Introduction
As the field of immunology continues to unravel the complexities of immune responses and their intersection with therapeutic interventions, the emergence of innovative drug delivery systems has sparked new avenues of exploration. The original research on FericipXT-coated PEGylated rutile TiO2 nanoparticles (NPs) in drug delivery has not only illuminated the realm of pharmaceutical science but also beckons the attention of immunologists, offering profound insights into the immunological implications of nanoparticle-based drug delivery systems [1]. This commentary seeks to delve deeper into the implications of the research, analyzing the issues addressed in the original article, while also exploring future prospects and challenges.
The Original Research Endeavor
The original research article focused on the utilization of FericipXT-coated PEGylated rutile TiO2 NPs as a potential drug delivery vehicle for imatinib, a tyrosine kinase inhibitor used in the treatment of various cancers. TiO2 NPs exhibit various phases out of which the rutile phase is considered to be the most stable one. By optimizing various synthesis parameters as discussed in the article, rutile TiO2 NPs were synthesized. The rutile TiO2 NPs were coated with iron supplement – FericipXT to enhance the magnetic responsiveness of the NPs so that they can be easily navigated through the magnetic field to achieve targeted drug delivery. FericipXT is an iron supplement readily available for human consumption in local pharmacies. The NPs were further PEGylated to improve the biocompatibility of the resulting NPs. The term ‘PEGylated’ refers to coating with polyethylene glycol (PEG) which is a polyether compound and finds various applications in medicine. The study undertook in vitro assessments to elucidate the release profile of imatinib from these NPs, shedding light on their potential as a means of pH-responsive drug release as well. The drug-loaded nanocarrier showed sustained drug release over the period of more than two days under acidic pH conditions which are associated with tumor microenvironment. However, under neutral pH conditions, the drug release was strongly suppressed. The findings of the original research presented compelling evidence of the efficacy of the NPs in sustaining the release of imatinib, thereby potentially enhancing its therapeutic outcomes. The theoretical implications of this research are broader and may suggest modifications to existing theories or models of the world. The practical implications of the research are multifaceted and encompass a spectrum of real-world impacts. Firstly, the development of FericipXT-coated PEGylated rutile TiO2 NPs as a targeted drug delivery system has the potential to revolutionize therapeutic interventions, particularly in the context of cancer treatment, where the non-specificity of the conventional therapeutic techniques results in many side-effects. Secondly, the sustained release profile of therapeutic agents facilitated by these NPs could lead to improved treatment outcomes and reduced adverse effects for patients.
Analyzing the Issues Addressed
The original research article raised several pertinent issues that continue to drive the discourse in the field of nanomedicine and drug delivery. One of the central issues pertains to the biocompatibility and safety profile of the NPs [2]. Addressing this concern, the study has placed emphasis on evaluating the biocompatibility and potential toxicity of these NPs, paving the way for the development of safe and effective drug delivery systems. However, the study has only discussed the in vitro aspect of the drug delivery. While the in vitro assessments provided valuable insights into the release kinetics of imatinib, the translation of these findings to in vivo settings necessitates a thorough understanding of the nanoparticle's interactions with biological systems [3]. Thus, the extension of this study must incorporate relevant in vivo studies to have a better understanding of the mechanism through which the drug-loaded nanocarrier interacts with the human body. Another critical issue highlighted in the original research is the need for sustained drug release from the NPs. In the context of chronic diseases such as cancer, the sustained and controlled release of therapeutics is paramount to achieving prolonged therapeutic effects while minimizing frequent dosing and associated side-effects. The original research has delved into the engineering of stimuli-responsive FericipXT-coated PEGylated rutile TiO2 NPs, enabling on-demand drug release in response to specific physiological cues which is pH. This approach holds promise in tailoring drug delivery to the dynamic microenvironments within the body, thereby optimizing therapeutic outcomes.
