Abstract
Background: Cat allergens are a major contributor to environmental allergens' overall burden, but efforts to reduce cat allergens are often unsuccessful.
Objective: To determine whether feeding cats a diet containing an egg product with anti-Fel d1 IgY would produce clinically relevant reductions in allergy symptoms of human subjects.
Methods: Following a priming exposure to blankets used for cat bedding, human subjects were subsequently exposed to environmental chambers primed with blankets from cats fed either a control diet or a test diet containing an egg product with polyclonal anti-Fel d1 IgY. 8 cats: 5 neutered male and 3 spayed females were used. Total Nasal Symptom Score and Total Ocular Symptom Score were assessed at regular intervals. Subjects were randomly exposed to the control or test condition on the first exposure and the opposite condition on the second exposure.
Results: The levels of immunologically active Fel d1 in chambers with blankets from cats fed the test diet were lower than those from control cats, and human subjects exposed to this condition showed significantly lower Total Nasal Symptom Scores and improvement in some ocular symptoms.
Conclusion: Fel d1 levels in the environment are significantly decreased by feeding cats a diet containing egg product with polyclonal anti-Fel d1 IgY. This decrease in allergen results in a significant in total nasal symptom scores and some ocular symptoms.
Keywords
Cat allergy, Fel d1, IgY, Cat dander, allergy, Allergen exposure, Cat
Abbreviations
aFel d1: Immunologically Active Fel d1; TNSS: Total Nasal Symptom Score; TOSS: Total Ocular Symptom Score
Introduction
Cat allergens are a major contributor to the overall burden of environmental allergens throughout the world. In the US, it is estimated that there are approximately 86.5 million cats residing in over 40 million households [1]. The ubiquitous nature of the primary cat allergen, Fel d1, within the indoor environment has made control of cat allergy difficult, if not impossible. Although several methods for decreasing the allergen load within the home have been described [2,3], the majority have limitations. For example, bathing cats regularly transiently decreases allergen levels on the hair. However, cats often resist bathing, and it is often challenging for cat owners. Allergists [3], often recommend removal of all cats from the house followed by comprehensive cleaning but this often fails because cat owners are unwilling to comply.
The exact function of Fel d1 is unknown; therefore, methods to reduce or stop its production by the cat may not be desirable. An alternative approach is to develop methods for decreasing the amount of immunologically active Fel d1 (aFel d1) in the environment. In a previous study [4], we have shown that the allergenicity of Fel d1 can be neutralized with anti-Fel d1 polyclonal immunoglobulin Y (IgY) from chicken egg yolks using both in vitro and ex vivo mast cell culture models. Immunoglobulin Y is avian immunoglobulins equivalent to mammalian IgG. Naturally produced by chickens, IgY is transferred and concentrated in egg yolks to provide passive immunity for the offspring. Consequently, large quantities of IgY accumulate in chicken egg yolks, extracted, purified, and delivered in food. Orally ingested chicken egg-derived IgY antibodies have proven safe and efficacious in reducing diarrhea in domestic animals in multiple studies [5]. In a recent study, Satyaraj et al. (unpublished observation) demonstrated that chickens exposed to cats naturally produce anti-Fel d1 IgY in their eggs.
Satyaraj et al. previously [6] demonstrated that feeding cats a diet with an egg product containing anti-Fel d1 IgY resulted in a significant decrease in the amount of immunologically active Fel d1 in the cats’ saliva. In a subsequent study, cats fed the same diet showed significantly reduced levels of immunologically active Fel d1 on their hair [7]. This provided the basis for this proof of concept study. In this study, we asked whether feeding cats a diet with egg product containing anti-Fel d1 IgY would produce clinically relevant reductions in allergy symptoms in human subjects.
Methods
The study protocols were reviewed and approved by the Institutional Animal Care and Use Committee. The study cats were outbred “Domestic Short-Hair”. Eight cats were used for this study (five neutered male cats and three spayed female cat). All cats were individually housed in accommodations that met or exceeded the requirements outlined in the Animal Welfare Act. Rooms were maintained between 50°F and 85°F and were assigned to a 12-hour light/dark cycle. Cats were individually fed to maintain body weight, with food available up to 22 hours daily, and water was available ad libitum. Body weight was monitored, and the amount of food provided was adjusted as needed to maintain ideal body weight. Cats were evaluated twice daily by trained personnel to ensure their good health and well-being. Veterinary care was provided as needed. All cats received a veterinary physical examination before the start and again after the studies. Following the completion of each study, all cats were returned to the facility’s general cat population.
