Abstract
Numerous animal studies have shown that the decrease in the tissue Nicotinamide Adenine Dinucleotide (NAD+) levels during aging are closely related to age-related physiological decline and that the administration of NAD+ precursors restore NAD+ levels and promotes health and prolongs lifespan. Therefore, in order to demonstrate whether NAD+ supplementation by NAD+ precursors mitigate age-related physiological dysfunction including muscle weakness in older men. We conducted a placebo-controlled, randomized, double-blind, parallel-group study in which Nicotinamide mononucleotide (NMN) was administered to healthy older men for 12 weeks. In this commentary, we overview reported human NAD+-related clinical trials including our NMN study.
Keywords
Nicotinamide Mononucleotide, Nicotinamide Adenine Dinucleotide+, Nicotinamide Riboside, Nicotinamide, Sirtuins
Introduction
Aging is a risk factor for all kinds of diseases, including diabetes, dyslipidemia, cardiovascular diseases and neurological diseases such as Alzheimer's disease. Suppression of age-related function decline could be an important approach in preventing these diseases. In this commentary, we focus on Nicotinamide Adenine Dinucleotide (NAD+) metabolism, one of critical regulators of aging and overview recent clinical trials in which NAD+ precursors, Nicotinamide mononucleotide (NMN) or Nicotinamide riboside (NR) were administered to increase NAD+ contents.
Calorie Restriction and Sirtuins
Caloric restriction is the only non-pharmacological intervention that suppresses age-related function decline among many species, and in particular, NAD+-dependent deacetylase sirtuin is one of the molecules which bring out the effect of caloric restriction on longevity. Sir2, an NAD+-dependent deacetylase, has been shown to be a regulatory factor of lifespan and senescence in budding yeast, C. elegans, and Drosophila. Mammals contain seven homologs of Sir2, SIRT1–7, which are involved in a wide range of cellular processes such as metabolism, cell proliferation, and apoptosis [1]. The degradation of NAD+ to nicotinamide (NAM) by sirtuins is conjugated with the deacetylation of lysine residues of specific proteins, which relates to health promotion and longevity [1].
NAD+ Metabolism
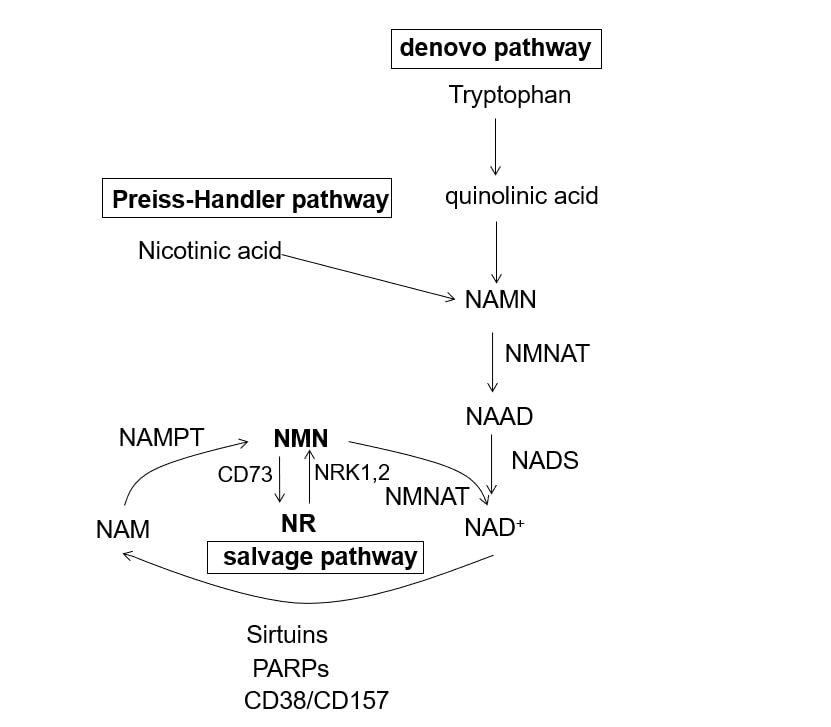
The NAD+ synthesis pathway consists of a de novo synthesis system originated from tryptophan, a Preiss-Handler pathway starting from nicotinic acid, and a salvage pathway that recycles NAM produced by the decomposition of NAD+ (Figure 1) [2,3]. Nicotinamide phosphoribosyltransferase (NAMPT), a rate-limiting enzyme in the NAD+ synthesis, plays an important role in the salvage pathway, which converts NAM to NMN. Following the conversion of NAM to NMN by NAMPT, NMN is resynthesized to NAD+ by a second enzyme, Nicotinamide/Nicotinic acid Mononucleotide Adenylyltransferase (NMNAT). The decrease of NAD+ levels and sirtuin activity during aging are closely related to age-related decline in physiological function [2,3]. Numerous preclinical studies have revealed that the elevation of NAD+ levels on the administration of an NAD+ precursor, NMN or NR, can mitigate age-related disorders; however, human data are under investigation [2,3].