Immunological Significance of Nanoparticle Interaction
The interaction of NPs with the immune system is a subject of intrinsic relevance to immunologists. The immunological implications of NPs extend from their biodistribution to their potential immunomodulatory effects. Understanding the interplay between NPs and the immune system is critical, as it can influence the safety, efficacy, and immune responses elicited by these drug delivery systems [4-6]. The biodistribution of NPs following administration is a pivotal aspect with direct immunological relevance. NPs can interact with immune cells, including macrophages, dendritic cells, and lymphocytes, within the reticuloendothelial system, potentially influencing immune cell function and activation [7]. Moreover, the potential for NPs to modulate the immune microenvironment at the administration site and in distal lymphoid organs warrants close scrutiny. The immune responses elicited by nanoparticle-drug complexes, such as cytokine release and antigen presentation, are of particular interest to immunologists, as they contribute to the immunomodulatory potential of these drug delivery systems [8].
Immunomodulatory Potential and Therapeutic Implications
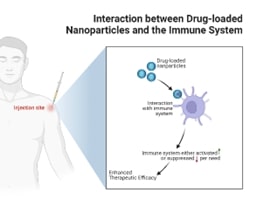
The immunomodulatory potential of FericipXT-coated PEGylated rutile TiO2 NPs holds significant implications for immunology and immunotherapy. Immunomodulatory potential refers to the ability of certain substances, known as immunomodulators, to modify or regulate the immune system's response. This can involve either enhancing or suppressing immune functions, depending on the therapeutic needs. NPs, through their physicochemical properties, have the potential to modulate immune responses, thereby influencing the therapeutic outcomes of co-administered drugs. The ability of NPs to influence the immune microenvironment, such as promoting immunogenic or tolerogenic responses, warrants exploration in the context of immunotherapies for cancer, autoimmune diseases, and vaccination strategies. Furthermore, the crosstalk between nanoparticle-drug complexes and the immune system can be leveraged to design targeted immunotherapies (Figure 1). Immunomodulatory payloads refer to therapeutic agents or substances that are designed to modify the immune response in a targeted manner. These payloads can be delivered through various means, such as in drug formulations or as part of biologic therapies, and are often used in the context of immunotherapy. By engineering NPs with immunomodulatory payloads or targeting ligands, researchers can potentially harness the immunological interactions of these drug delivery systems to elicit specific immune responses. This approach has implications for the development of next-generation immunotherapies that integrate drug delivery and immune modulation, offering new avenues for precision medicine and personalized immunotherapeutic interventions [9]. Khatua et al. [10] in their review article discussed essential immune elements and their regulation through therapeutic nanoplatforms. Nanotherapeutics can activate and recruit T cells to enhance immune responses, particularly in cancer treatment; strategies include blocking inhibitory signals and delivering immunomodulatory agents. NPs can either activate B cells to produce antibodies or suppress malfunctioning B cells to prevent autoimmune diseases. NPs are used to deliver therapeutic cytokines like IL-12 to stimulate immune responses, particularly in cancer therapy. Targeting components like C3 with siRNA NPs can modulate immune responses and treat conditions like autoimmune myocarditis. From their article, the step-by-step process how nanotherapeutics target and modulate T cells is described below (Figure 2):
Figure 1. Crosstalk between drug-loaded NPs and the immune system for increased therapeutic efficacy. Image created with BioRender.com.
Figure 2. General schematic showing how nanotherapeutics modulate T cell activity. The example of Kaliotoxin (KTX) loaded lipid nanoparticle is for understanding the concept only. No such work has been reported or cited anywhere yet. Image created with BioRender.com.
- Targeting T-cell receptors (TCRs): Engineered NPs are conjugated or encapsulated with therapeutic agents that specifically target TCRs. This allows for precise delivery of the therapeutic agents to T cells.
- Activation of T cells: In conditions like cancer, T cells are often suppressed. Nanotherapeutics can activate T cells by blocking inhibitory signals (e.g., PD-1/PDL1) and delivering activating agents directly to T cells.