The control and test diets were formulated and manufactured by Nestlé Purina PetCare Company. The formulations were nutritionally complete and balanced based upon the Association of American Feed Control Officials and Nestlé Purina PetCare Company’s standards. The control and test diets were identical except that the test diet was formulated with egg product containing anti-Fel d1 antibodies to provide 8 ppm anti-Fel d1 specific IgY in diet. Eight cats were enrolled in the feeding trial, of which four were put on the control diet and four on the test diet with egg product containing anti-Fel d1 antibodies for eight weeks. During the last four weeks of the study, these cats’ blankets were collected and used to load exposure chambers described below.
To determine the effect of decreasing immunologically active Fel d1 (aFel d1) in the environment, a series of portable exposure chambers were used [8]. Portable “Spring House Clear Growth” greenhouse chambers were purchased from FlowerHouse, Inc, Clio, MI. The chambers measured 6’-deep×6’-wide×6.5’ tall (1.8m×1.8m×2m) and were enclosed (Figure 1). The chambers had a single zippered entrance and were clear for observations. Each chamber contained a chair for the participants, a cat blanket pre-loaded with cat hair from test-fed or control-fed cats, a small fan providing circulation, and an area for collecting and measuring aFel d1 levels within the chamber to quantitate total aFel d1 exposure.
Figure 1: Exposure Chambers: Portable “SpringHouseClear Growth” greenhouse chambers (FlowerHouse, Inc, Clio, MI). The chambers measure 6’-deep x 6’-wide x 6.5’ tall (1.8m x 1.8m x 2m) and are totally enclosed, have a single zippered entrance and are clear for observation.
Six chambers were set up in a large open area in the allergy clinic at Washington University School of Medicine in St. Louis, MO. Blankets from the test- and control-fed cats housed in the Purina Petcare Center in St Joseph, MO were transported in sealed bags to the clinic area; control and test blankets were placed to load the appropriate chambers. The chambers were closed, and the fan was allowed to run for 36 hours to allow allergen loading of the chambers (Wedner et al, submitted). Petri dishes (100×15 mm BD Falcon) coated with 5% gelatin solution were set in each of the exposure chambers to collect airborne Fel d1.
These coated plates were placed in the chambers, lids removed, and allowed to collect dust for a set period. At the end of the collection period, 2 ml PBS-Tk (PBS, 0.1% tween-20) was added to the dish and plates were rotated overnight at room temperature at 300 RPM. The PBS-T solution was then removed, centrifuged at 1000xg for 10 min and Fel d1 levels were measured by ELISA assay (Indoor Biotechnologies, Charlottesville, VA) and expressed as ng of Fel d1/ml or ng of Fel 1/plate/hour to normalize for time. The amount of aFel d1 was measured periodically throughout this period. Immunologically active Fel d1 levels were measured by ELISA (Indoor Biotechnologies, Charlottesville, VA). Once loaded, the chambers were used to expose participants, as described below.
The Washington University approved the study approved the study approved the study in St. Louis Institutional Review Board, and each participant signed an informed consent before participating in the study. Participants, age 18 to 65 years, were identified as allergic to cats based upon a history of cat sensitivity and a positive prick skin test to the standardized cat allergen extract (Greer Labs, Lenoir, NC). A positive skin test was determined as 3 mm or greater than the negative control. Potential participants with a history of asthma were excluded from the study. The study was double-blinded as both the participants and the study personnel who observed the participants were unaware of each exposure's condition. Each participant served as their own control. As the participant were all polysensitized (see below) the exposures were all performed after the fall pollen season and before the spring pollen season in St. Louis. The participants were not exposed to cats in their home environment. During the exposure, periods participant refrained from taking antihistamines.
