Abstract
The advent of highly active antiretroviral therapy has significantly increased the longevity of people living with HIV infection. Consequently, the HIV patient population is maturing, and age-related diseases now have a much greater impact on their health and well-being than do HIV associated infections. Cigarette smoke exposure is highly prevalent in the HIV community and chronic smoke inhalation triggers the onset and progression of chronic obstructive pulmonary disease (COPD). Typically, COPD is an age-related disorder that only occurs after decades of smoke exposure. However, HIV infected patients exhibit heightened susceptibility to this disorder often developing this disease up to ten years earlier than smokers without HIV. COPD is a leading cause of death in the United States and worldwide, so it is important to decipher the causes of HIV-related respiratory disease. This review addresses the changing demographics of HIV infection in the post-HAART era and how these changes increase the importance of diseases like COPD. It also summarizes the evidence indicating that HIV is not merely a co-factor associated with smoking but rather a causal agent that independently promotes disease development. Lastly, we review the potential mechanisms by which the HIV virus and HIV medications alter lung biology to promote airflow and lung tissue destruction. Given the growing burden of COPD in people living with HIV, the medical community needs better treatment strategies to improve long term outcomes in this patient population. Thus, understanding how HIV infection fosters the onset of COPD is an urgent public health concern.
Keywords
COPD, airflow, lung, antiretroviral
Abbreviations
CDC: Centers for Disease Control; COPD: Chronic Obstructive Pulmonary Disease; HIV: Human Immunodeficiency Virus; HAART: Highly Active Antiretroviral Therapy; AIDS: Acquired Immunodeficiency Syndrome; FEV1: Forced Expiratory Volume in 1 second; FVC: Forced Vital Capacity; DLCO: Diffusing Capacity for Carbon monoxidase; PLWH: People Living with HIV; SHIV: Simian Human Immunodeficiency Virus; MMP: Matrix Metalloproteinase; MUC5AC: Mucin 5AC; TB: Tuberculosis; TUNEL: Terminal deoxynucleotidyl transferase dUTP Nick End Labeling
Overview of the Impact of HIV on COPD
The CDC estimates that 1,173,900 persons aged ≥13 years are currently living with HIV infection in the United States [1]. Approximately 161,800 (13.8%) of these individuals do not know that they carry the infection. The demographics of HIV changed over time and current statistics show the highest prevalence rate in those aged 45-54 years (733.3/100,000). High prevalence rates also occurred in those 55 or older (400.2/100,000) while the younger 13-24 age category exhibited the lowest disease prevalence (93.1/100,000) [1]. In 2016, people over the age of 50 accounted for 17% of the 39,782 new HIV diagnoses and more than half of all HIV cases nationally [1]. In 1990, only 10% of HIV patients were above the age of 50 but experts now predict that 70% of all HIV patients will be 50 or older by 2030 [2]. These numbers show how the HIV patient population in this country is aging with successful implementation of highly active antiretroviral therapy (HAART). Moreover, these trends parallel data from other developed nations indicating that the aging of the HIV patient population is a global phenomenon [3]. The widespread implementation of HAART in less developed countries markedly increased life expectancy and now more than 10% of the 35 million people infected with HIV globally are 50 or older [4]. Despite the effects of HAART, a gap of life expectancy of approximately six years still occurs in HIV-infected subjects [5]. Given the importance of HIV worldwide, understanding the cause of this decreased life expectancy is an urgent public health concern.
The number of deaths due to AIDS-related infections and malignancies dropped dramatically since 1996. By the early 2000s, more people were dying from non-AIDS related causes and this pattern is even more pronounced in older HIV patients [6]. With increasing life expectancies, the incidence of age-related co-morbidities in the general population rises so it is not surprising that these infirmities are now major health issues in the HIV community. Tobacco related illnesses are a continuing health crisis and cigarette smoking causes 11.5% of all global deaths with the majority of those occurring in China, India, the USA, and Russia [7]. Lung function declines in humans after the age of twenty-five and smoking accelerates this natural process often resulting in respiratory symptoms by the age of fifty [8]. Cigarette smoke and other exposures induce chronic obstructive pulmonary disease (COPD), which is defined by the GOLD criteria as a marked reduction in the forced expiratory volume in 1 second (FEV1) relative to the forced vital capacity (FVC), and an absolute FEV1/FVC ratio less than 0.70 [9]. The pathology of this disease involves excessive mucus production and airway remodeling which blocks the flow of air in and out of the lung [10]. This causes dyspnea by increasing the work of breathing and promoting lung hyperinflation. The over distension of the lung reduces chest wall compliance and flattens the diaphragm which decreases its contractile force [11]. In addition, chronic smoke exposure destroys pulmonary alveoli which are the gas absorbing units of the lung. The primary function of the lung is to absorb oxygen and eliminate carbon dioxide. Thus, the loss of alveoli reduces oxygen delivery to the circulation and makes it difficult to do even simple everyday tasks. COPD is the third leading cause of death in the United States [12] which underscores its public health relevance. In addition, it causes substantial morbidity by incapacitating patients and making them dependent of continuous oxygen to perform minimal levels of exertion.
Smoking is highly prevalent in the HIV community with surveys showing smoking rates between 40 and 70% [13-15]. Investigators showed that smoking was a major modifiable risk factor linked to non-AIDS related deaths in HIV subjects [16]. COPD accounts for a significant percentage of these non-AIDS related deaths in people living with HIV (PLWH) [17,18]. Given the high prevalence of cigarette smoking, it can be challenging to sort out the independent effects of HIV infection on the risk of COPD development. Nevertheless, several studies indicate that HIV infection, by itself, increases the susceptibility to the onset and progression of this disease [19] despite the use of highly active anti-retroviral (HAART) therapy [20,21]. A cross sectional analysis of 167 subjects from the HIV clinics at the University of Pittsburgh found that 21% of HIV subjects had airway obstruction and 64% showed a decreased diffusing capacity for carbon monoxide (DLCO) [22]. Though this study lacked a non-infected control group, this disease prevalence is much higher than expected given the age of these subjects (median age 46). Similarly, a Japanese case control study of 49 HIV positive and 257 HIV negative men found an almost five-fold higher COPD prevalence in HIV positive vs. age-matched HIV negative subjects [23]. This difference was highly significant even after adjusting for age and pack years of smoking. This study, however, was criticized for its relatively small sample size. A much larger study of 351 people living with HIV and an age-matched cohort of 702 uninfected individuals found a COPD prevalence of 16% in the HIV subjects vs. 9% in the uninfected comparison group. Multivariate analysis confirmed a strong, independent association between HIV infection and airflow obstruction [24].