- Artificial antigen-presenting cells (aAPCs): Nanoscale aAPCs can stimulate T cells by mimicking natural antigen-presenting cells, providing signals for T-cell activation.
- Recruitment of T cells: NPs can be designed to recruit T cells to specific sites, such as tumors. This is achieved by modifying NPs with antibodies or ligands that attract T cells to the desired location.
- Suppression of T cells: In autoimmune diseases, hyperactive T cells need to be suppressed. NPs can deliver agents that downregulate specific pathways (e.g., potassium Kv 1.3 channels) to reduce T cell activity and alleviate disease symptoms.
- Bispecific T cell engagers (nanoBiTEs): These NPs are designed to bind both T cells and cancer cells, facilitating the recruitment of T cells to the tumor site.
- Multi-specific T cell engagers (nanoMuTEs): These advanced NPs can target multiple antigens on cancer cells, enhancing the recruitment and activation of T cells.
- Regulatory T cells (Tregs): NPs can also be used to expand Tregs, which play a crucial role in maintaining immune tolerance and preventing autoimmune diseases.
The step-by-step process how nanotherapeutics target and modulate B cells is described below:
- B cell activation: NPs are loaded with disease-relevant antigens to activate B cells. These NPs are decorated with HIV-1 trimer spikes, for example, lead to enhanced B cell activation compared to soluble factors.
- B cell suppression: NPs are conjugated with antibodies targeting B cell receptors (BCRs) like CD38 and CD20 to inhibit B cell lymphoma progression. Therapeutic siRNA entrapped NPs downregulate tumorigenic biomarkers in B cells. NPs bearing both type I and type II anti-CD20 mAbs deplete antibody-resistant B cell lymphomas.
- Complement-mediated cytotoxicity: NPs are modified with peptides to activate the complement system, leading to targeted depletion of autoreactive B cells.
Autoimmune diseases are a group of disorders in which the immune system mistakenly attacks the body's own cells and tissues. Normally, the immune system protects the body from harmful substances such as viruses and bacteria. It does this by producing antibodies and immune cells that recognize and destroy these substances. However, in the case of autoimmune diseases, the immune system fails to distinguish between healthy cells and foreign invaders, leading to an attack on the body's own tissues.
There are more than 80 types of autoimmune diseases, and they can affect almost any part of the body. For example, autoimmune hepatitis affects the liver, while type 1 diabetes involves the immune system attacking the pancreas. The symptoms of autoimmune diseases can vary depending on the specific part of the body that is affected. Common symptoms include redness, swelling, heat, and pain, which are signs of inflammation. Diagnosing autoimmune diseases can be challenging, as there is usually not a specific test to confirm a particular autoimmune disease. Doctors often rely on a combination of symptoms, physical examinations, and specialized tests to make a diagnosis. Additionally, autoimmune diseases can have a significant impact on daily life, and managing flares is a crucial aspect of living with these conditions. The exact cause of autoimmune diseases is not fully understood, but it is believed to involve a combination of genetic and environmental factors. Researchers continue to investigate the mechanisms and risk factors associated with autoimmune diseases, aiming to develop better diagnostic tools and more targeted treatments. Advancements in the treatment of autoimmune diseases have been a subject of ongoing research and development. Autoimmune diseases present complex challenges due to their wide variability, different causative and pathogenic processes, and dynamic nature. Current treatment strategies often fall short of achieving complete remission, and there is a need for innovative approaches to address these challenges. While there are no definitive cures for autoimmune diseases, significant progress can be made with the help of nanoparticle-based drug delivery.