The demographic information for the participants is shown in Table 1. Eleven subjects, aged 26 to 59 years, and 1 male and 10 females were recruited for the study. All participants had a strong history of cat allergy and were skin prick test positive to standardized cat extract. None of the 11 participants were current cat owners. None had asthma.
|
Participant |
Age |
Sex |
Ethnicity |
Skin Test to cat |
Other Allergies by history |
|
1 |
39 |
F |
Cauc |
13/45 |
"pollen" |
|
2 |
42 |
F |
Cauc |
13/30 |
Dogs, Fall Pollen |
|
3 |
27 |
M |
Cauc |
7/15 |
Ragweed |
|
4 |
59 |
F |
Cauc |
13/30 |
"Dust", Mold |
|
5 |
30 |
F |
Cauc |
12/35 |
Mold, Pollen, Dust mites |
|
6 |
48 |
F |
Cauc |
9/19 |
Weeds, Grass, Dust mites |
|
7 |
26 |
F |
Cauc |
9/40 |
"Hay Fever" |
|
8 |
59 |
F |
Cauc |
10/25 |
Trees, Ragweed, Mold |
|
9 |
25 |
F |
Cauc |
9/35 |
Dogs, Mold, Ragweed, "Dust" |
|
10 |
34 |
F |
Cauc |
7/25 |
None reported |
|
11 |
33 |
F |
Cauc |
8/7 |
None reported |
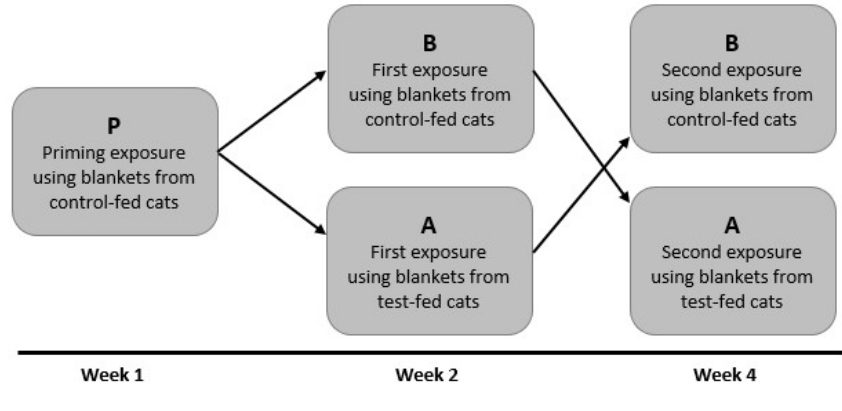
Each participant had three exposures in the environmental chambers. Based upon studies in large environmental chambers [9], we decided to perform a “priming exposure” in the chamber, followed by exposures to bedding from control fed cats or cats fed test diet (Figure 2). Thus, a series of three exposures, priming (unblinded) followed by exposure to blankets from cats fed control diet or test diets, was performed. All participants were exposed to bedding from control-fed cats for 3 hours (or until symptoms became intolerable) as a priming dose. The amount of aFel d1 was monitored throughout this priming exposure (P) period. During this and each subsequent exposure, each participant filled out a questionnaire for total nasal symptom score (TNSS) [10] and total ocular symptom score (TOSS) [10] every 15 minutes throughout the exposure period. Participants were asked to rank their individual symptoms of each scoring system using a scale of 0=None, 1=Mild, 2=Moderate, or 3=Severe. Neither the participants nor the study personnel were informed as to the amount of aFel d1 in the chambers.
Figure 2: Diagrammatic Representation of Study Protocol. Individuals who were cats sensitive but not exposed to cats at home were selected for the study.
As shown in Figure 2, one week following the priming dose, each participant was exposed to cat allergen in the chamber for 3 hours (or until symptoms became intolerable) in the chambers to blankets from cats fed either a control diet or test diet (Exposure 1). The blanket condition used was only known to the person who performed the loading of the individual chambers, and the participants and the study personnel who observed the participants during the exposure periods were blinded to the conditions. Participants were randomly assigned to one of the two exposures (A, test-fed cats or B, control-fed cats). The subsequent session (Exposure 2), which took place two weeks later, was a crossover exposure to the other condition; those subjected to condition A during Exposure 1 were subjected to condition B during Exposure 2, and vice versa. The participants and study observers were aware that it was a crossover design but were unaware of the specific conditions during each exposure session. As noted above, each participant filled out the TNSS and TOSS every 15 minutes until the end of the 3-hour study period or until symptoms became intolerable.
