmonella typhi infection.
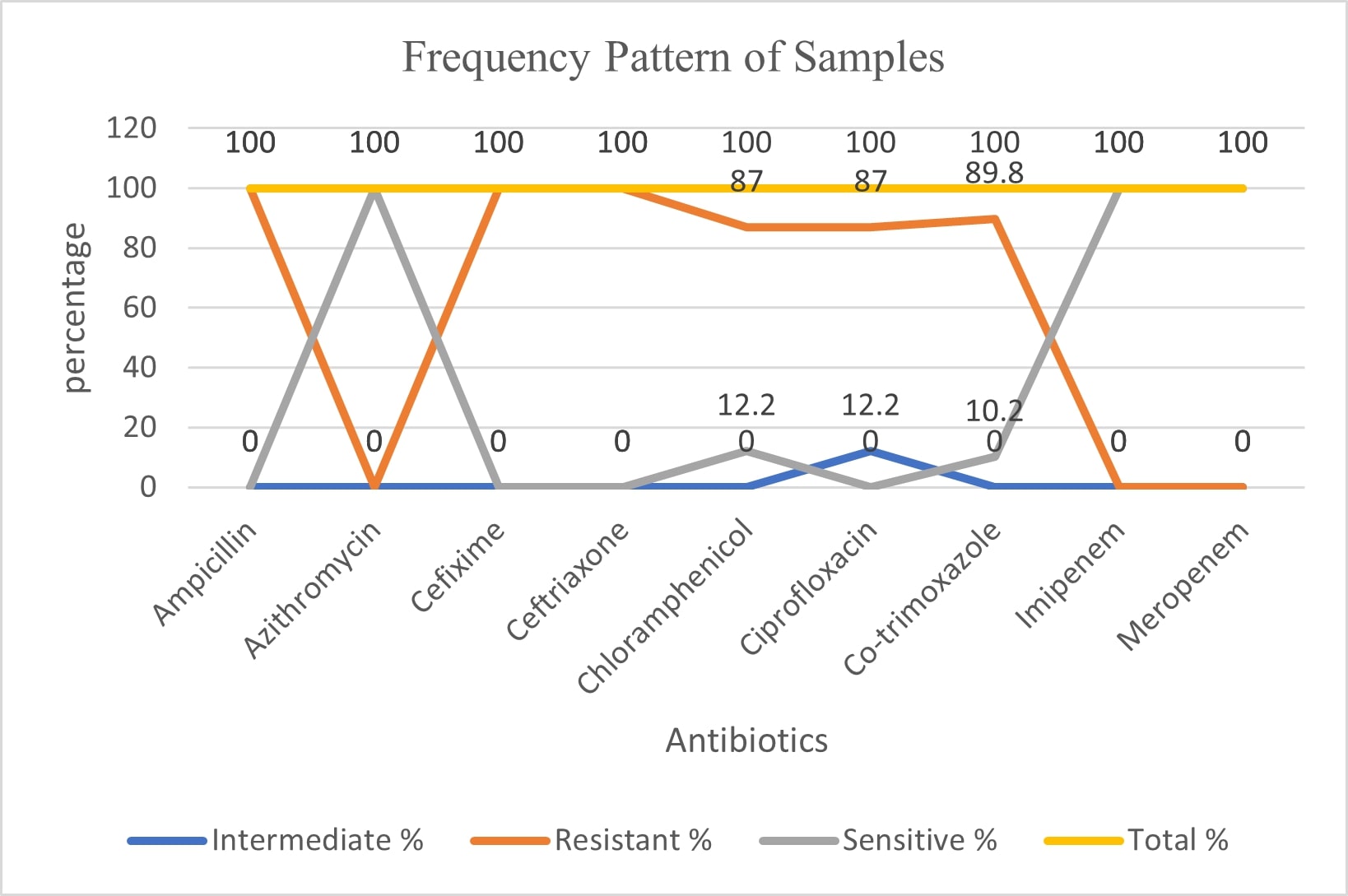
Figure 1. The frequency pattern of the clinical samples for Salmonella typhi and Salmonella paratyphi are shown in the graph. The data showed the samples used for working in the lab were XDR strains.
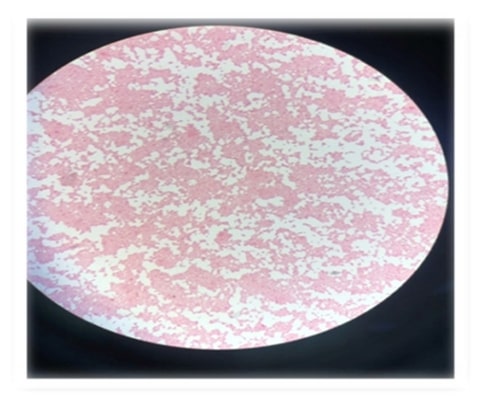
Figure 2. Salmonella negative rods confirmation under microscope.