Figure1. NAD+ synthesis in mammals. NAAD: Nicotinic Acid Adenine Dinucleotide; NADS: NAD Synthetase; NAMN: Nicotinic Acid Mononucleotide; NAM: Nicotinamide; NMN: Nicotinamide Mononucleotide; NR: Nicotinamide Riboside; NAMPT: Nicotimamide Phosphoribosyltransferase; NMNAT: Nicotinamide Mononucleotide Adenylyltransferase; PARPs: Poly ADP Ribose Polymerases; NRK: Nicotinamide Ribose Kinase.
NR Human Clinical Trials
NR is a vitamin B3 analog and a major vitamin component in milk (~1mg/L) [4]. In addition, NMN is contained in ingredients such as green soybeans, broccoli, and meat (~1mg/100g food) [5]. However, since its amount is extremely small, it is difficult to obtain sufficient intake from food. As such purified and concentrated NR and NMN are used in clinical trials. Human clinical trials using NR and NMN are underway around the world. In NR clinical trials, NR was administered at a maximum daily intake of 2000 mg for up to 12 weeks and its safety has been confirmed [6]. Increases in blood and tissue NAD+ concentrations also have been confirmed, although the report of the benefit of NR for the age-related function decline is limited at present. In a recent report, administration of 1000 mg NR to 30 patients with newly diagnosed Parkinson's disease (NADPARK study) increased NAD+ concentration in cerebrospinal fluid and mitochondrial-associated gene expression in peripheral blood and muscle, and mildly mitigated clinical symptoms of patients with the changes in NAD+ metabolism on Positron Emission Tomography [7].
NMN Human Clinical Trials
NMN human clinical trials started later than those of NR, but recently there have been a series of reports (Table 1). At first, the safety of single-day administration of NMN 100 mg, 250 mg, and 500 mg was reported [8]. Next, NMN 250 mg was administered to obese elderly postmenopausal women (BMI 25.3-39.1 kg/m2) for 10 weeks [9]. The safety of NMN administration was demonstrated, and an increase in blood NAD+ concentration was observed due to NMN administration [9]. Although no increase in NAD+ was observed in skeletal muscle, the glucose clamp test showed that NMN intake increased insulin sensitivity of skeletal muscle, and muscle biopsy also suggested that insulin signals (AKT and mTOR phosphorylation) in skeletal muscle were increased. On the other hand, no effects of NMN on body composition, muscle strength or blood test metabolic parameters were observed [9].