The above studies demonstrate a link between COPD and HIV. Uncertainty remains whether that association has implications on longevity in HIV infection. To address this question, a cohort of 396 HIV infected subjects (median age 49 years) were followed for 69 months. Those subjects with a ratio of forced expiratory volume in 1 second/forced vital capacity (FEV1/FVC) <0.7 and/or a diffusing capacity for carbon monoxide (DLCO) <60% exhibited a significantly increased mortality over time even after adjustment for smoking history [25]. One potential reason why airflow obstruction correlated with increased mortality is that HIV destabilizes pulmonary function in those with compromised lung function. COPD exacerbations are an acute worsening of the disease state which results in breathlessness, respiratory failure and in severe cases death. Exacerbations are important because once they occur, they promote disease progression and further physiologic impairment with increased morbidity and mortality [26]. To examine the link between HIV infection and COPD exacerbations, investigators utilized the ALIVE cohort, a study of former or current intravenous drug users with or without HIV infection [27]. After adjusting for co-factors, HIV infection, by itself, was associated with a 2.47-fold increased risk of COPD exacerbations. Of interest, those with highest CD4 counts were most at risk for COPD exacerbations suggesting that immune reconstitution could promote disease instability. The proportion of deaths due to COPD in the HIV patient population is increasing rapidly [18,28]. Several biological factors including airway cell apoptosis, indolent infection, inflammation and even HAART therapy itself, have been implicated in the enhanced susceptibility to COPD in HIV infected subjects [29-31]. However, the processes that accelerate COPD formation in HIV positive individuals remain poorly defined and this limits strategies to treat and prevent the disease in these patients. Given the growing prevalence of COPD in people living with HIV, new mechanistic insights are needed to address this important public health issue.
Researchers debate whether the HIV virus directly promotes COPD or is merely a correlative factor associated with higher smoking rates. Animal studies of HIV and cigarette smoke exposure could avoid these confounding issues, but HIV does not infect mice and exposing primates to cigarette smoke is challenging. The EcoHIV virus is a genetically modified version of HIV that infects mice but loses tropism for human tissues [32]. Co-infection with EcoHIV enhanced emphysema and airflow obstruction in mice exposed to chronic cigarette smoke [33]. These findings point to a direct causative role of HIV virus in the pathogenesis of this disease. Recently, a primate model combined cigarette smoke exposure with SHIV infection in cynomolgus macaques [34]. These researchers demonstrated that the virus directly infected the airway epithelium in these studies. The human bronchial epithelium expresses HIV receptor CD4 and co-receptors CCR5 and CXCR4. Both R5 and X4 HIV strains infect these cells and subsequently suppress key components needed for mucociliary clearance [35]. Moreover, SHIV was a strong independent risk factor for goblet cell metaplasia/hyperplasia and mucus formation and mucin5AC (MUC5AC) synthesis in the primate model. Above all, COPD is a physiologic disease and the exuberant production of airway mucus obstructs the airways and increases the work of breathing [36]. Thus, these results show that the virus promotes a key pathophysiologic feature of this disease. Indeed, researchers found that SHIV and CS synergistically decreased lung function and increased extrathoracic tracheal ring thickness. Studies show that airway wall thickness correlates well with FEV1 [37,38], so this provides further evidence that the virus engenders airway obstruction. Though the mechanisms remain uncertain, these animal studies denote a direct role for the virus itself in the onset and progression of chronic airway obstruction in this disease.
Adults living with HIV have high smoking prevalence rates. This makes it challenging to sort out cause and effect. Studies of perinatal infection provide an opportunity to examine the respiratory effects of HIV independent of cigarette smoke exposure. One study of perinatally infected youth on HAART demonstrated a significantly higher rate of irreversible airflow obstruction in HIV-infected vs. age-matched uninfected youth (9% vs. 17%; p<0.02) during late adolescence [39]. These cohorts were well matched and smoking prevalence was similarly low in both groups. The comparison group was exposed to HIV in utero but did not develop infection. Given the demographic similarity in these cohorts, it is unlikely that confounding factors like second-hand smoke exposure account for these differences. Consistent with these findings, a South African study of perinatally infected youth showed reduced lung function and diffusing capacity for carbon monoxide for HAART treated HIV infected youth compared to uninfected controls [40]. Together, these studies denote a direct causative role of HIV infection the development of respiratory disease.
Randomized, placebo-controlled trials provide the strongest evidence for causality. However, longitudinal well controlled cohort studies can effectively inform on potential disease risk factors when randomized trials are not feasible or ethical. One of the largest studies to address the pulmonary consequence of HIV infection is the Multicenter AIDS Cohort Study (MACS), a longitudinal cohort study of men who have sex with men (both HIV-positive and HIV-negative). In this cohort of 1,067 men, the median age was 57 years and 24% of the subjects were current smokers and 45% were former smokers. Interestingly, among HIV-positive participants, a higher current CD4+ T-cell count was associated with lower FEV1 % predicted and higher odds of FEV1 impairment (FEV1 <80% of predicted), but lower odds of DLCO impairment (DLCO <80% of predicted) [41]. This was a striking finding and shows poor viral control does not explain the link between HIV and airflow obstruction. Indeed, higher CD4 counts were also associated with increased risk of COPD exacerbation. This underscores the importance of research to identify the underlying biological factors responsible for COPD development in those infected with HIV.
