Abstract
The red imported fire ant, Solenopsis invicta Buren, poses a significant threat to public health due to its venomous stings. While the chemistry of fire ants has been well investigated, most studies have focused on ants from mature colonies. The dynamic changes in chemical compositions during colony development have been less explored. In this study, we established S. invicta colonies using newly collected queens from the field and then tracked the chemistry of workers as the colonies developed. Our findings reveal that the chemistry of fire ant workers undergoes significant changes with the development of the colonies. However, the change in chemical diversity was not necessarily in the same direction for all individual glands. For example, components in the venom gland became more diversified with colony development, whereas a number of compounds in the Dufour’s gland only existed at the early stage of colony development. Initially, trans-2-methyl-6-tridecenylpiperidine (trans-C13:1-piperidine) could account for up to 98% of all detected alkaloids; however, its percentage gradually, not stepwise, decreased with colony development due to the increase of other alkaloids. During days 50 to 100, production peaks in trans- and cis-2-methyl-6-undecylpiperidines (trans- and cis-C11-piperidine) were consistently observed. Piperidene and pyridine alkaloids were also detected within two months of colony age; however, piperidine alkaloids were always the first to appear, followed by piperidene and pyridine alkaloids. In addition to Z,E-α-farnesene, Z,E-α-homofarnesene, Z,Z-α-homofarnesene, heptadecane, and nonadecane, six geranyl esters of fatty acids were identified among the 32 new compounds. As expected, the origin of these compounds was the Dufour’s gland. Although these compounds were consistently detected in sting extractions, they seldom appeared in milked venom (2 out of 30 cases). These compounds may be unique to fire ants in the early stages of colony development.
Keywords
Colony development, Venom alkaloids, Incipient colony, Chemical profiles, Piperidine alkaloids, Piperidene alkaloids, Pyridine alkaloids, Poison gland, Dufour’s gland, Sting apparatus
Introduction
The red imported fire ant, Solenopsis invicta Buren, one of the most notorious invasive species, poses several medical problems for humans due to its aggressive behavior and venomous sting. The continuous expansion of its territory has made S. invicta a global concern [1-6]. Known for their painful stings, fire ants can cause immediate pain, swelling, redness, and itching at the site of the sting, leading to at least three types of local reactions: wheal-and-flare reaction, sterile pustule, and large local reaction. Some individuals may develop allergic reactions to fire ant venom, ranging from mild localized reactions to severe systemic reactions [7]. In severe cases, individuals may experience anaphylaxis, a life-threatening allergic reaction [8-11]. Fire ant stings can also lead to secondary infections if the skin is broken or scratched open, allowing bacteria to enter the wound [12].
Though systemic research is limited [13], it is evident that fire ant stings can have a psychological impact [14]. The fear of encountering fire ants can lead to psychological distress, anxiety, and avoidance behaviors [15], For example, people may worry about accidentally stepping on a fire ant mound during a backyard picnic.
Immunotherapy has proven effective and safe for individuals with severe allergic reactions [16], but fewer advances have been made regarding the treatment of local reactions [7,17]. Fire ant whole-body extracts are commercially used for immunotherapy due to the difficulty in extracting pure fire ant venom. Thankfully, both conventional and rush methods of administering whole-body extract immunotherapy have demonstrated effectiveness and safety [16]. Efforts have also been made to develop new rapid methods to collect fire ant venom [18].
Fire ants possess a complex sting apparatus composed of several structures that work together to deliver venom [19]. The primary structure used by fire ants to inject venom into their victims is the stinger, located at the tip of the abdomen. This stinger is connected to a venom sac, a specialized glandular structure within the ant's abdomen that stores the venom produced by the poison gland located near the sac. The venom sac is connected to a venom canal, which allows the venom to be injected into the victim. In addition to the venom gland, Dufour’s gland is also directly associated with the fire ant's sting apparatus. Both the venom sac and Dufour’s gland are directly connected to the stinger bulb. Muscles control the movement of the stinger and the opening and closing of a valve at the base of the sting bulb [19]. Since there is no muscle associated with the venom sac, it has been proposed that the propelling force for venom injection is provided by a strong contraction of the gaster for S. invicta and other Solenopsis species [20,21]. If this is the case, it might be possible that components from Dufour’s gland are injected into the victim along with the substance from the venom sac. Fire ant venom has been collected using a unique method involving centrifugation [22]. Some components from the Dufour's gland, such as α-farnesene, appeared in the collection. This could indicate that Dufour's gland components might be a source of venom components. However, it is still uncertain whether Dufour's gland components are actually injected into the victim.