|
|
Subjects |
Number of subjects (n) |
Dosage |
Duration |
Trial design |
Effect |
Reference |
|
1 |
Males aged 40-60 |
10 |
1) 100 mg |
Single day |
A single-arm non-randomized intervention |
Safety |
8 |
|
2 |
Females aged 55-75 |
25 |
250 mg |
10 weeks |
Placebo-controlled, randomized, double-blind, parallel-group trial |
Increase in NAD+ content in PBMCs , insulin- stimulated glucose disposal and skeletal muscle insulin signaling. |
9 |
|
3 |
|
48 |
1) 300 mg(+exercise)
|
6 weeks |
Placebo-controlled, randomized, double-blind, four-arm trial |
Increase in the aerobic capacity during exercise training |
15 |
|
4 |
|
42 |
250 mg |
12 weeks |
Placebo-controlled, randomized, double-blind, parallel-group trial |
Increase in NAD+ content in PBMCs. Improvements in gait speed and muscle function |
10 |
|
5 |
|
108 |
250 mg |
12 weeks |
Placebo-controlled, randomized, double-blind, parallel-group, trial |
Improvement in sleep quality, fatigue, and physical performance |
14 |
|
6 |
|
66 |
150 mg |
60 days |
Placebo-controlled, randomized, double-blind, parallel-group trial |
Increase in NAD+/NADH levels in the serum and SF36 score. |
16 |
|
7 |
|
80 |
1) 300 mg
|
60 days |
Placebo-controlled, randomized, double-blind, parallel-group, dose-dependent trial |
Increase in blood NAD+ concentration, |
17 |
|
8 |
|
31 |
1250 mg |
4 weeks |
Placebo-controlled, randomized, double-blind, parallel-group trial |
Safety |
19 |
NMN oral administration improves muscle performance in healthy older men.
In the meantime, we planned a randomized, double-blind, placebo-controlled, parallel-group comparative study of NMN administration in healthy elderly men aged 65 years or older [10]. We focused on muscle mass and strength because the previous trial already included insulin secretion and glucose intolerance as primary endpoints. Muscle weakness in the elderly has important medical implications as sarcopenia. Because sarcopenia is more prevalent in males, elderly men were selected as the subject. The intake of NMN was set at 250 mg, which is equivalent to the previous study [9], as an intake that is assumed to be safe. Enrolled forty-two healthy elderly men aged 65 years or older were randomly assigned to an NMN group (250 mg/day) or a placebo group, and NMN or placebo was administered for up to 12 weeks. After 6 weeks (NMN group n = 21, placebo group n = 21) or 12 weeks (NMN group n = 10, placebo group n = 10), hematological parameters (number of red blood cells, white blood cells, platelets, etc.), biochemical parameters (triglyceride, LDL-cholesterol, glucose, HbA1c, insulin, AST, ALT, γ-GTP, etc.), concentrations of NAD+ and NAD+-related metabolites such as NMN and NR in whole blood, exercise capacity and physical function (the 30-s chair-stand test, walking speed, grip strength), body composition by bioimpedance (BIA) method and computed tomography (CT), and a wide variety of outcomes indicative of sensory, vascular, and cognitive functions, were evaluated.
As a result, no obvious adverse events were observed in the NMN group for up to 12 weeks. Oral intake of NMN effectively increased blood levels of NAD+ and NAD+ precursors, such as NMN and NR, compared to the placebo group.
On the other hand, in order to investigate the effect of oral NMN administration on skeletal muscle mass in healthy elderly men, the skeletal muscle mass index (SMI) and site-specific muscle mass were measured by BIA method as primary endpoints. Statistical analysis was performed on baseline, week 6, and week 12 measurements using a mixed-effect model or mixed-effect model for repeated measures (MMRM). As a result, there was no significant difference in skeletal muscle mass changes in any analysis. In addition, no change in fatty liver or visceral fat was observed by CT.
To examine the effects of NMN on motor function, walking speed, 30-second chair standing test and grip strength were analyzed using the same statistical methods. As a result, mixed effects model or MMRM showed significant improvement in walking speed and left grip strength after NMN administration. These results suggest that oral NMN administration may improve motor function in healthy elderly men, although it does not affect skeletal muscle mass. In addition, a significant difference in the mean values of walking speed was observed between the placebo group and the NMN one after 6 weeks of NMN administration as well as after 12 weeks. Furthermore, a significant difference in the mean values of the 30-s chair-stand test was observed between each group after 6 weeks, which supports the efficacy of NMN in motor function.
