Abstract
Objectives: Treignac natural mineral water has a unique filmogenic property. The objective of this study was to test its anti-inflammatory activity on bronchial cells in vitro and its toxicity on rabbit lungs in vivo.
Material and methods: Treignac® mineral water concentrated by reverse osmosis (European patent) and a Volvic® mineral water control were prepared by the same process. The aerosol generator used for patients in pneumology produces standardized particles (<3 µ). Normal bronchial cells cultured in microplates were submitted to the aerosol and stimulated by poly (I:C). IL6 (interleukin 6), TNFα (Tumor Necrosis Factor α) and IL8 (Interleukin 8) were then measured in the supernatants by ELISA. Adult rabbits were exposed to an aerosol of concentrated Treignac® water in a closed cage for 10 min. Clinical signs were noted and histopathological analysis were performed.
Results: The first series of experiments suggested that concentrated Treignac® water inhibited cytokine release by bronchial cells compared to both saline and concentrated Volvic mineral waterr. The second series of experiments confirmed the effect of concentrated Treignac® water on IL6 release. As compared to saline, rabbits exposed to the aerosols presented no clinical signs and the histopathological analysis was negative.
Conclusions: These experiments demonstrated that concentrated Treignac water exhibited anti-inflammatory properties without causing any toxic effect on rabbit lungs, despite its hypo-osmolarity.
Keywords
Mineral water, Bronchial cells, Reverse osmosis, Cytokines, Poly (I:C)
Introduction
Hydrotherapy is a common technique used in thermal centers, primarily for the external treatment of skin pathologies [1]. Various centers specializing in the management of infectious and inflammatory respiratory pathologies use hydrotherapy techniques internally, as well as through nasally, and bronchially [2-7]. These techniques involve exposing patients to an aerosol of thermal water either in a common room, an individual cabin, or through the use of specialized masks. The aerosols generated by various current processes consist of droplets with diameters less than 3 µm, thus allowing them to penetrate more deeply into the bronchial tree. Recently [8] a meta-analysis supported the improvement of key bronchial asthma parameters following mineral water inhalation.
For many years, the biological effects of Treignac natural mineral water, sourced from an artesian spring in Corrèze, have been studied [9-11]. This water is characterized by very low minerality (22 mg.L-1), a high proportion of hydroxysilicic acid (32%) and a slightly acidic pH (5.7). Due to this high proportion of soluble silica and very low minerality, it was possible to design the production of concentrated water by reverse osmosis (European Patent) containing a higher concentration of hydroxysilicic acid while maintaining the same electrolytic balance as the natural mineral water, ultimately preserving its very low mineral content. Due to the solubility limits of hydroxysilicic acid, the maximum concentration factor applied was 8, resulting in a final hydroxysilicic acid concentration of approximately 55-60 mg/L. This concentrated water showed in vitro and in vivo properties similar to those of other mineral waters used in cosmetics (hydrating, soothing). However, it exhibited particularly pronounced soothing and film-forming properties, which are considered unique [10]. Indeed, the drying of an aerosol of concentrated Treignac water—though less markedly with natural water—on a conductive carbon support generates a film composed of fractal forms of hydroxysilicic acid condensation, which can be observed under scanning electron microscopy. This is in contrast to many other mineral waters used in cosmetology. The hypothesis can therefore be proposed that these fractal forms of condensed silica are the source of the unique protective, soothing, and water loss-inhibiting properties of this water [10].
To evaluate the potential benefit of using this concentrated water in aerosol therapy for respiratory inflammatory conditions, its anti-inflammatory activity was assessed by measuring the release of cytokines IL-6, TNFα, and IL-8 from activated normal bronchial cells in culture. Additionally, the effect of concentrated water on the bronchial epithelium was investigated in healthy rabbits exposed to an aerosol, considering its hypoosmolarity. These two series of experiments are the focus of this article.