Fire ants orchestrate a series of behaviors when delivering their venom, which may be mediated by an alarm pheromone produced by mandibular gland. Using their powerful mandibles, fire ants grasp the victim to deliver the venom. The mandibular gland, connected to the mandibles, releases a pyrazine alarm pheromone [23] when fire ants are in an attack mode. Although pyrazine is a well-known irritant, since it is not injected like the venom, its impact on the victim is likely minimal.
The venom chemistry of S. invicta has been extensively studied, demonstrating its complexity [21,24-25]. Fire ant venoms contain a high proportion of water-insoluble alkaloids and a small amount of water-soluble proteins. A series of 2-methyl-6-alkyl or alkenyl piperidines, 2-methyl-6-alkyl or alkenyl piperidenes, and 2-methyl-6-alkyl or alkenyl pyridines were identified in S. invicta venom. The alkyl or alkenyl side chains on position six of the piperidine, piperidene, or pyridine ring can consist of 9, 11, 13, 15, or 17 carbons. Piperidine alkaloids, commonly named solenopsins, are the major components of S. invicta venom. Based on the length of the alkyl side chain on position six of the piperidine ring, they are further categorized as solenopsin A (C11), B (C13), C (C15), and D (C17). Piperidene alkaloids include both Δ1,6 and Δ1,2 isomers. Pyridine alkaloids were the newest addition to the list of fire ant venom components. The discovery was made possible through the use of a unique technique called solid-phase microextraction (SPME) coupled with gas chromatography–mass spectrometry (GC–MS) and a modified thermal desorption process [26].
The chemistry of S. invicta Dufour’s gland is also well-studied. Z,E-α-farnesene, E,E-α-farnesene, Z,E-α-homofarnesene, and Z,Z-α-homofarnesene, heptadecane, and an unidentified terpene were reported in S. invicta Dufour’s gland as the source of trail pheromone components [27-29].
The composition and volume of venom in S. invicta can undergo changes depending on various factors such as the size and age of the ant [30] and environmental condition [31,32]. It was observed that the relative abundance of each of the six primary alkaloids was strongly associated with worker size in most instances. Additionally, the ratios of saturated to unsaturated alkaloids showed a positive correlation with worker size. Likewise, there were significant differences in both the abundance and ratios of alkaloids between small and large workers [30].
During the early stages of a fire ant colony, several key changes occur as the colony establishes itself and begins to grow, which may significantly affect ant chemistry. The colony begins with a single queen or a small group of founding queens. The queen(s) lay eggs, which hatch into worker ants. These worker ants start constructing the nest and tunnels, caring for the brood, venturing out from the nest in search of food sources, and participating in brood raids among colonies [33]. As the colony grows, foraging becomes more organized and efficient. The queen(s) continue to lay eggs to replenish the worker ant population and to produce new reproductives (alates) for future colony expansion. As the worker ant population increases and the nest grows in size, the colony expands its territory. Venom plays a crucial role in various aspects of colony development, including catching large prey, sanitizing the nest, and protecting the brood from infectious diseases. Like the age of individual ants, colony development may also have a significant effect on venom composition. Surprisingly, except for several early investigations [34,35], very few studies have been conducted on the dynamics of venom chemistry in relation to colony development, despite significant advances in understanding fire ant chemistry. Understanding the dynamics of venom components during colony development is important not only for understanding fire ant biology, but also for understanding venom toxicity. The venom components determine the potency of the venom, measured by its ability to induce pain, irritation, or other physiological effects in potential victims. Although the unique property of pheromone trails built by ants in brood raids has been noticed for many years [36], no studies have examined the dynamics of chemistry of Dufour’s gland in relation to colony development.