While any significant differences in the other endpoints evaluated in this clinical trial by NMN administration were not observed, a trend toward improvement in hearing ability was observed, although not statistically significant. Animal studies have shown that administration of NAD+-related metabolites improves age-related hearing loss and noise-induced hearing loss through activation of mitochondrial SIRT3 in the inner ear [11-13].
Other NMN Clinical Trials
Recently, another Japanese group also have reported that NMN administration improved motor functions of the elderly. 250 mg NMN for 12 weeks improved some of the motor functions (standing test) and the quality of sleep according to the Pittsburgh Sleep Quality Index for the elderly [14]. In addition, the report from China showed that NMN administration (300 mg, 600 mg, 1200 mg, 6 weeks) combined with exercise training (running, cycling) increased ventilatory performance threshold (VT) dose-dependently [15]. In the other report from China, administration of 150 mg NMN for 60 days in middle-aged and older adults aged 40-65 significantly improved SF-36, the indicator of health-related Quality of Life [16]. Also, in a report from India, administration of 300 mg, 600 mg, and 900 mg NMN for 60 days in middle-aged and older adults aged 40-65 significantly improved the results of the 6-minute walk test and SF-36 [17]. The improvement of motor function by NMN may be caused by the improvement of not only muscle strength but also all functions of the elderly, such as cardiopulmonary function, vitality and mental health. On the other hand, a recent report analyzed muscle metabolomes of the subjects aged 20-30 and 65-80, respectively, and identified NAD+ as one of the metabolites that declines with aging. In addition, while the NAD+ levels of the elderly who did exercise training were similar to those of the young, NAD+ of the elderly whose motor function declined decreased further [18]. We consider there is a close relationship between muscle NAD+ levels and motor function, and NMN administration may increase muscle NAD+ and improve motor function in the same way as exercise training.
Recently, the safety of high-dose NMN (1250 mg) for 4 weeks was demonstrated in men and women aged 20-65 [19]. We currently conduct a new NMN clinical trial to examine the beneficial effect of high-dose (1250 mg), long-term (24 weeks) NMN administration on aging.
Conclusion
Recent human clinical trials have tested whether NMN administration and its subsequent activation of sirtuins could be an effective means of restoring the physiological function that declines during aging and controlling age-related diseases. Based on these results, the effects of oral NMN administration in humans currently appear to be limited compared to animal studies. However, NMN administration can become an effective means of improving the performance of the elderly by adjusting the dosage and administration period in the near future. In addition, since the mechanism by which NMN improves motor function in elderly people is unclear, further elucidation of the detailed mechanism is required.
In particular, as the prevention of sarcopenia is a major issue in a super-aging society, NMN administration can be an effective means of preventing sarcopenia. Since NMN can be ingested as a supplement, it has the potential to be put into practical use as an intervention method for aging, and is highly expected to contribute to the realization of a healthy and long-lived society.
Author Contributions
MI wrote the first draft of the commentary. This was revised by TY with valuable comments and suggestions. All authors contributed to the article and approved the submitted version.
Conflict of Interest
The authors declare no conflict of interest.
References
2. Yoshino J, Baur JA, Imai S. NAD+ intermediates: The Biology and Therapeutic Potential of NMN and NR. Cell Metab. 2018;27:513-528.
3. Okabe K, Yaku K, Tobe K, Nakagawa T. Implications of altered NAD metabolism in metabolic disorders. J Biomed Sci. 2019;26:34.
4. Trammell SA, Yu L, Redpath P, Migaud ME, Brenner C. Nicotinamide Riboside Is a Major NAD+ Precursor Vitamin in Cow Milk. J Nutr. 2016;146:957-963.
5. Mills KF, Yoshida S, Stein LR, Grozio A, Kubota S, Sasaki Y, et al. Long-Term Administration of Nicotinamide Mononucleotide Mitigates Age-Associated Physiological Decline in Mice. Cell Metab. 2016;24:795-806.