Material and Methods
Concentrated water
Concentrated water was prepared by reverse osmosis from natural water (patent No. EP3432989 [12]) under the following technical conditions: Reverse osmosis system by Applied Industrial Techniques (TIA), using the BL 200 membrane process model. The process was carried out at a pressure of 106 Pa (10 bars) with refrigeration by tap water at 12°C. CSM (Customer Satisfaction Membrane) membranes, made of polyamide and thin-film composite (TFC), were used in the system. Due to the solubility limits of hydroxysilicic acid (which is typically above 100-120 mg/L under normal temperature and pressure conditions), the concentration was limited to 8 times, resulting in a final concentration of 55 to 60 mg/L. The average compositions are shown in Table 1. The batches of concentrated water were then sterilized using gamma irradiation. For studies on bronchial cells, the activity of concentrated Treignac® mineral water (from the French village of Treignac in High Corrèze) was compared to that of Volvic® water (from Puy-de-Dôme, France). The concentration by reverse osmosis was adjusted so that both concentrated waters had silica contents of 54 mg/L, or 18 mg/L after dilution to 1/3 (Table 2). Preliminary studies showed that, on a dry support, the film-forming effect was optimal at this concentration. On the other hand, in vivo experiments were carried out using 8X concentrated water.
|
Calcium |
8.33 mg/L |
Chloride |
27 mg/L |
|
Magnesium |
3.52 mg/L |
Sulfates |
5.05 mg/L |
|
Sodium |
20.40 mg/L |
Nitrates |
26.8 mg/L |
|
Potassium |
2.34 mg/L |
Dry residue at 180°C |
182 mg/L |
|
Silica |
53.8 mg/L |
pH |
6.9 |
|
Hydrogencarbonate |
26.2 mg/L |
Resistivity (ρ) |
47 ?.m |
|
Treignac |
Volvic |
||||
|
|
Natural |
Concentrated diluted to 1/3 |
Natural |
Concentrated diluted to 1/3 |
|
|
pH |
5.7 |
6.8 |
7.4 |
8.1 |
|
|
Silica mg/L |
6.9 |
18.3 |
33 |
18 |
|
|
Conductivity |
30.3 |
72 |
174 |
94 |
|
|
Na+ mg/L |
2.9 |
6.2 |
11.6 |
7 |
|
|
Cl- mg/L |
3.2 |
9 |
13.5 |
8 |
|
Aerosol generator
The aerosol generator used for both the in vitro and in vivo experiments was an ultrasonic generator used in the treatment of respiratory pathologies (Ultraneb, Drive Devillbiss Healthcare, France) which produces particles with a diameter of less than 3 µm.
Cytokine release by normal bronchial cells in culture
A normal human bronchial cell line (internal reference HBEpC, Qima laboratory, Gençay, France) was seeded, with the culture medium being the basal medium for "Airway Epithelial Cells." After 24 hours of culture, the plates were placed for one minute in an enclosure saturated with aerosol (pure water, physiological water, saline solution at 55 mg/L of NaCl, concentrated Treignac water diluted 1/3, concentrated Volvic water diluted 1/3) produced by aerosol generator. This allowed 1 µL of the test solution to be deposited per cm². The plates were then incubated for 15 minutes at 37°C with 10% CO2. The culture medium, with or without the poly(I:C) activator (Poly inosinic-polycytidylic acid) at 0.3 µg/mL (Sigma Laboratory reference P 9582) was then added and the plates were incubated for 48 hours. After incubation, the supernatants were collected to measure the secreted cytokines. The cytokines IL6, IL8, and TNFα were measured by flow cytometry (Flex set multiplex system, BD Biosciences laboratory). The results were expressed in pg/ml of cytokine released or in percentages relative to the control without activator. At our request, the Qima laboratory conducted two successive series of experiments. The first series involved testing the different products in triplicates, while the second series, focused solely on the release of IL-6, was carried out in hexaplicates.
Bronchial aerosolization in rabbits
Experiments were conducted by the EMIS Research technical platform (Limoges University Hospital, France). Rabbits were sourced from an approved breeder and were brought into the facility at least 5 days prior to the start of the procedures to allow for acclimatization.