The objective of this study is to investigate the effect of colony development on fire ant chemistry, with a focus on compounds from the sting apparatus. We first established S. invicta colonies using new queens collected in the field and then tracked the chemistry of workers. The occurrence of various ant-derived compounds was recorded and quantified using Gas Chromatography-Mass Spectrometry (GC-MS).
Materials and Methods
Ant samples
Over 300 S. invicta colonies were established from newly collected queens after nuptial flights during April and June of 2022 and 2023, respectively. All the queens were collected from Greenville, Washington County, Mississippi. A compartmentalized test tube was utilized to develop new colonies from a new queen [37]. The test tube was divided into queen chamber and water storage section using two cotton balls [38]. The new queen was placed in the queen chamber, and the water storage section was filled with distilled water. No food was provided until the colony had minim workers because the queen provided all the nutrients needed for the colony. After the first minim worker appeared, the test tube was placed in a plastic container with the inner walls coated with Fluon, and the cotton plug was removed. These colonies were maintained at 28°C. All dead workers were collected from each colony and frozen in a -80°C freezer. The chemical profiles of live workers were closely monitored for three colonies established from new queens. At least twice a month, chemical analysis was conducted on extracts of whole body for three workers for each of three colonies.
To compare the chemical profiles of laboratory colonies to matured colonies in the field, ants from 10 mature colonies (colonies with female alates) were collected on the same day or one day before the chemical analysis. Ants were collected around 8:30–9:00 am by taking part of the mound using a small shovel and placing ants with mound soil in a plastic container (22 × 14 × 8 cm [L × W × H]). The inner wall of the container was coated with Fluon to prevent ants from escaping. Three sting apparatus were dissected from each colony, extracted, and analyzed using GC-MS.
To determine whether Dufour's gland contributes to the venom constituents in S. invicta, venom released from workers was collected and directly loaded onto a solid-phase microextraction (SPME) fiber, which was inserted into the injection port of a GC-MS for chemical analysis. Three ants from each of 10 colonies were used. The sting apparatuses of the same ants were dissected, and the poison sacs and poison glands were removed from each sting apparatus. These sting apparatuses were then subjected to chemical analysis to determine the chemical components of the Dufour's glands.
Sample preparation for chemical analysis
- Worker whole-body. A worker was first frozen at -80°C for at least 5 minutes and then weighed. The ant was then transferred into a 200 µl insert, pushed to the bottom of the insert, and crushed using a disposable pipet (Drummond Scientific Company, Broomall, PA).
- Sting apparatus. A worker was first rinsed using approximately 2 ml acetone, and the sting apparatus was dissected using two pairs of fine-tipped forceps. The gaster was cut from the body using a blade and torn apart on a filter paper under a microscope. The tissue around the sting apparatus was removed without damaging the poison sac. The sting apparatus was then transferred into a 200 µl insert and crushed using a disposable pipet.
- Sting apparatus without poison sac and poison gland. Sting apparatus was dissected as described above. The poison sac and the poison gland were removed using a fine tipped forceps. The sting apparatus without poison sac and gland was then placed into a 200 µl insert and crushed using a disposable pipet.
Fifty microliters of a 5.2 µg/ml trans-2-methyl-6-dodecylpiperidine in hexane:ethyl acetate solution (1:1) was added into each of above samples. Two microliters of the solution were then injected into gas chromatography–mass spectrometry (GC-MS).
- Direct load of milked venom on SPME fiber. Under a dissecting microscope, an ant was held by the petiole with forceps, and the tip of the abdomen was touched repeatedly by an extended SPME fiber. Drops of venom on the tip of the protruded sting were directly loaded onto the SPME fiber. Three to four drops of venom from a single ant were collected. The fiber was then injected into GC-MS. Gray SPME fibers (DVB/CAR/PDMS, 50/30 μm) and a manual holder (Supelco Inc., Bellefonte, PA) were used. Before the first use, fibers were thermally cleaned at 250°C in the injection port of a GC for 12 hours. The fiber was cleaned for 1 hour between each sample.