The primary outcome variable was the change in symptoms scores comparing the initial high dose priming condition and the test or control exposures. Statistical analysis was performed across the three levels of exposure and the differences were stated using the Kruskal-Wallis and the Dwass, Chrichlow-Fligner methods for 2-sided pair-wise comparisons [11,12]. A variable accosting to the cross-over design and the sequence of allocating to the three exposures priming (P), exposure 1 (A) and exposure 2 (B) was created and testing with the exposure arm in a multivariable mixed-effect regression models were examined to evaluate predictors. The differences in some of the variables were then evaluated using the Chi-square analysis.
For each analysis the differences between the total symptom score either TNSS or TOSS were calculated, and this comparison was then extended to each of the sub-categories for the symptoms scores. These were runny-nose, sneezing, nasal-itching and congestion for the TNSS and itchy-eyes, scratchy-eyes and watery eyes for the TOSS. For each of the total scores and the sub-scores the comparisons were carried out, and p-values were calculated. For each of the comparison a p-value of 0.05 or less was considered significant. When comparing the individual components of the TNSS and the TOSS the Bonferoni adjustment was made to correct for multiple observations.
Results
The present study noted that the amount of immunologically active Fel d1 (aFel d1) that was loaded into the chambers was significantly reduced when using blankets from cats fed the test diet versus blankets from cats fed the control diet. This demonstrated that feeding cats the diet with egg product containing anti-Fel d1 IgY antibodies was sufficient to decrease participants' exposure to cat allergen.
Demographic Data
The demographic information for the participants is shown in Table 1.
Eleven subjects, aged 26 to 59 years, and 1 male and 10 females were recruited for the study. All participants had a strong history of cat allergy and were skin prick test positive to standardized cat extract. None of the 11 participants were current cat owners. None had asthma.
Symptom Scores
Although our preliminary studies suggested that ‘priming’ exposure to cat allergen would be necessary, this did not prove to be the case. Indeed, the symptoms scores for TNSS, TOSS, and each sub-group tended to be higher in the priming exposure than the subsequent exposure periods. The priming exposure data, exposure A (test-fed cats) and exposure B (control fed cats) are shown in Tables 2a, 2b and 2c, respectively. It should be noted that by design, the amount of Fel d1 in the priming chambers was high and, indeed, the amount of Fel d1 in the priming chambers was more elevated than either the control or treated cat chambers. Fel d1 levels in priming exposure are shown in Table 3.