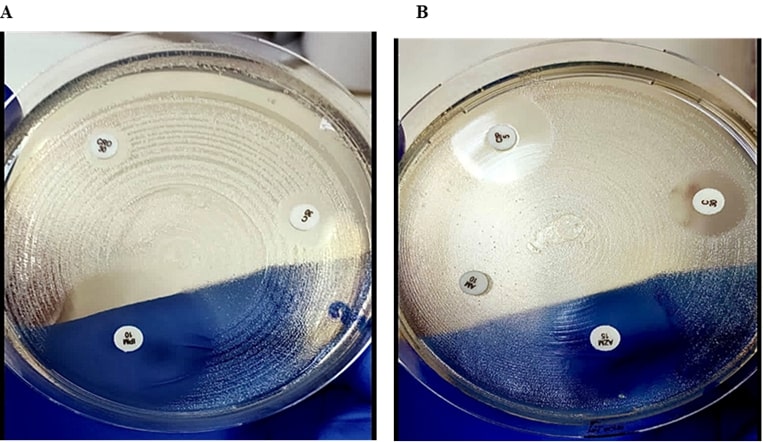
Figure 3. a) The MH plates showing Ampicillin, Ciprofloxacin and Ceftriaxone resistance while the zone of inhibition around azithromycin measures no resistance. b) The resistant zones of inhibition can be seen for Cefixime, Co-trimoxazole while imipenem shows full sensitivity.
Discussion
Talking about cephalosporin-reported cases, after azithromycin and cephalosporin complete resistance, carbapenems will be the possible treatment left for S. typhi as well as for other gram-negative bacteria [5]. During their acute illness, infected people can transmit bacteria to others, and 1 to 5% of typhoid fever survivors become carriers [7]. Salmonella typhi has developed antibiotic resistance, leading to a rise in MDR and XDR cases each year. The overuse and misuse of first-line antibiotics have resulted in resistance against third-line antibiotics as well [8]. S. typhi cases reported from 2009–2011 were MDR S. typhi with 91.7% cases in children [9]. The XDR resistance in Pakistan was reported in Hyderabad in November 2016 with 486 ceftriaxone-resistant cases [9].
Conclusion
Workers in clinical microbiology laboratories are exposed to a wide range of harmful bacteria. Salmonella species is one of the most often documented bacterial causes of laboratory-acquired illnesses. The laboratory examination comprised phenotypic and genotypic characterization of isolates using standard microbiological identification techniques, serotyping and antibiotic susceptibility tests, and whole-genome sequencing. While working in the lab, the patient became infected with XDR Salmonella paratyphi A, prompting the use of azithromycin as an oral treatment.
References
2. Chang MS, Woo JH, Kim S. Management of Typhoid Fever - Clinical and Historical Perspectives in Korea. Infect Chemother. 2019 Sep;51(3):330-5.
3. Duff N, Steele AD, Garrett D. Global Action for Local Impact: The 11th International Conference on Typhoid and Other Invasive Salmonelloses. Clin Infect Dis. 2020 Jul 29;71(Suppl 2):S59-S63.
4. Langridge GC, Nair S, Wain J. Nontyphoidal Salmonella serovars cause different degrees of invasive disease globally. J Infect Dis. 2009 Feb 15;199(4):602-3.
5. Marchello CS, Birkhold M, Crump JA; Vacc-iNTS consortium collaborators. Complications and mortality of non-typhoidal salmonella invasive disease: a global systematic review and meta-analysis. Lancet Infect Dis. 2022 May;22(5):692-705.
6. Waddington CS, Darton TC, Woodward WE, Angus B, Levine MM, Pollard AJ. Advancing the management and control of typhoid fever: a review of the historical role of human challenge studies. J Infect. 2014 May;68(5):405-18.
7. Ferreccio C, Morris JG Jr, Valdivieso C, Prenzel I, Sotomayor V, Drusano GL, et al. Efficacy of ciprofloxacin in the treatment of chronic typhoid carriers. J Infect Dis. 1988 Jun;157(6):1235-9.
8. Mandal S, Debmandal M, Pal NK. Antibiotic resistance of Salmonella enterica serovar Typhi in Kolkata, India, and in vitro experiments on effect of combined chemotherapy. ScientificWorld Journal. 2012;2012:454059.
9. Akhtar S, Sarker MR, Jabeen K, Sattar A, Qamar A, Fasih N. Antimicrobial resistance in Salmonella enterica serovar typhi and paratyphi in South Asia-current status, issues and prospects. Crit Rev Microbiol. 2015;41(4):536-45.