The specific autoimmune diseases that could be targeted by this research include:
- Lupus (Systemic Lupus Erythematosus): Lupus is a systemic autoimmune disease that can affect multiple organs and tissues in the body. The targeted and controlled delivery of therapeutic agents using NPs could offer new possibilities for managing the diverse manifestations of lupus. The study by Khiewkamrop et al. [11] focuses on dendritic cells, which are crucial players in our immune system, helping to activate immune responses and maintain tolerance to harmless substances. In conditions like systemic lupus erythematosus, these cells can become overly active or lose their ability to promote tolerance, contributing to disease development. To address this, researchers developed a new type of NPs made from a biodegradable material called PDMAEMA-PLGA. This NP is designed to deliver treatments directly to dendritic cells in the body. The PDMAEMA-PLGA NPs can release drugs over time, which is beneficial for treatment. These NPs showed the ability to suppress immune responses in specific types of dendritic cells derived from bone marrow. When combined with a drug called dexamethasone, the NPs helped convert certain dendritic cells from promoting immune responses to inducing tolerance, which is crucial for managing autoimmune diseases like lupus. The treatment led to an increase in regulatory T cells (Tregs), which help control immune responses and prevent autoimmunity. In experiments with mice that model lupus, the nanoparticle therapy showed promise in reducing disease symptoms by promoting the expansion of Tregs in a targeted manner. Overall, this research supports the potential of using PDMAEMA-PLGA NPs as a new therapy for SLE and possibly other autoimmune conditions, allergies, and transplant rejections by inducing specific immune tolerance.
- Rheumatoid Arthritis (RA): RA is a chronic inflammatory disorder that primarily affects the joints. Nanoparticle-based drug delivery systems may offer a targeted approach to deliver anti-inflammatory or disease-modifying drugs to the affected joints, potentially improving treatment outcomes. Treating RA is challenging, which is why researchers are looking into using natural substances as delivery systems for medications. They are exploring options like albumin, extracellular vesicles, cell membranes, and even bacteria to create these delivery systems. These natural carriers are preferred because they are less likely to cause immune reactions and can stay in the body longer without being cleared out by the immune system. These delivery systems can enhance the stability and targeting of RNA-based treatments, which are important for new therapies. Non-viral carriers, like lipid nanoparticles (LNPs), are particularly promising for delivering mRNA vaccines due to their safety and ability to carry large amounts of material. On the other hand, viral carriers like AAV vectors are effective at targeting and delivering genes but have limitations, such as a smaller capacity for the genetic material they can carry [12].
- Ulcerative colitis: These are inflammatory bowel diseases characterized by chronic inflammation of the digestive tract. The precise delivery of therapeutic agents to the inflamed tissues using NPs could offer a novel approach to managing these conditions. Zhao et al. [13] developed a pH-sensitive oral nanotherapeutic system called MC@Cel-NPs to deliver celastrol for treating ulcerative colitis. A chitosan/mannose coating enhanced the stability in the gastrointestinal tract, while the negatively charged surface helps target inflamed colon areas. The released celastrol effectively reduced inflammation and removed excess reactive oxygen species (ROS) in immune cells and inflamed tissues. This research demonstrates that even toxic natural compounds can be formulated into nanomedicines using a simple emulsification method. The process is easy to replicate and offers flexibility.
- Type 1 diabetes: This autoimmune disease targets the pancreas, leading to the destruction of insulin-producing cells. Nanoparticle-based drug delivery systems may hold promise in delivering agents aimed at modulating the immune response against pancreatic cells. Hyperglycemia is a key characteristic of diabetes mellitus that can lead to diabetic foot ulcers (DFU), affecting a significant portion of the population. Understanding the molecular mechanisms behind diabetic wound healing is crucial for developing effective treatments. Nanoscaffolds and nanotherapeutics, which range from 1 to 100 nm in size, offer a promising approach to enhance wound healing in diabetes patients. Their small size allows NPs to interact with biological components and penetrate wound sites effectively. They aid in crucial healing processes such as vascularization, cell proliferation, and cell signaling. Additionally, nanomaterials can deliver various pharmacological agents—including nucleic acids, growth factors, antioxidants, and antibiotics—to targeted tissues, providing sustained release that benefits the healing process [14].