The procedure involved aerosol therapy administered to the animals using either the selected 8X concentrated mineral water (n=5) or physiological saline (n=5), with the same aerosol generator used for cultured bronchial cells. To perform the procedure, the animal was placed in a transport cage or a similar enclosure. The nebulizer tip was attached to the cage, and a film was placed over the cage to create a maximally enclosed environment. The nebulizer was then turned on to administer the aerosol and nebulization was maintained for 5 minutes, delivering an aerosol volume of 3 mL. The animal was then removed and returned to its housing cage. This aerosol therapy protocol was performed twice daily (separated by at least 6 hours) for two sessions of 5 consecutive days, spaced 2 days apart. The rabbits were then monitored for 5 weeks (± 1 week). Two rabbits from each group were euthanized starting the week following the last exposure to assess any early reactions. Bronchoalveolar lavage (BAL) was performed on the animals immediately post-mortem. All rabbits were euthanized for lung sampling and analysis, which included the search for inflammatory and infectious parameters and lesions. Throughout the protocol, the animals were monitored daily. During the post-treatment follow-up phase, extensive clinical monitoring was performed, focusing on the occurrence of general signs, fever, and respiratory symptoms.
Statistical analysis
The statistical analysis of the experiments on cultured bronchial cells was performed using the Student’s t-test for the first series of experiments and the non-parametric Wilcoxon test for the global analysis of IL-6 release. For the experiments on rabbits, qualitative data were analyzed using the Fisher's exact test, while quantitative data were analyzed using the Wilcoxon rank test.
Results
Cytokine release by cultured bronchial cells
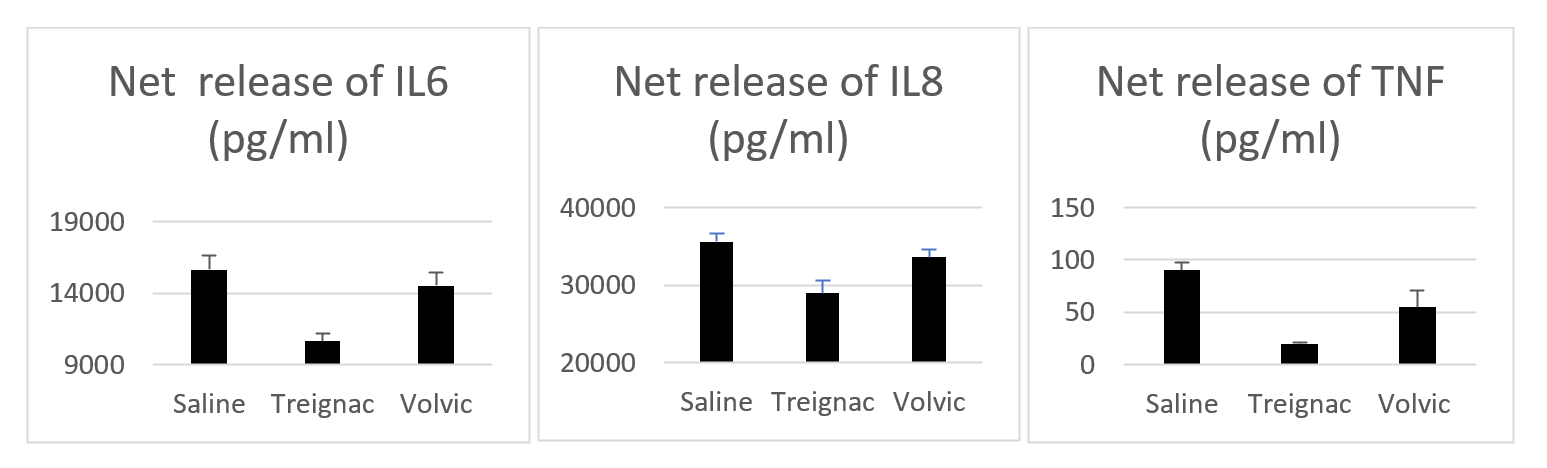
Cytokine release by poly(I:C): Triplicate experiments: For the three cytokines IL6, IL8, and TNFα, concentrated Treignac water induced inhibition of 32%, 19%, and 78%, respectively. The small-sample t-test showed a significant difference for concentrated Treignac water compared to saline for all three cytokines. In contrast, the effect of concentrated Volvic water, tested in parallel, was not significant (Figure 1).
Figure 1. Net release of IL6, IL8, and TNFα by poly(I:C) in the presence of physiological saline, concentrated Treignac water diluted 1/3 and concentrated Volvic water diluted 1/3. Comparison of triplicates using Student's t-test shows a significant difference for Treignac water (n=3).