Gas chromatography–mass spectrometry (GC-MS)
An Agilent 7890A gas chromatograph (GC) paired with an Agilent 5975 mass selective detector (MS) was utilized (Santa Clara, CA). A DB-5 capillary column (30 m × 0.25 mm i.d., 0.25 μm film thickness) was employed. The GC temperature program consisted of an initial temperature of 60°C, held for 1 minute, then increased to 240°C at a rate of 3°C/min and held for 20 minutes, followed by an increase to 280°C held for 8 minutes. Injection and transfer line temperatures were 250°C and 270°C, respectively. Splitless mode was used for both SPME and solvent extraction samples. The mass spectrometer operated at 70 eV in electron impact mode, employing full scan mode for all the samples.
Synthesis of 2-methyl-6-alkylpyridines and geraniol fatty acid esters
The syntheses of 2-methyl-6-alkylpyridines followed the method described by Bandara Herath and Nanayakkara [39]. These syntheses are based on diastereoselective electrophilic substitution of enantiomerically-pure α-lithiated 2-alkylpiperidine. Six medium-chain (6–10 carbon atoms) and long-chain (12–26 carbon atoms) fatty acids were used to synthesize fatty acid esters of geraniol. Twenty microliters of liquid fatty acid, or 20 mg solid fatty acid, was mixed with 20 µl geraniol in a 200 µl insert in a 2-ml vial. After adding 40 µl of 3M sulfuric acid into the insert, the vial was sealed with a cap and heated at 100°C in a water bath for 30 min. After the vial cooled down, 200 µl distilled water was added to the insert. Two layers were formed. Since the synthesized ester was insoluble in water, the upper layer was collected. No effort was made to separate the ester from the excessive geraniol and fatty acid, and the collected upper layer was directly used in GC-MS analysis.
Data analysis
The concentrations of each alkaloid in the samples were measured using the ratio of the peak area of the target alkaloid to that of the internal standard, trans-2-methyl-6-dodecanoylpiperidine. To assess differences in alkaloid concentrations among different age groups, an analysis of variance was conducted using PROC ANOVA in SAS software, version 9.4 (SAS Institute Inc., 2004), with age group as the independent variable and alkaloid concentration as the dependent variable. Post-hoc tests, such as Tukey's HSD, were performed to identify specific age groups that differed significantly in alkaloid concentrations. To analyze the trend of the percentage data of trans-2-methyl-6-tridecenylpiperidine over ages, a second-degree polynomial curve was fitted to the percentage data using PROC REG in SAS. This allowed for the exploration of nonlinear relationships between age and percentage data. The model included age as the independent variable and percentage data as the dependent variable. The adequacy of the curve fit was assessed based on goodness-of-fit measures and visual inspection of the fitted curve against the observed data points.
Results
Chemical complexity of fire ant workers in relation to colony development
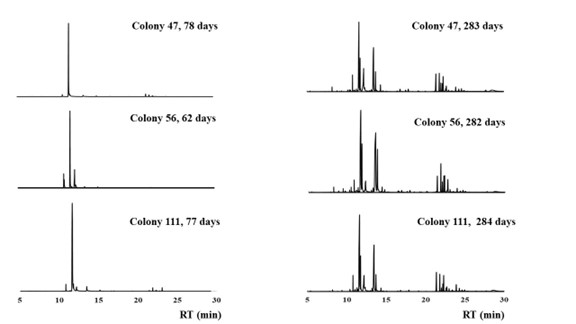
The chemical complexity of fire ants gradually increased with the development of the colony (Figure 1). The number of detected compounds in the body extraction increased until the colony reached approximately 150 days of age (from the date of queen collection) (Figure 2). Over 60 peaks were detected in the body extractions after 150 days of age.
Figure 1. Chromatograms of hexane ethyl acetate (1:1) extracts of whole bodies of worker ants from colonies of different ages, showing the chemical profiles of the workers become more complicated with the development of the colonies.
Figure 2. The relationship of number of compounds detected in worker body extractions with the age of the colonies. The number of detected compounds increased with the development of the colony, which reached a plateau after about 150 days.