- Multiple sclerosis (MS): MS is a neurological autoimmune disease characterized by the immune system's attack on the protective myelin sheath covering nerve fibers. Targeted delivery of immunomodulatory agents using NPs could potentially influence the course of the disease. Modifying stem cells to express brain-specific markers and enhancing chemokines through nano-engineered exosomes could provide effective solutions. The therapeutic potential of nanoscripts and exosomes derived from stem cells shows great promise for tackling neurodegenerative diseases [15].
- Psoriatic arthritis: This autoimmune condition affects the joints and skin, and the targeted delivery of therapeutic agents using NPs could offer a localized approach to managing the symptoms of this condition. Rahangdale et al. [16] fabricated apremilast-loaded zinc oxide-mesoporous silica NPs which showed improved therapeutic efficacy for psoriasis treatment. Both in vitro and in vivo studies showed enhanced anti-psoriatic activity of these NPs.
- Sjögren's syndrome: This autoimmune disease primarily affects the moisture-producing glands, leading to symptoms such as dry eyes and dry mouth. Nanoparticle-based drug delivery systems may offer a targeted approach to managing the glandular manifestations of the disease. Intravenously administered carbon quantum dots (CQDs) reduced immunopathological effects in non-obese diabetic mice over six weeks. Specifically, levels of autoantibodies (anti-SSA and anti-SSB) decreased, and saliva flow rates increased significantly in the CQD-treated group. Additionally, there was a reduction in apoptotic cells and inflammatory cytokine TNF-α in the submandibular glands. CQDs appeared to mitigate inflammation by decreasing infiltration of CD3+ and CD4+ T cells while increasing Treg cells. These findings highlight the therapeutic potential of CQDs derived from Setaria viridis for treating Sjögren's Syndrome [17].
Challenges and Future Directions
The integration of advanced nanotechnology, materials science, and pharmaceutical engineering is anticipated to pave the way for the development of next-generation drug delivery systems with enhanced precision and efficacy. However, the translation of these innovations from bench to bedside necessitates rigorous preclinical and clinical evaluations, ensuring the safety, efficacy, and scalability of the nanoparticle-based drug delivery platforms. Despite the promising immunological implications of nanoparticle-based drug delivery systems, several challenges warrant attention. The potential immunotoxicity of NPs, their long-term effects on immune function, and the risk of unintended immune activation or suppression necessitate comprehensive evaluation. Moreover, understanding the immunological fate of NPs following systemic administration, their interactions with immune cells, and their impact on immune memory and tolerance are areas that require in-depth investigation. Nanoparticle-based drug delivery systems have shown great promise in enhancing the efficacy of immunotherapy, particularly in cancer treatment. However, concerns regarding their long-term effects and potential toxicity remain significant. Here are some strategies to mitigate these issues:
1.Design and engineering of NPs
a. Tailored Properties: The design of NPs can be optimized to enhance biocompatibility and reduce toxicity. This includes modifying their size, shape, and surface chemistry to improve their interaction with biological systems and minimize adverse effects [18].
b. Protective Coatings: Applying biocompatible coatings can help shield the NPs from immune recognition and degradation, thereby prolonging their circulation time and reducing systemic toxicity [19].
2.Targeted delivery
a. Enhanced targeting: By engineering NPs to specifically target tumor cells or the tumor microenvironment, it is possible to increase the concentration of therapeutic agents at the desired site while minimizing exposure to healthy tissues. This targeted approach can significantly reduce off-target effects and systemic toxicity [20].
b. Controlled release mechanisms: Developing NPs that can release their payload in a controlled manner allows for sustained therapeutic effects while reducing the risk of toxicity associated with high initial doses [21].