|
Total Nasal Symptom Score |
Mean |
Standard deviation |
|
TNSS |
32.6 |
20.3 |
|
Runny Nose |
10 |
9 |
|
Sneezing |
1.9 |
3.8 |
|
Nasal Itching |
11.4 |
9.7 |
|
Nasal Congestion |
9.5 |
9 |
|
TOSS |
14 |
15.4 |
|
Itchy Eyes |
8.7 |
8.4 |
|
Scratchy Eyes |
2.4 |
3.5 |
|
Watery Eyes |
3 |
5.4
|
|
Total Nasal Symptom Score |
Mean |
Standard deviation |
|
TNSS |
17.9 |
17.15 |
|
Runny Nose |
4.27 |
8.27 |
|
Sneezing |
0.18 |
0.6 |
|
Nasal Itching |
7.64 |
7.8 |
|
Nasal Congestion |
5.82 |
6.4 |
|
TOSS |
9.36 |
10.3 |
|
Itchy Eyes |
6.6 |
6.19 |
|
Scratchy Eyes |
0.18 |
0.4 |
|
Watery Eyes. |
2.64 |
7.19 |
|
Total Nasal Symptom Score |
Mean |
Standard deviation |
|
TNSS |
14.64 |
10.39 |
|
Runny Nose |
3.91 |
4.76 |
|
Sneezing |
0.27 |
0.65 |
|
Nasal Itching |
5.64 |
5.05 |
|
Nasal Congestion |
4.82 |
5.25 |
|
TOSS |
6.09 |
4.83 |
|
Itchy Eyes |
4.27 |
4.84 |
|
Scratchy Eyes |
0.45 |
1.21 |
|
Watery Eyes. |
1.18 |
3.6 |
|
Levels of Fel d1 in the priming chambers |
|
|
Chamber # |
Mean Fel d1 levels |
|
Chamber 1 |
8.5 |
|
Chamber 2 |
8 |
|
Chamber 3 |
12 |
|
Chamber 4 |
12.1 |
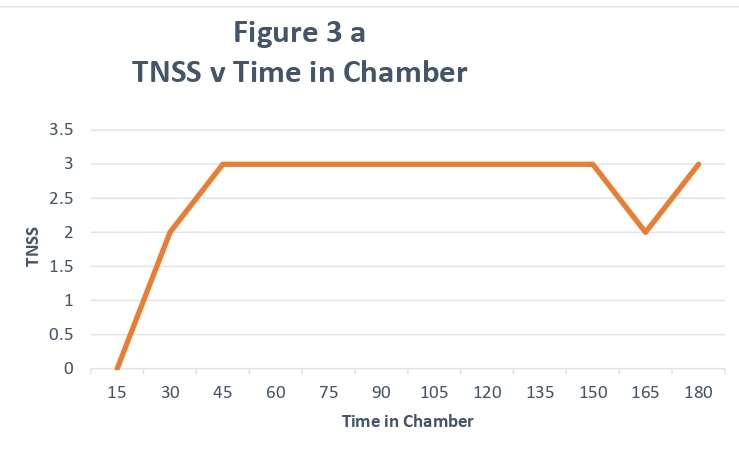
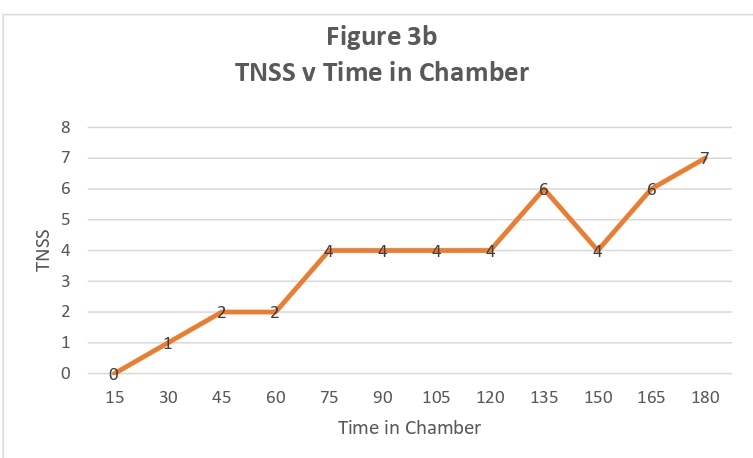
For most participants, the change in TNSS and TOSS was noted rapidly, usually within 30 minutes and, for the majority of subjects, remained constant for the remainder of the study period (representative subject data shown in Figure 3a). By contrast, several participants had an early response to the allergen exposure, which gradually increased during the exposure period (representative subject data shown in Figure 3b). Although several participants had TNSS above 7, no participant left the chamber early.
Figure 3A: Representative TNSS Scores showing rapid increase in the first 30 min: X- axis denotes time in chamber and Y-axis denotes TNSS score.
Figure 3B: Representative TNSS Scores showing gradual increase: X- axis denotes time in chamber and Y-axis denotes TNSS score.
As can be noted from the size of the standard deviations shown in Table 2, there was significant variability among the 11 participants. This variability was not related to the participants' age or sex and was not related to the size of the wheal or flare of the positive skin tests. In the following data for comparing the two exposures, it was noted that although the variability continued, those participants who had higher scores tended to remain high, while those with lower scores tended to stay lower.