Mechanism of HIV Induced Lung Injury
Persons living with HIV (PLWH) appear to have a more aggressive, and earlier presenting form of COPD when compared to their non-HIV infected counterparts [42]. HIV positive patients are more likely to demonstrate increased cough symptoms and sputum production as well as reduced DLCO than HIV negative individuals [17,43]. Those with very poorly controlled HIV as indicated by high viral RNA load and low CD4 count, have a more rapid clinical deterioration than those with well-controlled HIV [44]. The mechanism of HIV induced lung injury correlates with several factors including elevated HIV RNA viral load and increased immunosuppression, CD8 T-cell activation and elevated levels of inflammatory cytokines including proteases. These elements create a pro inflammatory environment which increases the susceptibility to lung injury [45]. The pathogenesis of COPD in long term smokers involves the release of inflammatory mediators such as leukotriene B4, IL-8, and TNF-alpha from alveolar epithelial cells and macrophages, amplifying the inflammatory process, and inducing structural changes in the lung [46].
The overlapping immunomodulatory factors found in both pathologies posit a reasonable theory for the development of emphysematous changes in PLWH. While CD4 T-cell counts decline in advanced HIV, the amount of cytotoxic CD8 lymphocyte numbers increase in both BAL and lung biopsy specimens [47, 48]. These CD8 T-cells cause a lymphocytic alveolitis that may be ultimately responsible for causing COPD in those infected with HIV [49, 50]. As discussed previously, the EcoHIV murine smoke exposure model replicates the progression of HIV in a previously immunocompetent host [33]. When combined with smoke inhalation, this model induces obstructive disease coupled with severe emphysematous changes identified on lung pathology. In mice that were both EcoHIV+, and smoke-exposed, there were increased levels of TUNEL+ cells, and LDH release indicating enhanced cell damage in the lung [33]. Indeed, HIV viral proteins Nef and gp120 both induce endothelial cell apoptosis even in the lungs of aviremic patients [51, 52]. Endothelial cell apoptosis plays a major role in emphysema [53] and this could account for the decreased diffusing capacity which is frequently identified in PLWH.
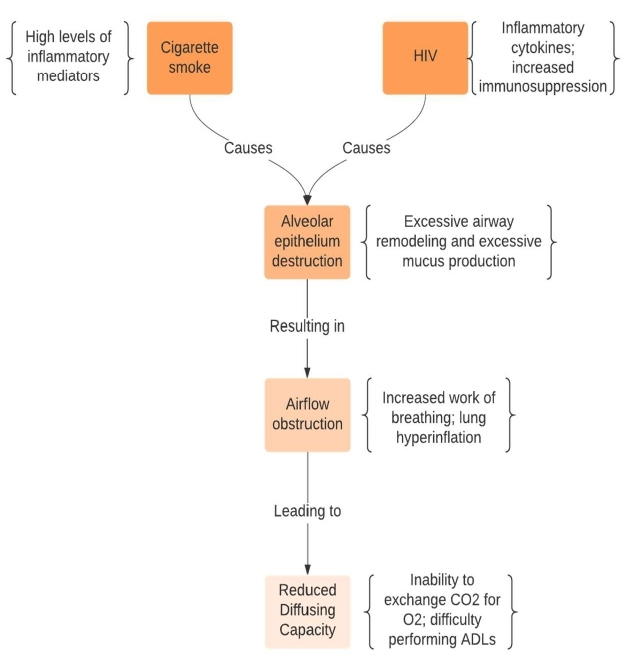
Aside from the increases in apoptosis, the Eco-HIV infected and smoke exposed mice exhibited elevated levels of metalloproteinases, specifically MMP-9, MMP-10, and MMP-13 which indicates that a protease-elastase imbalance contributes to lung disease progression in HIV [33]. Researchers recently demonstrated that HIV reprograms basal cells within the airways to promote a tissue invasive phenotype with increased MMP-9 expression [54]. This protease has activity against key lung structural elements like elastin and its enhanced expression promoted emphysema development in mice [55]. In the Eco-HIV mouse model, viral infection activated mitogen activated protein kinase signaling, which is linked to MMP induction in the lung [56]. Importantly, HIV appears to selectively up regulate MMP expression in the lung in humans. Indeed, alveolar macrophages from HIV positive smokers with early emphysema exhibited significantly higher MMP mRNA expression compared to alveolar macrophages from HIV negative smokers with early emphysema [57]. Furthermore, HIV positive individuals exhibited increased protein levels of MMP-2, -7, -9 and -12 within the epithelial lining fluid [57]. Thus, the virus promotes a proteolytic milieu, which when coupled with cigarette smoke exposure, induces alveolar epithelial destruction and airway remodeling. These changes cause airflow obstruction and reduced diffusing capacity (Figure 1) which leads to worsened COPD outcomes in patients who are both HIV-positive and smokers.
Figure 1: Mechanisms of HIV related COPD. Cigarette smoke and HIV virus interact to enhance protease and mucus production within the airways. This causes airway remodeling that obstructs normal air flow making it more difficult to breathe. HIV and smoke also synergize to release proteases and induce apoptosis that destroys alveolar tissue. This greatly decreases the oxygen absorbing capacity of the lung.
Antiretroviral Therapy: A cause of COPD
Chronic obstructive pulmonary disease (COPD) is a recognized complication of HIV affecting about 25% of HIV patients. The sustained increase in the prevalence of COPD in HIV patients is attributable to a multifaceted interaction of immune activation and dysfunction, HIV viremia, pulmonary inflammation, tuberculosis (TB), increased tobacco smoking, socioeconomic status, and environmental exposures [58-60].
The introduction of HAART has been a medical breakthrough as it effectively suppresses viral load and subsequently increases the chances for patient’s survival [61]. However, prolonged use of these pharmacologic agents leads to drug resistance, drug-drug interactions, drug toxicity and immune reconstitution inflammatory syndrome [62]. The downside of prolonged HAART has notably been associated with cardiovascular disease, metabolic syndrome, rheumatologic disorders, thyroid disease, lipoatrophy, osteoporosis and low sperm count [63,64]. Additionally, some antiretroviral medications have been linked to increased body mass index which plays a role in causing lung disease [20,31,65,66].