3.Comprehensive toxicity assessments
a. Rigorous testing: Implementing thorough preclinical and clinical toxicity assessments is crucial. This includes evaluating immune cell function and potential immunotoxicity through established guidelines, such as those from the FDA [22]. Understanding the cause-effect relationships of nanoparticle interactions with biological systems can help identify and mitigate risks early in the development process.
b. Long-Term Studies: Conducting long-term studies to monitor the effects of NPs on immune function and overall health can provide insights into potential chronic toxicity and guide adjustments in formulation and delivery strategies [23].
4.Combination therapies
a. Synergistic approaches: Combining nanoparticle-based therapies with other treatment modalities, such as traditional chemotherapy or immunotherapy, can enhance therapeutic efficacy while potentially reducing the required doses of each agent, thereby minimizing toxicity [24].
b. Inducing immunogenic cell death (ICD): Utilizing NPs to promote ICD can enhance the immune response against tumors while reducing the risk of systemic toxicity associated with conventional therapies [25].
Looking ahead, the integration of immunological considerations into the design and assessment of nanoparticle-based drug delivery systems is paramount. Multidisciplinary collaborations between immunologists, pharmacologists, and material scientists are essential to unravel the immunological complexities of these drug delivery platforms. In conclusion, the original research on FericipXT-coated PEGylated rutile TiO2 NPs in drug delivery holds great significance for the field of immunology, offering a rich tapestry of immunological implications. The exploration of nanoparticle-immune interactions and their immunomodulatory potential paves the way for a new frontier in immunotherapy and immunomodulatory drug delivery systems. Embracing these immunological insights will undoubtedly shape the future landscape of nanoparticle-based therapeutics and foster innovative approaches in immunology and immunotherapy.
References
2. Wang YL, Lee YH, Chou CL, Chang YS, Liu WC, Chiu HW. Oxidative stress and potential effects of metal nanoparticles: A review of biocompatibility and toxicity concerns. Environ Pollut. 2024 Apr 1;346:123617.
3. Kunrath MF, Shah FA, Dahlin C. Bench-to-bedside: Feasibility of nano-engineered and drug-delivery biomaterials for bone-anchored implants and periodontal applications. Mater Today Bio. 2022 Dec 30;18:100540.
4. Guo C, Yuan H, Wang Y, Feng Y, Zhang Y, Yin T, et al. The interplay between PEGylated nanoparticles and blood immune system. Adv Drug Deliv Rev. 2023 Sep;200:115044.
5. Boraschi D, Canesi L, Drobne D, Kemmerling B, Pinsino A, Prochazkova P. Interaction between nanomaterials and the innate immune system across evolution. Biol Rev Camb Philos Soc. 2023 Jun;98(3):747-74.
6. Aljabali AA, Obeid MA, Bashatwah RM, Serrano-Aroca Á, Mishra V, Mishra Y, et al. Nanomaterials and Their Impact on the Immune System. Int J Mol Sci. 2023 Jan 19;24(3):2008.
7. Pondman K, Le Gac S, Kishore U. Nanoparticle-induced immune response: Health risk versus treatment opportunity? Immunobiology. 2023 Mar;228(2):152317.
8. Salthouse D, Novakovic K, Hilkens CMU, Ferreira AM. Interplay between biomaterials and the immune system: Challenges and opportunities in regenerative medicine. Acta Biomater. 2023 Jan 1;155:1-18.
9. Zeng YY, Gu Q, Li D, Li AX, Liu RM, Liang JY, et al. Immunocyte membrane-derived biomimetic nano-drug delivery system: a pioneering platform for tumour immunotherapy. Acta Pharmacol Sin. 2024 Jul 31.
10. Khatua R, Bhar B, Dey S, Jaiswal C, J V, Mandal BB. Advances in engineered nanosystems: immunomodulatory interactions for therapeutic applications. Nanoscale. 2024 Jul 11;16(27):12820-56.