Exposure Sequence
We next evaluated the sequence of exposures following priming (P), i.e., whether exposure to condition A or B during the first exposure session affected the data. This was done for both the TNSS and its sub-scales and the TOSS and it sub-scales. For each comparison, the sequence of exposure did not affect the overall data. Using a mixed model analysis, the p-values for the A v. B comparison were not significant and varied from 0.90 for watery eyes to 0.23 for scratchy eyes. The p-values for the other scales were between these two values. These are shown in Table 4.
|
Symptom |
P value |
|
Watery eyes |
90.9 |
|
Scratchy eyes |
0.23 |
|
Itchy eyes |
0.46 |
|
Nasal Congestion |
0.62 |
|
Nasal Itching |
0.43 |
|
Sneezing |
0.36 |
|
Runny Nose |
0.88 |
|
TNSS |
0.75 |
|
TOSS |
0.64 |
We then compared the scores to compare the priming exposure (P) and the two subsequent exposures (A, blankets from test-fed cats; B, blankets from control-fed cats). The data demonstrated that both exposures A and B had lower scores than the priming values and the chi-square analysis revealed that this was significant for the TNSS (p=0.035) but not for the TOSS (p=0.72). When exposure A and exposure B were compared to the priming values, the difference was significant for the test-fed cats, but not for the control-fed cats. The subgroup analysis did not demonstrate that one or more of the subgroups was significant and thus the outcome was the result of the sum of the 4 sub scores in the TNSS.
Subscale Comparisons
Using the mixed model analysis, we then compared each of the sub scales to see if this would demonstrate each group's contribution to the overall scores. This comparison did indicate that there were significant differences among the sub-groups. For the ocular sub-groups, the difference between priming and test-fed cats (A) was significant for scratchy eyes (p=0.015), but not for control-fed cats (B; p=0.051). This was also true for the itchy eyes comparison (P v. A, p=0.0072; P v. B, p=0.136).
Among the nasal subgroups, congestion was significant for the P v A group was substantial (p=0.0055), while the P v B comparison was not (p=0.170). There was a trend favoring the test-fed cats versus control-fed cats for nasal itching, sneezing, and runny nose, but this did not reach statistical significance.
Discussion
Fel d1 is a uteroglobin-like protein that comprises the major allergen among the known 10 cat allergens [13]. Fel d1 is produced primarily by the salivary and sebaceous glands and then deposited on the haircoat by grooming and by direct deposition on the hair shafts by the glands at their base [13]. Fel d1 easily becomes airborne, primarily carried by small particles (less than 5 microns in diameter), and persists in the environment [3,14,15].
All cats produce Fel d1 regardless of breed, gender or neuter status; there are no truly allergen-free or hypoallergenic cats [14-18]. Unfortunately, the mythical allergen-free or hypoallergenic cat has not been seen in nature nor developed using various methods [15]. It should be noted that allergen production by individual cats varies widely [19]. Male cats in general produce more allergen than females [2]. But female cats also produce a significant amount of allergen, we noted that Fel d1 allergen levels varied within the cat colony used in this study, and this was a significant confounder. Indeed, Fel d1 was significantly greater in the P group than in either the test or control diet-fed cats. For this reason, we chose to compare the change in symptom scores between the P exposure and either the A or B exposures. This demonstrated that going from a very high Fel d1 environment to a lower Fel d1 level produced by feeding cats the test diet was clinically relevant.
A standard recommendation for managing human allergies to cats is to remove the cats from the home environment [3]. This has been effective in some studies, but the reduction in allergen levels is modest and takes a significant amount of time. Wood et al [20]. Observed that 8 of 15 homes (53%) reached Fel d1 levels consistent with control homes (homes without cats) by 20-24 weeks after cat removal.
Many, if not most, cat owners struggle to comply with recommendations to remove their cat from home, necessitating approaches to mitigate the environmental allergen load. Bjjarnsdottir et al. [21]. Reported significant reductions of Fel d1 in dust samples from cat-owning homes with stringent environmental control measures, including regular and thorough cleaning; removal of carpeting and upholstered furniture from bedrooms; weekly vacuuming of floors and furniture; weekly laundering of bedding; placement of impermeable mattress and pillow covers; weekly bathing of the cat(s); keeping cats out of the bedroom; and sleeping with the bedroom windows open. Although effective, the intensive effort required for these measures may be difficult to maintain long-term. Therefore, methods that allow the cat to remain in the home and minimize onerous cleaning burdens while reducing allergic symptoms would be appealing to cat owners.