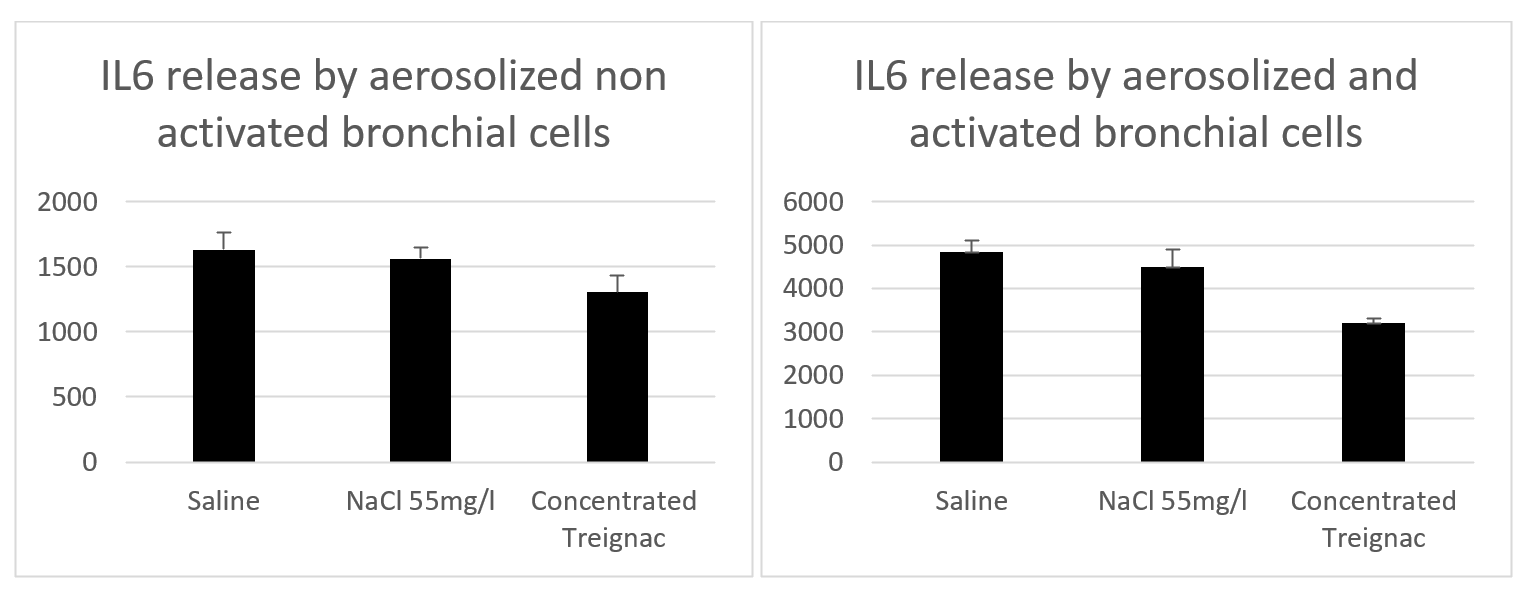
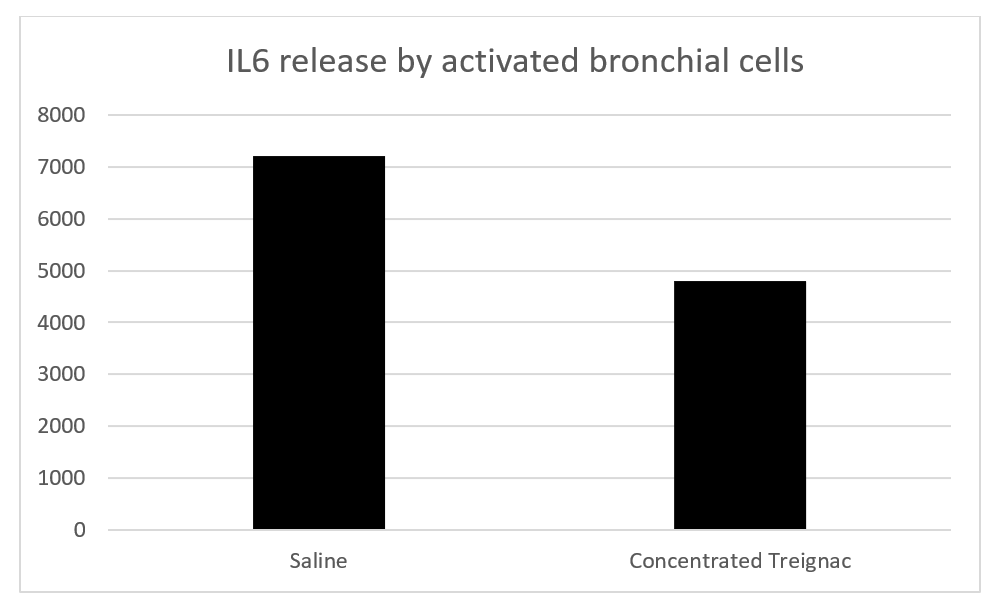
Hexaplicate experiments: To approximate the total mineral content of concentrated Treignac water diluted 1/3, we compared the effect of Treignac water not only to physiological saline (9 g/L NaCl) but also to a 55 mg/L NaCl solution. Treignac water induces a weak but significant inhibition of the spontaneous release of IL-6 (21% compared to physiological water and 17% compared to NaCl 55 mg/L, p=0.031). In bronchial cells activated by poly(I:C), concentrated Treignac water induces an inhibition of IL-6 release of 35% compared to NaCl 55 mg/L and 41% compared to physiological water (p<0.01 in both cases) (Figure 2). Taking into account the two previous experiments for IL-6, one in triplicate and the other in hexaplicate, the results show that for all 9 values, concentrated Treignac water induced an inhibition of 35% (p<0.01) (Figure 3).
Figure 2. IL6 release by normal bronchial cells in culture after misting, with and without activator. The difference between concentrated Treignac water and NaCl solution is significant in both cases, 17%, p=0.031 in the first and 35% p<0.01 in the second (n=6).
Figure 3. Effect of concentrated Treignac water on IL6 release from activated bronchial cells. The data represents the sum of triplicate and hexaplicate experiments (35% inhibition, p=0.004, as determined by the non-parametric Wilcoxon test).
Cellular toxicity: We compared the spontaneous release of IL6 (without activator) by bronchial cells in the presence of saline and concentrated Treignac water diluted 1:3. The results show (Figure 2) that there is no statistical difference between the results corresponding to the two products tested. Figure 2 also confirms that the activator induces significantly higher IL6 release compared to its absence, thereby validating the feasibility of the model.
Bronchial aerosolization in rabbits