Venom alkaloid profiles of fire ant workers in relation to colony development
There were significant variations in the concentration of total venom alkaloids per body weight (Figure 3). No significant difference was found in total alkaloid concentration per body weight among age groups for colony 56 (DF = 9, F = 1.75, P = 0.11) and colony 111 (DF = 11, F = 1.04, P = 0.44). While the ANOVA test suggested some differences among group means for colony 47 (DF = 9, F = 2.33, P = 0.043), the lack of significant mean comparisons after adjusting for multiple comparisons indicates that these differences are not reliable. Initially, trans-2-methyl-6-tridecenylpiperidine (trans-C13:1 piperidine) dominated and could account for up to 98% of all detected alkaloids. The percentage of trans- C13:1 piperidine gradually decreased due to the increase of other alkaloids (Figure 4). The trend quite fit to the second-degree polynomial model for all three colonies. Approximately 57-75% of the variability in the percentage of trans- C13:1 piperidine alkaloids could be explained by the colony age. In other words, the colony age collectively accounted for 57-75% of the variation in the percentage of trans- C13:1 piperidine alkaloids.
Figure 3. The concentrations of total trans-piperidine alkaloid in fire ant workers in relation to the colony age (mean ± SE). There were great variations among individuals, colonies and ages; however, there was always a concentration drop in the early stage (around 100 days) for all three colonies.
Figure 4. The percentage of trans-C13:1- piperidine alkaloid in the total trans-piperidine alkaloid in fire ant workers in relation to the colony age (mean ± SE). Initially, trans-C13:1 piperidine dominated and could account for up to 98% of all detected alkaloids. Its percentage gradually decreased due to the increase of other alkaloids.
Production peaks for the percentages of cis-2-methyl-6-undecylpiperidine (cis-C11 piperidine) (Figure 5) and trans-2-methyl-6-undecylpiperidine (trans-C11 piperidine) (Figure 6) were observed in the early stage (before 150 days). Within two months, piperidine, piperidene (both Δ1,6 and Δ1,2 isomers), and pyridine alkaloids were identified in the venom. Interestingly, piperidine alkaloids were always the first to appear, followed by piperidene and pyridine alkaloids (see all identified venom alkaloids in Table S1in Supplementary Materials).
Figure 5. The percentage of cis-C11- piperidine alkaloid in the total piperidine alkaloid in fire ant workers in relation to the colony age (mean ± SE). There was a peak between 100 and 150 days of the colony age for all three colonies.
Figure 6. The percentage of trans-C11- piperidine alkaloid in the total piperidine alkaloid in fire ant workers in relation to the colony age (mean ± SE). There were peaks before 150 days of the colony age for all three colonies.
Chemical profiles of Dufours’ gland in relation to colony development
Three farnesenes were detected in the extractions of body, sting apparatus, and sting apparatus with poison sac and poison gland removed. Z, E-α-farnesene was the dominant one, followed by Z,E-α-homofarnesene and E,E-α-homofarnesene (Figure 7). Six fatty acid esters of geraniol were identified (Table 1 and Figure S1-S6 in Supplementary Materials), with up to 26 unidentified compounds existing (Figure S7 to S32 in Supplementary Materials). All these compounds were determined to originate from Dufours’ glands. A full chromatogram of hexane ethyl acetate (1:1) extract of dissected worker sting apparatus without poison sac and gland was shown in Figure S33 and its peak assignment was shown in Table S2. Although quite unusual, some of these compounds did show up in the milked venom (in 2 of 30 samples), indicating that sometimes Dufours’ gland does contribute to the venom chemical makeup. The chemistry of Dufours’ glands of workers in the early stage of colony development seems more diversified than workers in mature colonies.
Figure 7. Partial chromatogram of hexane ethyl acetate (1:1) extracts of sting apparatus, showing three farnesenes and heptadecane. See Table 1 and Supplementary Material for other compounds in sting apparatus with poison gland removed.
Discussion
The chemistry of fire ant workers undergoes significant changes with the development of the colonies. However, the change in chemical diversity was not necessarily in the same direction for all individual glands. For example, components in the venom gland became more diversified with colony development, whereas a number of compounds in the Dufours’ gland only existed at the early stage of colony development.