11. Khiewkamrop P, Kaewraemruaen C, Manipuntee C, Saengruengrit C, Insin N, Leelahavanichkul A, et al. Immunosuppressive Polymeric Nanoparticles Targeting Dendritic Cells Alleviate Lupus Disease in Fcgr2b-/- Mice by Mediating Antigen-Specific Immune Tolerance. Int J Mol Sci. 2023 May 5;24(9):8313.
12. Li J, Li W, Zhuang L. Natural biomimetic nano-system for drug delivery in the treatment of rheumatoid arthritis: a literature review of the last 5 years. Front Med (Lausanne). 2024 May 9;11:1385123.
13. Zhao Y, Yao Y, Fan S, Shen X, Chai X, Li Z, et al. Oral delivery of pH-sensitive nanoparticles loaded Celastrol targeting the inflammatory colons to treat ulcerative colitis. J Tissue Eng. 2024 Aug 10;15:20417314241265892.
14. Hosseinzadeh A, Zamani A, Johari HG, Vaez A, Golchin A, Tayebi L, et al. Moving beyond nanotechnology to uncover a glimmer of hope in diabetes medicine: Effective nanoparticle-based therapeutic strategies for the management and treatment of diabetic foot ulcers. Cell Biochem Funct. 2023 Jul;41(5):517-41.
15. Ghosh S, Bhatti GK, Sharma PK, Kandimalla R, Mastana SS, Bhatti JS. Potential of Nano-Engineered Stem Cells in the Treatment of Multiple Sclerosis: A Comprehensive Review. Cell Mol Neurobiol. 2023 Dec 17;44(1):6.
16. Rahangdale M, Solanki S, Patil P, Bhavsar D, Sawant K. Fabrication and characterization of apremilast-loaded zinc oxide-mesoporous silica nanoparticles for psoriasis treatment. Ther Deliv. 2024;15(6):449-62.
17. Fu C, Qin X, Shao W, Zhang J, Zhang T, Yang J, et al. Carbon quantum dots as immune modulatory therapy in a Sjögren's syndrome mouse model. Oral Dis. 2024 Apr;30(3):1183-97.
18. Wu P, Han J, Gong Y, Liu C, Yu H, Xie N. Nanoparticle-Based Drug Delivery Systems Targeting Tumor Microenvironment for Cancer Immunotherapy Resistance: Current Advances and Applications. Pharmaceutics. 2022 Sep 21;14(10):1990.
19. Arcos Rosero WA, Bueno Barbezan A, Daruich de Souza C, Chuery Martins Rostelato ME. Review of Advances in Coating and Functionalization of Gold Nanoparticles: From Theory to Biomedical Application. Pharmaceutics. 2024 Feb 9;16(2):255.
20. Zhang J, Wang S, Zhang D, He X, Wang X, Han H, et al. Nanoparticle-based drug delivery systems to enhance cancer immunotherapy in solid tumors. Front Immunol. 2023 Aug 3;14:1230893.
21. Elumalai K, Srinivasan S, Shanmugam A. Review of the efficacy of nanoparticle-based drug delivery systems for cancer treatment. Biomedical Technology. 2024 Mar 1;5:109-22.
22. Sharma A, Madhunapantula SV, Robertson GP. Toxicological considerations when creating nanoparticle-based drugs and drug delivery systems. Expert Opinion on Drug Metabolism & Toxicology. 2012 Jan 1;8(1):47-69.
23. Sharma S, Parveen R, Chatterji BP. Toxicology of nanoparticles in drug delivery. Current pathobiology Reports. 2021 Nov 24:1-12.
24. Lang X, Wang X, Han M, Guo Y. Nanoparticle-Mediated Synergistic Chemoimmunotherapy for Cancer Treatment. International Journal of Nanomedicine. 2024 Dec 31:4533-68.
25. Peng J, Li S, Ti H. Sensitize Tumor Immunotherapy: Immunogenic Cell Death Inducing Nanosystems. International Journal of Nanomedicine. 2024;19:5895.