Our group's separate investigation validated the portable exposure chambers utilized in this study as an effective and economical method for studying human allergic symptoms related to changes in environmental aFel d1 levels [8]. Portable environmental chambers were used to better control the allergen exposure levels, as compared to the variability of allergen levels in in-home studies such as that of Bjarnsdottir and colleagues [21]. Our previous study demonstrated that the chambers can effectively be loaded with aFel d1 using cat blankets and once loaded, aFel d1 levels are maintained at relatively constant levels throughout the participant exposure period [8]. The current study demonstrated that the levels of immunologically active aFel d1 from cats fed the test diet with egg product containing anti-Fel d1 IgY antibodies ingredient were lower than those of the control-fed cats.
Additional work by our group has demonstrated that feeding cats a diet with egg product containing anti-Fel d1 IgY significantly decreased the levels of aFel d1 in cat hair and dander [6,7]. The purpose of the current study was to determine whether the decrease in Fel d1 shed by cats fed a diet with egg product containing anti-Fel d1 Igy antibodies is sufficient to reduce nasal and ocular symptoms in a chamber study. Although studies now suggest that the amount of allergen produced by the sebaceous glands exceeds that of the saliva [17], decreasing the amount of salivary aFel d1 significantly decreases the amount of allergen on the cat’s hair and, as demonstrated here, is sufficient to be clinically relevant. In addition, any unbound anti-Fel d1 IgY antibody in the saliva can bind and neutralize sebaceous-origin Fel d1 on the haircoat as the cat deposits saliva during grooming.
In the current study, there was a statistically significant decrease in TNSS compared to the priming exposure when participants were exposed to an environment loaded with blankets with hair from test-fed cats and no difference when participants were exposed to an environment loaded with blankets with hair from control-fed cats. The comparison for TOSS was not significant. However, using a mixed-exposure model we were able to demonstrate that several of the nasal and ocular subscales did reach statistical significance.
We have not compared the component analysis of the cat allergens among the group of cat-sensitive patients used in this study. In an ongoing in-home study, we are evaluating the component sensitivity of the participants to eliminate this as a confounder to the data.
The present study demonstrates that the amount of aFel d1 deposited in a portable environmental chamber is significantly decreased by feeding cats a diet with egg product containing anti-Fel d1 IgY antibodies, and this decrease in allergen results in a significant reduction in total nasal symptom scores and in some ocular symptoms in human subjects.
The most common recommendation for managing cat allergies is to remove the cat from home, which may cause emotional stress for the cat owner and result in poor compliance. Alternatively, allergic cat owners are instructed to follow rigorous cleaning methods that may be difficult for many owners to implement and maintain long-term. Given that the exact role of Fel d1 in the cat is currently unknown, the method described in this study reduces allergenic Fel d1 in the environment without altering its production. This radically different approach reduces the immunologic activity of the Fel d1 allergen without adversely affecting the cat’s physiology and offers a solution for the management of cat allergies while allowing cats to remain in the household.
Conflict of Interest
Feeding cats egg product with polyclonal-anti-Fel d1 antibodies decreases environmental Fel Dd1 and allergic response: a proof-of-concept study.
H James Wedner receives research funds from Nestle Purina Research and consulting fees from Nestle Purina Research He has no other conflicts related to this article. Terisa Mantia receives research funds from Nestle Purina Research. Ebenezer Satyaraj, Cari Gardner and Scott Sherrill are employees of Nestle Purina Research. They report no other conflicts. Noor Al-Hammadi reports no conflicts.
Funding
This study was funded by Nestlé Purina Research.
References
2. Jalil-Colome J, de Andrade AD, Birnbaum J, Casanovab D, Mège JL, Lanteaume A, Charpin D, Vervloet D. Sex difference in Fel d 1 allergen production. Journal of allergy and clinical immunology. 1996 Jul 1;98(1):165-8.
3. Dávila I, Domínguez‐Ortega J, Navarro‐Pulido A, Alonso A, Antolín‐Amerigo D, González‐Mancebo E, Martín‐García C, Núñez‐Acevedo B, Prior N, Reche M, Rosado A. Consensus document on dog and cat allergy. Allergy. 2018 Jun;73(6):1206-22.
4. Satyaraj E, Sun P, Sherrill S. Fel d1 blocking antibodies in cat saliva as a novel strategy to reduce IgE mediated allergy to cats. Forthcoming 2019.