6. Dollerup OL, Christensen B, Svart M, Schmidt MS, Sulek K, Ringgaard S, et al. A randomized placebo-controlled clinical trial of nicotinamide riboside in obese men: safety, insulin-sensitivity, and lipid-mobilizing effects. Am J Clin Nutr. 2018;108:343-353.
7. Brakedal B, Dölle C, Riemer F, Ma Y, Nido GS, Skeie GO, et al. The NADPARK study: A randomized phase I trial of nicotinamide riboside supplementation in Parkinson's disease. Cell Metab. 2022 ;34:396-407.e6.
8. Irie J, Inagaki E, Fujita M, Nakaya H, Mitsuishi M, Yamaguchi S, et al. Effect of oral administration of nicotinamide mononucleotide on clinical parameters and nicotinamide metabolite levels in healthy Japanese men. Endocr J. 2020;67:153-160.
9. Yoshino M, Yoshino J, Kayser BD, Patti GJ, Franczyk MP, Mills KF, et al. Nicotinamide mononucleotide increases muscle insulin sensitivity in prediabetic women. Science. 2021;372:1224-1229.
10. Igarashi M, Nakagawa-Nagahama Y, Miura M, Kashiwabara K, Yaku K, Sawada M, et al. Chronic nicotinamide mononucleotide supplementation elevates blood nicotinamide adenine dinucleotide levels and alters muscle function in healthy older men. npj Aging. 2022;8:5.
11. Someya S, Yu W, Hallows WC, Xu J, Vann JM, Leeuwenburgh C, et al. Sirt3 mediates reduction of oxidative damage and prevention of age-related hearing loss under Caloric Restriction. Cell. 2010;143:802-812.
12. Brown KD, Maqsood S, Huang JY, Pan Y, Harkcom W, Li W, et al. Activation of SIRT3 by the NAD+ Precursor Nicotinamide Riboside Protects from Noise-Induced Hearing Loss. Cell Metab.2014;20:1059-1068.
13. Han S, Du Z, Liu K, Gong S. Nicotinamide riboside protects noise-induced hearing loss by recovering the hair cell ribbon synapses. Neurosci Lett. 2020;725:134910.
14. Kim M, Seol J, Sato T, Fukamizu Y, Sakurai T, Okura T. Effect of 12-Week Intake of Nicotinamide Mononucleotide on Sleep Quality, Fatigue, and Physical Performance in Older Japanese Adults: A Randomized, Double-Blind Placebo-Controlled Study. Nutrients. 2022;14:755.
15. Liao B, Zhao Y, Wang D, Zhang X, Hao X, Hu M. Nicotinamide mononucleotide supplementation enhances aerobic capacity in amateur runners: a randomized, double-blind study. J Int Soc Sports Nutr. 2021;18:54.
16. Huang H. A Multicentre, Randomised, Double Blind, Parallel Design, Placebo Controlled Study to Evaluate the Efficacy and Safety of Uthever (NMN Supplement), an Orally Administered Supplementation in Middle Aged and Older Adults. Front Aging. 2022;3:851698.
17. Yi L, Maier AB, Tao R, Lin Z, Vaidya A, Pendse S, et al. The efficacy and safety of β-nicotinamide mononucleotide (NMN) supplementation in healthy middle-aged adults: a randomized, multicenter, double-blind, placebo-controlled, parallel-group, dose-dependent clinical trial. Geroscience. 2022;8:1-15.
18. Janssens GE, Grevendonk L, Perez RZ, Schomakers BV, de Vogel-van den Bosch J, Geurts JMW, et al. Healthy aging and muscle function are positively associated with NAD+ abundance in humans. Nature Aging. 2022;2:254-263.
19. Fukamizu Y, Uchida Y, Shigekawa A, Sato T, Kosaka H, Sakurai T. Safety evaluation of β-nicotinamide mononucleotide oral administration in healthy adult men and women. Sci Rep. 2022;12:14442.