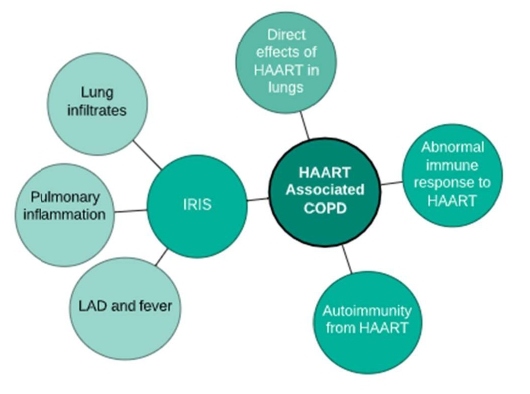
Airway obstruction has been directly associated with HAART; however, the link between COPD and these medications remain poorly understood. Potential explanations include direct effects of HAART in the lung tissue, abnormal immune response to HAART, and autoimmunity from HAART use [60]. Studies have recently included these treatments as a cause of obstructive patterns on spirometry [66]. The authors found that the relationship between use of HAART and increased airway obstruction persisted despite adjustment for confounders such as the presence and duration of HIV infection, age, intravenous drug use, history of bacterial pneumonia and tobacco use [20,66]. Kunisaki et al. also found no protective effect of antiretroviral medications on lung function after adjusting for smoking [31,67]. Hence, screening spirometry might be valuable in HIV-infected people with a history of tobacco exposure or respiratory symptoms [68].
Immune reconstitution inflammatory syndrome (IRIS) is a well-documented side-effect of HAART, which occurs in response to known or occult pathogen. IRIS could potentially lead to airway obstruction as a result of changes in the immune system induced by a combination of HAART and the presence of infectious antigens. Effects of IRIS include a collection of lung infiltrates, lymphadenopathy and fever likely resulting from the release of memory CD4+ T-cells occurring several months after HAART commencement. A modified IRIS mechanism that stimulates pulmonary inflammation in response to occult micro-organisms also occurs and results in a minor form of airway obstruction [69]. Although no explanation has been elucidated as to how HAART leads to of immune reconstitution inflammatory syndrome (IRIS), it is thought that the immune response is a principal factor in causing COPD [70].
HIV patients are generally susceptible to developing autoimmunity which is another probable cause of COPD in patients after initiation of HAART [71]. Prolonged HAART use has led to other organ-specific autoimmunity such as sarcoidosis, arthritis, autoimmune thyroid disease and autoimmune hepatitis. Immune reaction occurs by the release of autoreactive naive T-cells and lung antigens, and usually occurs about six months after commencement of HAART [72]. HAART also weakens production of T-regulatory cells leading to a weakened suppression of autoimmunity along with a decreased mucosal T-regulatory cells [73]. These changes may occur in the lungs resulting in decreased ability to suppress the autoimmune response due to specific lung antigens produced by ART leading to autoimmunity [22]. The susceptibility of HIV-infected patients on HAART to developing autoimmunity and the discoveries supporting autoimmune pathogenesis in COPD indicate that autoimmune mechanisms may also be involved in HAART-related COPD [20].
Trans-Activator of Transcription (Tat) is a small nuclear protein consisting of 86–101 amino acids encoded by the tat gene in HIV-1. Tat allows for rapid HIV growth by recruiting kinases like Cdk9 and RNA polymerase to the transcriptional complex [74,75]. It further enhances viral replication by attracting histone acetyltransferases that release transcriptional inhibition of viral promoters [76]. Infected cells secrete Tat and it then accumulates in the extracellular compartment where it is taken up by neighboring cells [77]. Tat concentrations within the sera of HIV infected patients range from 2 ng/ml to 40 ng/ml [78]. Once inside a cell, Tat modulates the host gene expression and protein translation by interacting with transcription factors [79] or promoter regions [80] and by binding the 5’ untranslated region (UTR) of mRNA like TNF-β and IL-6 [81,82]. Of interest, Tat antagonizes the CXCR4 receptor and this shifts infection away from T cells and towards CCR5 expressing macrophages [78]. Viral integration into lung macrophages creates long-term latency in this organ and highly active anti-retroviral therapy (HAART) does not eradicate these reservoirs [83]. Infected macrophages dramatically increase local Tat concentrations in their vicinity [84]. Moreover, virally infected macrophages release exosomes containing HIV proteins like Tat [85] and HAART fails to suppress this release [86]. Macrophages reside in close proximity to the airways, so the release of these Tat-containing exosomes has the potential to significantly alter airway epithelial cell biology. Understanding how Tat modulates these cells will provide important insights into the mechanisms of airflow obstruction in HIV related COPD.
COPD in HIV patients could also be a caused by the direct toxic effects of antiretroviral drugs. A similar mechanism occurs in cardiovascular disease, metabolic syndrome and osteoporosis due to HAART-associated decreased expression of peroxisome-proliferator activated receptor. This receptor provides an anti-inflammatory effect in the respiratory system and decreased levels could contribute to the development of airway obstruction after the initiation of antiretrovirals [87]. Oxidative stress is also an effect of prolonged HAART use and could have a similar effect on the respiratory system [88]. Indeed, both animal and human studies show that oxidative injury plays a key role in the onset and progression of COPD [89-92]. Of note, Ritonavir interacts with the cytochrome p450 system to elevate systemic steroid levels in patients that use inhaled corticosteroids [93]. This can cause adrenal suppression and Cushing’s symptoms in users and would place patients at increased risk for respiratory infections. Together, these studies show that HAART could promote airway obstruction by direct lung toxicity or by inducing abnormal immune responses that injury the lung (Figure 2). Additional research is warranted to explore the pulmonary effects of HAART [88]. It is important that health care workers who treat HIV patients should be aware of the manifestations of obstructive lung diseases given the potential link between COPD and prolonged use of HAART [29,94].