Dufours’ gland plays a crucial role in fire ant society by providing pheromones for building foraging trails [40-42]. In fact, in the early stage of the colony development, S. invicta workers engage in brood raid [36,43-44], which also requires building trails [36]. It was observed that the trail used for brood raid can be reused even the next day, suggesting the presence of long-lasting components in the trail pheromone [36]. All the new compounds detected in this study are likely less volatile than farnesenes due to their greater molecular weight (Table 1), which may explain the long-lasting property of the trail built by workers participating in brood raid.
|
Compound Name |
RT (min)* |
KI** |
|
Geranyl decanonate |
12.233 |
2161.6 |
|
Geranyl dodecanonate |
14.397 |
2354.4 |
|
Geranyl tetradecanonate |
17.944 |
2553.1 |
|
Geranyl hexadecanonate |
22.041 |
2758.8 |
|
Geranyl oleate |
24.297 |
2931.5 |
|
Geranyl octadecanonate |
24.673 |
2965.4 |
Since the minim workers have a very simplified chemical profile and timid behaviors, they have been proposed as another caste in fire ant colonies [45]. This study demonstrated that there was a gradual, rather than stepwise, transition in alkaloid profiles with colony development (Figure 1), suggesting it might be difficult to define this ‘caste’ solely by using the alkaloid profile of the ant. It is important to determine whether there is a stepwise transition in behavior and morphology, which could be used to delineate this caste.
It has been proposed that piperidine alkaloid is formed from long-chain fatty acids, followed by the introduction of an amino group, intramolecular cyclization, and reduction of the imino group [46]. Since piperidine alkaloids consistently appeared before piperidene alkaloids, and pyridine alkaloids always appeared last, it may be possible that S. invicta synthesize piperidine alkaloids using alternative biosynthesis pathways and then oxidize the piperidines to piperidenes and pyridine alkaloids.
Although quite unusual, compounds from Dufours’ glands did sometimes show up in fire ant venom. Compared to venom alkaloids, these compounds must be minimal in abundance. However, minor compounds do not necessarily mean minor functions. It may be necessary to check how those compounds affect the toxicity of fire ant venom.
Extensive research has been conducted to identify proteins and peptides in fire ant venom [24]. In terms of their roles in allergic reactions, studying the dynamics of peptides and proteins in fire ant venom concerning colony age is equally important. In fact, this is also an unexplored research area. With significant advances in genomics, proteomics, and mass spectrometry techniques, investigating the dynamics of these proteins and peptides in relation to colony development should be a future research direction.
Acknowledgments
I thank Dr. Dhammika Nanayakkara in the National Center for Natural Products Research, School of Pharmacy, University of Mississippi for synthesizing fire ant venom alkaloids and Dr. Yuzhe Du, USDA-ARS, Southern Insect Management Research Unit for reviewing the early version of this manuscript. I thank Mr. Leon Hicks and Ms. Priya Chatakondi in USDA-ARS, Biological Control of Pests Research Unit for technical assistance. Mention of trade names or commercial products in this publication is solely for the purpose of providing specific information and does not imply recommendation or endorse-ment by the U.S. Department of Agriculture.
Statements & Declarations
Funding
The authors declare that no funds, grants, or other support were received during the preparation of this manuscript.
Competing interests
The authors have no relevant financial or non-financial interests to disclose.
Ethical approval
This article does not contain any studies with human participants or vertebrates performed by any of the authors.
Informed consent
Informed consent was obtained from all individual participants included in the study.
References
2. Bertelsmeier C. Globalization and the anthropogenic spread of invasive social insects. Curr Opin Insect Sci. 2021 Aug;46:16-23.
3. Ascunce MS, Yang CC, Oakey J, Calcaterra L, Wu WJ, Shih CJ, et al. Global invasion history of the fire ant Solenopsis invicta. Science. 2011 Feb 25;331(6020):1066-8.
4. Zhang R, Li Y, Liu N, Porter SD. An overview of the red imported fire ant (Hymenoptera: Formicidae) in mainland China. Florida Entomologist. 2007 Dec;90(4):723-31.
5. Wylie R, Yang CC, Tsuji K. Invader at the gate: The status of red imported fire ant in Australia and Asia. Ecological Research. 2020 Jan;35(1):6-16.
6. Menchetti M, Schifani E, Alicata A, Cardador L, Sbrega E, Toro-Delgado E, et al. The invasive ant Solenopsis invicta is established in Europe. Curr Biol. 2023 Sep 11;33(17):R896-R897.