The mean weight increase was not significantly different between the two groups (21.8% for Treignac water and 22.4% for saline) and the histopathological analysis of rabbit’s lungs (Table 3) showed no significant difference between concentrated Treignac water and saline. Additionally, no clinical signs, such as bronchospasms, were observed. Finally, the morphology of BAL macrophages was normal for both aerosols tested.
|
ID animal |
Group |
Lung structure |
Lymphocytes islets |
|
Eosinophiles |
Heart |
BAL |
|
1291 |
Treignac |
Normal |
Many |
|
Few |
Normal |
Macrophages |
|
1292 |
Treignac |
Normal |
Rare |
|
Few |
Normal |
Macrophages, lymphocytes |
|
1296 |
Treignac |
Normal |
Rare |
|
Rare |
Normal |
Macrophages |
|
1297 |
Treignac |
Normal |
Rare |
|
Rare |
Normal |
Macrophages |
|
1299 |
Treignac |
Normal |
Rare |
|
Rare |
Normal |
Macrophages |
|
1293 |
Saline |
Normal |
Many |
|
Rare |
Normal |
Macrophages |
|
1294 |
Saline |
Normal |
Rare |
|
Rare |
Normal |
Macrophages |
|
1295 |
Saline |
Normal |
Rare |
|
Rare |
Normal |
Macrophages, lymphocytes |
|
1298 |
Saline |
Normal |
Rare |
|
Rare |
Normal |
Macrophages |
|
1300 |
Saline |
Normal |
Rare |
|
Rare |
Normal |
Macrophages |
Discussion
Concentrated Treignac water exhibited anti-inflammatory activity on cultured activated bronchial cells by inhibiting IL6 secretion. The results also support inhibition of TNFα and IL8, three cytokines with a major pro-inflammatory role [13], particularly in bronchial inflammatory and infectious pathologies. Previous in vivo and in vitro results (unpublished) demonstrated by flow cytometry that concentrated Treignac water, as well as natural water, induced inhibition of the expression of the activation marker CD11b on human basophils, eosinophils, and neutrophils activated by the fMLP peptide. The composition of Treignac water and the experiments carried out using scanning electron microscopy (SEM) [3] point towards hydroxysilicic acid as the active principle. However, based on the SEM experiments, in which Treignac water was compared to mineral waters of similar compositions and demonstrated unique characteristics, particularly in the crystallization of hydroxysilicic acid into silica, it is likely that this active ingredient is not the only one contributing to its effects.