Figure 2: HAART Associated COPD. The imitation of HAART could mediate lung injury by direct effects of the drug on the lung. It also reconstitutes immune responses that could lead to IRIS related lung injury or promote autoimmune responses that damage the lung.
Conclusion
The HIV patient population is aging and the incidence of age-related diseases like COPD increases in this cohort over time [95]. This trend will likely continue as more patients are surviving with this disease and new infections continue to decline [96]. Improving long-term pulmonary outcomes and longevity in people living with HIV will require greater smoking cessation initiatives along with an enhanced understanding of the effects of HIV and HAART medications on lung biology. Indeed, continued smoking markedly decreases lung function and accelerates the onset of COPD in HIV infected subjects [97]. Additionally, understanding the biological factors responsible for the increased incidence of COPD in HIV will potentially lead to new strategies to better treat this condition. Aside from cigarette smoke, a host of factors cause COPD including urban pollution, biomass use, malnutrition and childhood infections [98,99]. HIV infected subjects are disproportionately affected by these risk factors so addressing the issue of COPD in HIV infection will have a major public health impact in this community [100-102]. It is important to note that COPD causes respiratory symptoms that dramatically impair quality of life. Thus, improving COPD disease prevention and treatment will ensure that our aging HIV population lives longer and healthier lives.
Acknowledgment
This work was supported by grants made available to Robert Foronjy (Flight Attendant Medical Research Institute (CIA160028) and the Alpha-1 Foundation)
Author Contributions Statement
Elaine Fletcher wrote the manuscript and composed the figures. Robert Foronjy and Chimuanya Okoli wrote and edited the manuscript.
References
2. Wing EJ. HIV and aging. International Journal of Infectious Diseases. 2016 Dec 1;53:61-8.
3. Mary-Krause M, Grabar S, Lievre L, Abgrall S, Billaud E, Boué F, et al. Cohort profile: French hospital database on HIV (FHDH-ANRS CO4). International Journal of Epidemiology. 2014 Oct 1;43(5):1425-36.
4. 4HIV and aging: a special supplement to the UNAIDS report on the global AIDS epidemic 2013.
5. Marcus JL, Chao CR, Leyden WA, Xu L, Quesenberry Jr CP, Klein DB, et al. Narrowing the gap in life expectancy between HIV-infected and HIV-uninfected individuals with access to care. Journal of Acquired Immune Deficiency Syndromes (1999). 2016 Sep 1;73(1):39.
6. Antiretroviral Therapy Cohort Collaboration. Causes of death in HIV-1—infected patients treated with antiretroviral therapy, 1996–2006: collaborative analysis of 13 HIV cohort studies. Clinical Infectious Diseases. 2010 May 15;50(10):1387-96.
7. Reitsma MB, Fullman N, Ng M, Salama JS, Abajobir A, Abate KH, et al. Smoking prevalence and attributable disease burden in 195 countries and territories, 1990–2015: a systematic analysis from the Global Burden of Disease Study 2015. The Lancet. 2017 May 13;389(10082):1885-906.
8. Fletcher JR, Ramwell PW. Altered lung metabolism of prostaglandins during hemorrhagic and endotoxin shock. InSurgical forum 1977; 28:184.
9. O'Shea MK, Ryan MA, Hawksworth AW, Alsip BJ, Gray GC. Symptomatic respiratory syncytial virus infection in previously healthy young adults living in a crowded military environment. Clinical Infectious Diseases. 2005 Aug 1;41(3):311-7.
10. Ramos FL, Krahnke JS, Kim V. Clinical issues of mucus accumulation in COPD. International Journal of Chronic Obstructive Pulmonary Disease. 2014;9:139.
11. Cherniack RM, Hodson A. Compliance of the chest wall in chronic bronchitis and emphysema. Journal of Applied Physiology. 1963 Jul 1;18(4):707-11.
12. Soriano JB, Kendrick PJ, Paulson KR, Gupta V, Abrams EM, Adedoyin RA, et al. Prevalence and attributable health burden of chronic respiratory diseases, 1990–2017: a systematic analysis for the Global Burden of Disease Study 2017. The Lancet Respiratory Medicine. 2020 Jun 1;8(6):585-96.
13. Arday DR, Tomar SL, Nelson DE, Merritt RK, Schooley MW, Mowery P. State smoking prevalence estimates: a comparison of the Behavioral Risk Factor Surveillance System and current population surveys. American Journal of Public Health. 1997 Oct;87(10):1665-9.
14. Crothers K. Chronic obstructive pulmonary disease in patients who have HIV infection. Clinics in Chest Medicine. 2007 Sep 1;28(3):575-87.
15. Patel N, Talwar A, Reichert VC, Brady T, Jain M, Kaplan MH. Tobacco and HIV. Clin Occup Environ Med. 2006;5(1):193-207
16. Wada K, Funada M, Matsumoto T, Shimane T. Current status of substance abuse and HIV infection in Japan. Journal of Food and Drug Analysis. 2013 Dec 1;21(4):S33-6.
17. Triplette M, Justice A, Attia EF, Tate J, Brown ST, Goetz MB, et al. Markers of chronic obstructive pulmonary disease are associated with mortality in people living with HIV. AIDS (London, England). 2018 Feb 20;32(4):487.
18. Schwarcz SK, Vu A, Hsu LC, Hessol NA. Changes in causes of death among persons with AIDS: San Francisco, California, 1996–2011. AIDS Patient Care and STDs. 2014 Oct 1;28(10):517-23.
19. Popescu I, Drummond MB, Gama L, Coon T, Merlo CA, Wise RA, et al. Activation-induced cell death drives profound lung CD4+ T-cell depletion in HIV-associated chronic obstructive pulmonary disease. American Journal of Respiratory and Critical Care Medicine. 2014 Oct 1;190(7):744-55.
20. George MP, Kannass M, Huang L, Sciurba FC, Morris A. Respiratory symptoms and airway obstruction in HIV-infected subjects in the HAART era. PloS One. 2009 Jul 21;4(7):e6328.