5. Diraviyam T, Zhao B, Wang Y, Schade R, Michael A, Zhang X. Effect of chicken egg yolk antibodies (IgY) against diarrhea in domesticated animals: a systematic review and meta-analysis. PloS one. 2014 May 20;9(5):e97716.
6. Satyaraj E, Li Q, Sun P, Sherrill S. Anti-Fel d1 immunoglobulin Y antibody-containing egg ingredient lowers allergen levels in cat saliva. Journal of feline medicine and surgery. 2019 Oct;21(10):875-81.
7. Satyaraj E, Gardner C, Filipi I, Cramer K, Sherrill S. Reduction of active Fel d1 from cats using an antiFel d1 egg IgY antibody. Immunity, inflammation and disease. 2019 Jun;7(2):68-73.
8. Wedner J, Mantia T, Satyaraj E. Using individual exposure chambers to study cat allergy. J Allergy Clin Immunol. Forthcoming 2019.
9. Day JH, Horak F, Briscoe MP, Canonica GW, Fineman SM, Krug N, Leynadier F, Lieberman P, Quirce S, Takenaka H, Van Cauwenberge P. The role of allergen challenge chambers in the evaluation of anti‐allergic medication: an international consensus paper. Clinical & Experimental Allergy Reviews. 2006 Feb;6(2):31-59.
10. Litvyakova LI, Baraniuk JN. Nasal provocation testing: a review. Annals of Allergy, Asthma & Immunology. 2001 Apr 1;86(4):355-65.
11. McDonald JH. Handbook of Biological Statistics (3rd ed.). Sparky House Publishing, Baltimore, Maryland; 2014.
12. Douglas CE, Michael FA. On distribution-free multiple comparisons in the one-way analysis of variance. Communications in Statistics-Theory and Methods. 1991 Jan 1;20(1):127-39.
13. Bonnet B, Messaoudi K, Jacomet F, Michaud E, Fauquert JL, Caillaud D, Evrard B. An update on molecular cat allergens: Fel d 1 and what else? Chapter 1: Fel d 1, the major cat allergen. Allergy, Asthma & Clinical Immunology. 2018 Dec 1;14(1):14.
14. Satyaraj E, Gardner C, Filipi I, Cramer K, Sherrill S. Reduction of active Fel d1 from cats using an antiFel d1 egg IgY antibody. Immunity, inflammation and disease. 2019 Jun;7(2):68-73.
15. Salo PM, Cohn RD, Zeldin DC. Bedroom allergen exposure beyond house dust mites. Current allergy and asthma reports. 2018 Oct 1;18(10):52.
16. Butt A, Rashid D, Lockey RF. Do hypoallergenic cats and dogs exist?. Annals of Allergy, Asthma & Immunology. 2012 Feb 1;108(2):74-6.
17. Kelly LA, Erwin EA, Platts-Mills TA. The indoor air and asthma: the role of cat allergens. Current opinion in pulmonary medicine. 2012 Jan;18(1):29.
18. Kelly SM, Karsh J, Marcelo J, Boeckh D, Stepner N, Santone B, Yang J, Yang WH. Fel d 1 and Fel d 4 levels in cat fur, saliva, and urine. Journal of Allergy and Clinical Immunology. 2018 Dec 1;142(6):1990-2.
19. Nicholas C, Wegienka G, Havstad S, Ownby D, Johnson CC. Influence of cat characteristics on Fel d 1 levels in the home. Annals of Allergy, Asthma & Immunology. 2008 Jul 1;101(1):47-50.
20. Bastien BC, Gardner C, Satyaraj E. Influence of time and phenotype on salivary Fel d1 in domestic shorthair cats. Journal of feline medicine and surgery. 2019 Oct;21(10):867-74.
21. Wood RA, Chapman MD, Adkinson Jr NF, Eggleston PA. The effect of cat removal on allergen content in household-dust samples. Journal of allergy and clinical immunology. 1989 Apr 1;83(4):730-4.
22. Björnsdottir US, Jakobinudottir S, Runarsdottir V, Juliusson S. The effect of reducing levels of cat allergen (Fel d 1) on clinical symptoms in patients with cat allergy. Annals of Allergy, Asthma & Immunology. 2003 Aug 1;91(2):189-94.