Regarding the cellular toxicity of concentrated Treignac water, previous experiments conducted out by IDEA laboratories (Marcillac, France) showed, through in vitro ocular tolerance tests on SIRC cells and on the skin of healthy subjects, that both natural and concentrated Treignac water did not induce any toxicity or irritation. These toxicity tests were necessary due to the hypoosmolarity of Treignac water, even in its concentrated state, which could potentially present cellular toxicity. The results obtained from normal bronchial cells in culture confirm the absence of cellular toxicity and even suggest a mild protective effect.
These results are further supported by the absence of any clinical reaction to exposure to an aerosol of concentrated Treignac water, as confirmed by pathological analyses. In particular, the normal appearance of BAL macrophages is particularly significant because it confirms the bronchial non-toxicity of silica, in the form of soluble hydroxysilicic acid contained in concentrated Treignac water, with no activation of macrophages.
These initial promising results encourage further clinical studies in humans. We are planning to assess Treignac mineral water aerosol treatment in patients with chronic inflammatory pathologies, such as COPD (Chronic Obstructive Pulmonary Disease), which is often associated with bacterial and viral infections.
References
2. Keller S, König V, Mösges R. Thermal water applications in the treatment of upper respiratory tract diseases: a systematic review and meta-analysis. J Allergy (Cairo). 2014;2014:943824.
3. Rabago D, Zgierska A. Saline nasal irrigation for upper respiratory conditions. Am Fam Physician. 2009 Nov 15;80(10):1117-9.
4. Salami A, Dellepiane M, Strinati F, Guastini L, Mora R. Sulphurous thermal water inhalations in the treatment of chronic rhinosinusitis. Rhinology. 2010 Mar;48(1):71-6.
5. Passali D, De Corso E, Platzgummer S, Streitberger C, Lo Cunsolo S, Nappi G, et al. Spa therapy of upper respiratory tract inflammations. Eur Arch Otorhinolaryngol. 2013 Feb;270(2):565-70.
6. Calzetta L, Di Daniele N, Chetta A, Vitale M, Gholamalishahi S, Cazzola M, et al. The Impact of Thermal Water in Asthma and COPD: A Systematic Review According to the PRISMA Statement. J Clin Med. 2024 Feb 14;13(4):1071-90.
7. Margelidon-Cozzolino V, Chbini K, Freymond N, Devouassoux G, Belaaouaj A, Pacheco Y. La BPCO : une maladie qui commence précocement [COPD: An early disease]. Rev Pneumol Clin. 2016 Feb;72(1):49-60.
8. Fesyun AD, Solimene U, Grishechkina IA, Lobanov AA, Andronov SV, Popov AI, et al. Mineral water inhalations for bronchial asthma: a meta-analysis. Eur J Transl Myol. 2023 Jun 23;33(2):11460.
9. Sainte-Laudy J., Redziniak G., Pons A., Devers T. A study by SEM of the filmogenic effect of mineral waters. Particular case of the water of Treignac. 4e Workshop Expert'Labs, Fuveau, June, 2016.
10. Smith A, Abir FZ, El Hafiane Y, Launay Y, Faugeron-Girard C, Gloaguen V, et al. Fractal structures and silica films formed by the Treignac water on inert and biological surfaces. Nanoscale Adv. 2020 Aug 12;2(9):3821-8.
11. Fatima-Zarha Abir. Etude des phases minérales des eaux de Treignac, Projet de fin d’étude, Génie des procédés et matériaux céramiques, Limoges 2017.
12. Sainte-Laudy J, Pons A, Redziniak G, Kita W et Chéron M. Eau de source ou minérale concentrée enrichie en acide hydroxysilicique naturel. Brevet N° EP3432989 (10 Janvier 2024).
13. Tanaka T, Narazaki M, Kishimoto T. IL-6 in inflammation, immunity, and disease. Cold Spring Harb Perspect Biol. 2014 Sep 4;6(10):a016295.