21. Cui Q, Carruthers S, McIvor A, Smaill F, Thabane L, Smieja M. Effect of smoking on lung function, respiratory symptoms and respiratory diseases amongst HIV-positive subjects: a cross-sectional study. AIDS Research and Therapy. 2010 Dec 1;7(1):6.
22. Gingo MR, George MP, Kessinger CJ, Lucht L, Rissler B, Weinman R, et al. Pulmonary function abnormalities in HIV-infected patients during the current antiretroviral therapy era. American Journal of Respiratory and Critical Care Medicine. 2010 Sep 15;182(6):790-6.
23. Nakamura H, Tateyama M, Tasato D, Haranaga S, Ishimine T, Higa F, et al. The prevalence of airway obstruction among Japanese HIV-positive male patients compared with general population; a case–control study of single center analysis. Journal of Infection and Chemotherapy. 2014 Jun 1;20(6):361-4.
24. Makinson A, Hayot M, Eymard-Duvernay S, Ribet C, Raffi F, Pialoux G, et al. HIV is associated with airway obstruction: a matched controlled study. Aids. 2018 Jan 14;32(2):227-32.
25. Gingo MR, Nouraie M, Kessinger CJ, Greenblatt RM, Huang L, Kleerup EC, et al. Decreased lung function and all-cause mortality in HIV-infected individuals. Annals of the American Thoracic Society. 2018 Feb;15(2):192-9.
26. Soler-Cataluña JJ, Sánchez-Sánchez L, Martínez-García MÁ, Sánchez PR, Salcedo E, Navarro M. Mid-arm muscle area is a better predictor of mortality than body mass index in COPD. Chest. 2005 Oct 1;128(4):2108-15.
27. Lambert AA, Kirk GD, Astemborski J, Mehta SH, Wise RA, Drummond MB. HIV infection is associated with increased risk for acute exacerbation of COPD. Journal of Acquired Immune Deficiency Syndromes (1999). 2015 May 1;69(1):68.
28. Louie M, Markowitz M. Goals and milestones during treatment of HIV-1 infection with antiretroviral therapy: a pathogenesis-based perspective. Antiviral Research. 2002 Jul 1;55(1):15-25.
29. Morris A, George MP, Crothers K, Huang L, Lucht L, Kessinger C, et al. HIV and chronic obstructive pulmonary disease: is it worse and why?. Proceedings of the American Thoracic Society. 2011 Jun 1;8(3):320-5.
30. Madeddu G, Fois AG, Calia GM, Babudieri S, Soddu V, Becciu F, et al. Chronic obstructive pulmonary disease: an emerging comorbidity in HIV-infected patients in the HAART era?. Infection. 2013 Apr 1;41(2):347-53.
31. Kunisaki KM. Will expanded ART use reduce the burden of HIV-associated chronic lung disease?. Current Opinion in HIV and AIDS. 2014 Jan;9(1):27.
32. He H, Sharer LR, Chao W, Gu CJ, Borjabad A, Hadas E, et al. Enhanced human immunodeficiency virus Type 1 expression and neuropathogenesis in knockout mice lacking Type I interferon responses. Journal of Neuropathology & Experimental Neurology. 2014 Jan 1;73(1):59-71.
33. Geraghty P, Hadas E, Kim BH, Dabo AJ, Volsky DJ, Foronjy R. HIV infection model of chronic obstructive pulmonary disease in mice. American Journal of Physiology-Lung Cellular and Molecular Physiology. 2017 Apr 1;312(4):L500-9.
34. Chand HS, Vazquez-Guillamet R, Royer C, Rudolph K, Mishra N, Singh SP, et al. Cigarette smoke and HIV synergistically affect lung pathology in cynomolgus macaques. The Journal of Clinical Investigation. 2018 Dec 3;128(12):5428-33.
35. Chinnapaiyan S, Parira T, Dutta R, Agudelo M, Morris A, Nair M, et al. HIV infects bronchial epithelium and suppresses components of the mucociliary clearance apparatus. PloS One. 2017 Jan 6;12(1):e0169161.
36. Kim AJ, Boylan NJ, Suk JS, Hwangbo M, Yu T, Schuster BS, et al. Use of Single‐Site‐Functionalized PEG Dendrons To Prepare Gene Vectors that Penetrate Human Mucus Barriers. Angewandte Chemie. 2013 Apr 2;125(14):4077-80.
37. Mohamed Hoesein FA, de Jong PA, Lammers JW, Mali WP, Mets OM, Schmidt M, et al. Contribution of CT quantified emphysema, air trapping and airway wall thickness on pulmonary function in male smokers with and without COPD. COPD: Journal of Chronic Obstructive Pulmonary Disease. 2014 Sep 1;11(5):503-9.
38. Shearer WT, Jacobson DL, Yu W, Siberry GK, Purswani M, Siminski S, et al. Long-term pulmonary complications in perinatally HIV-infected youth. Journal of Allergy and Clinical Immunology. 2017 Oct 1;140(4):1101-11.
39. Shearer WT, Jacobson DL, Yu W, Siberry GK, Purswani M, Siminski S, et al. Long-term pulmonary complications in perinatally HIV-infected youth. Journal of Allergy and Clinical Immunology. 2017 Oct 1;140(4):1101-11.
40. Githinji LN, Gray DM, Hlengwa S, Myer L, Zar HJ. Lung function in South African adolescents infected perinatally with HIV and treated long-term with antiretroviral therapy. Annals of the American Thoracic Society. 2017 May;14(5):722-9.
41. Kunisaki KM, Nouraie M, Jensen RL, Chang D, D'Souza G, Fitzpatrick ME, et al. Lung function in men with and without HIV. AIDS. 2020 Jul 1;34(8):1227-35.
42. Effros RB, Fletcher CV, Gebo K, Halter JB, Hazzard WR, Horne FM, et al. Workshop on HIV infection and aging: what is known and future research directions. Clinical Infectious Diseases: An Official Publication of the Infectious Diseases Society of America. 2008 Aug 15;47(4):542.