7. deShazo RD, Soto-Aguilar M. Reactions to imported fire ant stings. Allergy Proc. 1993 Jan-Feb;14(1):13-6.
8. deShazo RD, Butcher BT, Banks WA. Reactions to the stings of the imported fire ant. N Engl J Med. 1990 Aug 16;323(7):462-6.
9. Solley GO, Vanderwoude C, Knight GK. Anaphylaxis due to Red Imported Fire Ant sting. Med J Aust. 2002 Jun 3;176(11):521-3.
10. Tankersley MS. The stinging impact of the imported fire ant. Curr Opin Allergy Clin Immunol. 2008 Aug;8(4):354-9.
11. Prahlow JA, Barnard JJ. Fatal anaphylaxis due to fire ant stings. Am J Forensic Med Pathol. 1998 Jun;19(2):137-42.
12. Stablein JJ, Lockey RF. Adverse reactions to ant stings. Clin Rev Allergy. 1987 May;5(2):161-75.
13. Wang L, Lu Y, Li R, Zeng L, Du J, Huang X, et al. Mental health effects caused by red imported fire ant attacks (Solenopsis invicta). PLoS One. 2018 Jun 25;13(6):e0199424.
14. Vinson SB. Invasion of the red imported fire ant (Hymenoptera: Formicidae): spread, biology, and impact. American Entomologist. 1997;43(1):23-39.
15. Liu Y, Huang J, Zhang J, Xu Y, Li X, Lu Y. Sensitization of Guinea Pig Skin to Imported Fire Ant Alkaloids and Establishment of an Inflammatory Model. Int J Environ Res Public Health. 2023 Jan 20;20(3):1904.
16. Neaves BI, Coop CA. Imported fire ant immunotherapy. Ann Allergy Asthma Immunol. 2024 Jan 26:S1081-1206(24)00013-9.
17. Hile DC, Coon TP, Skinner CG, Hile LM, Levy P, Patel MM, et al. Treatment of imported fire ant stings with mitigator sting and bite treatment--a randomized control study. Wilderness Environ Med. 2006 Spring;17(1):21-5.
18. Gonçalves Paterson Fox E, Russ Solis D, Delazari Dos Santos L, Aparecido Dos Santos Pinto JR, Ribeiro da Silva Menegasso A, Cardoso Maciel Costa Silva R, et al. A simple, rapid method for the extraction of whole fire ant venom (Insecta: Formicidae: Solenopsis). Toxicon. 2013 Apr;65:5-8.
19. Callahan PS, Blum MS, Walker JR. Morphology and histology of the poison glands and sting of the imported fire ant (Solenopsis saevissima v. richteri Forel). Annals of the Entomological Society of America. 1959 Sep 1;52(5):573-90.
20. Fox EG, Bueno OC, Yabuki AT, Jesus CM, Solis DR, Rossi ML, et al. General morphology and ultrastructure of the venom apparatus and convoluted gland of the fire ant, Solenopsis saevissima. Journal of Insect Science. 2010 Jan 1;10(1):24:11.
21. Xu G, Chen L. Biological Activities and Ecological Significance of Fire Ant Venom Alkaloids. Toxins (Basel). 2023 Jul 3;15(7):439.
22. Fox EGP, Xu M, Wang L, Chen L, Lu YY. Speedy milking of fresh venom from aculeate hymenopterans. Toxicon. 2018 May;146:120-123.
23. Vander Meer RK, Preston CA, Choi MY. Isolation of a pyrazine alarm pheromone component from the fire ant, Solenopsis invicta. J Chem Ecol. 2010 Feb;36(2):163-70.
24. Chen J. Chemistry and Functions of Imported Fire Ant Venom. Toxins (Basel). 2023 Aug 3;15(8):489.
25. Fox EGP, Adams RMM. On the Biological Diversity of Ant Alkaloids. Annu Rev Entomol. 2022 Jan 7;67:367-385.
26. Chen J, Zhao Y, Li XC, Zhao JH. Pyridine Alkaloids in the Venom of Imported Fire Ants. J Agric Food Chem. 2019 Oct 16;67(41):11388-11395.