43. Diaz PT, King MA, Pacht ER, Wewers MD, Gadek JE, Nagaraja HN, et al. Increased susceptibility to pulmonary emphysema among HIV-seropositive smokers. Annals of Internal Medicine. 2000 Mar 7;132(5):369-72.
44. Drummond MB, Merlo CA, Astemborski J, Marshall MM, Kisalu A, Mcdyer JF, et al. The effect of HIV infection on longitudinal lung function decline among injection drug users: a prospective cohort. AIDS (London, England). 2013 May 15;27(8):1303.
45. Drummond MB, Kirk GD, Astemborski J, Marshall MM, Mehta SH, McDyer JF, et al. Association between obstructive lung disease and markers of HIV infection in a high-risk cohort. Thorax. 2012 Apr 1;67(4):309-14.
46. Wejnert C, Raymond HF, Robbins T, Prejean J, Hall HI, McCray E, et al. Achieving greater HIV prevention impact through CDC's National HIV Behavioral Surveillance System. Journal of Acquired Immune Deficiency Syndromes. 2017 Jul 1;75:S249-52.s
47. Twigg III HL, Soliman DM, Day RB, Knox KS, Anderson RJ, Wilkes DS, et al. Lymphocytic alveolitis, bronchoalveolar lavage viral load, and outcome in human immunodeficiency virus infection. American Journal of Respiratory and Critical Care Medicine. 1999 May 1;159(5):1439-44.
48. Semenzato G. Immunology of interstitial lung diseases: cellular events taking place in the lung of sarcoidosis, hypersensitivity pneumonitis and HIV infection. European Respiratory Journal. 1991 Jan 1;4(1):94-102.
49. Popescu I, Drummond MB, Gama L, Lambert A, Hoji A, et al. HIV suppression restores the lung mucosal CD4+ T-cell viral immune response and resolves CD8+ T-cell alveolitis in patients at risk for HIV-associated chronic obstructive pulmonary disease. The Journal of Infectious Diseases. 2016 Nov 15;214(10):1520-30.
50. Gingo MR, Morris A, Crothers K. Human immunodeficiency Virus–Associated obstructive lung diseases. Clinics in Chest Medicine. 2013 Jun 1;34(2):273-82.
51. Chelvanambi S, Bogatcheva NV, Bednorz M, Agarwal S, Maier B, Alves NJ, et al. HIV-Nef protein persists in the lungs of aviremic patients with HIV and induces endothelial cell death. American Journal of Respiratory Cell and Molecular Biology. 2019 Mar;60(3):357-66.
52. Green LA, Yi R, Petrusca D, Wang T, Elghouche A, Gupta SK, et al. HIV envelope protein gp120-induced apoptosis in lung microvascular endothelial cells by concerted upregulation of EMAP II and its receptor, CXCR3. American Journal of Physiology-Lung Cellular and Molecular Physiology. 2014 Feb 15;306(4):L372-82.
53. Kasahara YA, Tuder RM, Cool CD, Lynch DA, Flores SC, Voelkel NF. Endothelial cell death and decreased expression of vascular endothelial growth factor and vascular endothelial growth factor receptor 2 in emphysema. American Journal of Respiratory and Critical Care Medicine. 2001 Mar 1;163(3):737-44.
54. Chung NP, Ou X, Khan KF, Salit J, Kaner RJ, Crystal RG. HIV reprograms human airway basal stem/progenitor cells to acquire a tissue-destructive phenotype. Cell Reports. 2017 May 9;19(6):1091-100.
55. Foronjy R, Nkyimbeng T, Wallace A, Thankachen J, Okada Y, Lemaitre V, et al. Transgenic expression of matrix metalloproteinase-9 causes adult-onset emphysema in mice associated with the loss of alveolar elastin. American Journal of Physiology-Lung Cellular and Molecular Physiology. 2008 Jun;294(6):L1149-57.
56. Mercer BA, Kolesnikova N, Sonett J, D'Armiento J. Extracellular regulated kinase/mitogen activated protein kinase is up-regulated in pulmonary emphysema and mediates matrix metalloproteinase-1 induction by cigarette smoke. Journal of Biological Chemistry. 2004 Apr 23;279(17):17690-6.
57. Kaner RJ, Santiago F, Crystal RG. Up‐regulation of alveolar macrophage matrix metalloproteinases in HIV1+ smokers with early emphysema. Journal of Leukocyte Biology. 2009 Oct;86(4):913-22.
58. Lalloo UG, Pillay S, Mngqibisa R, Abdool‐Gaffar S, Ambaram A. HIV and COPD: a conspiracy of risk factors. Respirology. 2016 Oct;21(7):1166-72.
59. Triplette M, Crothers K, Attia EF. Non-infectious pulmonary diseases and HIV. Current HIV/AIDS Reports. 2016 Jun 1;13(3):140-8.
60. Gingo MR, Morris A. Pathogenesis of HIV and the lung. Current HIV/AIDS Reports. 2013 Mar 1;10(1):42-50.
61. Pau AK, George JM. Antiretroviral therapy: current drugs. Infectious Disease Clinics. 2014 Sep 1;28(3):371-402.
62. Almodovar S. The complexity of HIV persistence and pathogenesis in the lung under antiretroviral therapy: challenges beyond AIDS. Viral immunology. 2014 Jun 1;27(5):186-99.
63. Ammassari A, Murri R, Pezzotti P, Trotta MP, Ravasio L, De Longis P, et al. Self-reported symptoms and medication side effects influence adherence to highly active antiretroviral therapy in persons with HIV infection. Journal of Acquired Immune Deficiency Syndromes (1999). 2001 Dec 1;28(5):445-9.
64. Heath KV, Singer J, O'Shaughnessy MV, Montaner JS, Hogg RS. Intentional nonadherence due to adverse symptoms associated with antiretroviral therapy. Journal of Acquired Immune Deficiency Syndromes (1999). 2002 Oct;31(2):211-7.