27. Vander Meer RK, Alvarez F, Lofgren CS. Isolation of the trail recruitment pheromone ofSolenopsis invicta. J Chem Ecol. 1988 Mar;14(3):825-38.
28. Vander Meer RK. Semiochemicals and the red imported fire ant (Solenopsis invicta Buren)(Hymenoptera: Formicidae). Florida Entomologist. 1983 Mar 1:139-61.
29. Xu T, Zhang N, Xu M, Glauser G, Turlings TCJ, Chen L. Revisiting the trail pheromone components of the red imported fire ant, Solenopsis invicta Buren. Insect Sci. 2023 Feb;30(1):161-172.
30. Deslippe RJ, Guo YJ. Venom alkaloids of fire ants in relation to worker size and age. Toxicon. 2000 Feb;38(2):223-32.
31. Papillion AM, Hooper-Bùi LM, Strecker RM. Flooding increases volume of venom sac in Solenopsis invicta (Hymenoptera: Formicidae). Sociobiology. 2011 Jan 1;57(2):301-8.
32. Haight KL. Defensiveness of the fire ant, Solenopsis invicta, is increased during colony rafting. Insectes Sociaux. 2006 Feb;53:32-6.
33. Tschinkel WR. The fire ants. Cambridge, MA: Harvard University Press; 2006. p. 747, 16 p. of plates.
34. Meer RK. Behavioral and Biochemical Variation in the Fire Ant, Solenopsis invicta. In: Jeanne RL, editor. Interindividual behavioral variability in social insects. Westview studies in Insect Biology. Boulder, CO: Westview Press; 1988. p. 223-55.
35. Vander Meer RK. Chemical taxonomy as a tool for separating Solenopsis spp. In: Lofgren CS, Vander Meer RK, editors. Fire ants and leaf cutting ants: biology and management. Boulder, CO: Westview Press; 1986. p. 316-26.
36. Tschinkel WR. Brood raiding in the fire ant, Solenopsis invicta (Hymenoptera: Formicidae): laboratory and field observations. Annals of the Entomological Society of America. 1992 Sep 1;85(5):638-46.
37. Bales MT, Adams ES. Intraspecific usurpation of incipient fire ant colonies. Behavioral Ecology. 1997 Jan 1;8(1):99-103.
38. Chen J, Du Y. Fire ants feed their nestmates with their own venom. J Insect Physiol. 2022 Oct;142:104437.
39. Bandara Herath HM, Dhammika Nanayakkara NP. Synthesis of enantiomerically pure fire ant venom alkaloids: Solenopsins and isosolenopsins A, B and C. Journal of Heterocyclic Chemistry. 2008 Jan;45(1):129-36.
40. Vander Meer RK, Williams FD, Lofgren CS. Hydrocarbon components of the trail pheromone of the red imported fire ant, Solenopsis invicta. Tetrahedron Letters. 1981 Jan 1;22(18):1651-4.
41. MEER RK, Lofgren CS, Alvarez FM. The orientation inducer pheromone of the fire ant Solenopsis invicta. Physiological Entomology. 1990 Dec;15(4):483-8.
42. Vander Meer RK. The trail pheromone complex of Solenopsis invicta and Solenopsis richteri. In: Lofgren CS, Vander Meer RK, editors. Fire ants and leaf cutting ants: Biology and management. Boulder, CO: Westview Press; 1986. p. 201-10.
43. Tschinkel WR, Howard DF. Colony founding by pleometrosis in the fire ant, Solenopsis invicta. Behavioral Ecology and Sociobiology. 1983 May;12:103-13.
44. Tschinkel WR. The fire ant, Solenopsis invicta, as a successful 'weed'. In: Eder J, Rembold H, editors. Chemistry and Biology of Social Insects. Verlag: München; 1987. 757p.
45. Bosworth J, Vander Meer RK. Colony founding minims: A new Solenopsis invicta caste. Proceedings of the 1984 Imported Fire Ant Conference, March 27-28, 1984, Gainesville, Florida, 233 p.; 1984.
46. Leclercq S, Braekman JC, Daloze D, Pasteels JM, Van der Meer RK. Biosynthesis of the solenopsins, venom alkaloids of the fire ants. Naturwissenschaften. 1996 May;83:222-5.