65. Gingo MR, Balasubramani GK, Rice TB, Kingsley L, Kleerup EC, Detels R, et al. Pulmonary symptoms and diagnoses are associated with HIV in the MACS and WIHS cohorts. BMC Pulmonary Medicine. 2014 Dec 1;14(1):75.
66. Estébanez-Muñoz M, Soto-Abánades CI, Ríos-Blanco JJ, Arribas JR. Updating our understanding of pulmonary disease associated with HIV infection. Archivos de Bronconeumología. 2012 Apr 1;48(4):126-32.
67. Kunisaki KM, Niewoehner DE, Collins G, Aagaard B, Atako NB, Bakowska E, et al. Pulmonary effects of immediate versus deferred antiretroviral therapy in HIV-positive individuals: a nested substudy within the multicentre, international, randomised, controlled Strategic Timing of Antiretroviral Treatment (START) trial. The Lancet Respiratory Medicine. 2016 Dec 1;4(12):980-9.
68. Mayaud C, Cadranel J. HIV in the lung from 1982 to 2013. Revue des Maladies Respiratoires. 2014 Feb;31(2):119.
69. Sharma A, Makrandi S, Modi M, Sharma A, Marfatia Y. Immune reconstitution inflammatory syndrome. Indian Journal of Dermatology, Venereology, and Leprology. 2008 Nov 1;74(6):619.
70. Mori S, Levin P. A brief review of potential mechanisms of immune reconstitution inflammatory syndrome in HIV following antiretroviral therapy. International Journal of STD & AIDS. 2009 Jul;20(7):447-52.
71. Vega LE, Espinoza LR. Human immunodeficiency virus infection (HIV)–associated rheumatic manifestations in thepre-and post-HAART eras. Clinical Rheumatology. 2020 Apr 15:1-8.
72. Wilson EM, Sereti I. Immune restoration after antiretroviral therapy: the pitfalls of hasty or incomplete repairs. Immunological Reviews. 2013 Jul;254(1):343-54.
73. Moreno-Fernandez ME, Presicce P, Chougnet CA. Homeostasis and function of regulatory T cells in HIV/SIV infection. Journal of Virology. 2012 Oct 1;86(19):10262-9.
74. Frankel AD, Young JA. HIV-1: fifteen proteins and an RNA. Annu Rev Biochem. 1998; 67: 1-25.
75. Clark E, Nava B, Caputi M. Tat is a multifunctional viral protein that modulates cellular gene expression and functions. Oncotarget. 2017 Apr 18;8(16):27569.
76. Marcello A, Zoppé M, Giacca M. Multiple modes of transcriptional regulation by the HIV‐1 Tat transactivator. IUBMB Life. 2001 Mar 1;51(3):175-81.
77. Ensoli B, Barillari G, Salahuddin SZ, Gallo RC, Wong-Staal F. Tat protein of HIV-1 stimulates growth of cells derived from Kaposi's sarcoma lesions of AIDS patients. Nature. 1990 May 3;345(6270):84-6.
78. Xiao H, Neuveut C, Tiffany HL, Benkirane M, Rich EA, Murphy PM, et al. Selective CXCR4 antagonism by Tat: implications for in vivo expansion of coreceptor use by HIV-1. Proceedings of the National Academy of Sciences. 2000 Oct 10;97(21):11466-71.
79. Reeder JE, Kwak YT, McNamara RP, Forst CV, D'Orso I. HIV Tat controls RNA Polymerase II and the epigenetic landscape to transcriptionally reprogram target immune cells. Elife. 2015 Oct 21;4:e08955.
80. Kim N, Kukkonen S, Martinez-Viedma MD, Gupta S, Aldovini A. Tat engagement of p38 MAP kinase and IRF7 pathways leads to activation of interferon-stimulated genes in antigen-presenting cells. Blood, The Journal of the American Society of Hematology. 2013 May 16;121(20):4090-100.
81. Scala G, Ruocco MR, Ambrosino C, Mallardo M, Giordano V, Baldassarre F, et al. The expression of the interleukin 6 gene is induced by the human immunodeficiency virus 1 TAT protein. The Journal of Experimental Medicine. 1994 Mar 1;179(3):961-71.
82. Sastry KJ, Reddy HR, Pandita R, Totpal K, Aggarwal BB. HIV-1 tat gene induces tumor necrosis factor-beta (lymphotoxin) in a human B-lymphoblastoid cell line. Journal of Biological Chemistry. 1990 Nov 25;265(33):20091-3.
83. Cribbs SK, Lennox J, Caliendo AM, Brown LA, Guidot DM. Healthy HIV-1-infected individuals on highly active antiretroviral therapy harbor HIV-1 in their alveolar macrophages. AIDS Research and Human Retroviruses. 2015 Jan 1;31(1):64-70.
84. András IE, Pu H, Deli MA, Nath A, Hennig B, Toborek M. HIV‐1 Tat protein alters tight junction protein expression and distribution in cultured brain endothelial cells. Journal of Neuroscience Research. 2003 Oct 15;74(2):255-65.
85. Sharma H, Chinnappan M, Agarwal S, Dalvi P, Gunewardena S, O'Brien-Ladner A, et al. Macrophage‐derived extracellular vesicles mediate smooth muscle hyperplasia: role of altered miRNA cargo in response to HIV infection and substance abuse. The FASEB Journal. 2018 Sep;32(9):5174-85.
86. Mediouni S, Darque A, Baillat G, Ravaux I, Dhiver C, Tissot-Dupont H, et al. Antiretroviral therapy does not block the secretion of the human immunodeficiency virus tat protein. Infectious Disorders-Drug Targets (Formerly Current Drug Targets-Infectious Disorders). 2012 Feb 1;12(1):81-6.
87. Mallon PW, Unemori P, Sedwell R, Morey A, Rafferty M, William K, et al. In vivo, nucleoside reverse-transcriptase inhibitors alter expression of both mitochondrial and lipid metabolism genes in the absence of depletion of mitochondrial DNA. Journal of Infectious Diseases. 2005 May 15;191(10):1686-96..
88. Williams AA, Sitole LJ, Meyer D. HIV/HAART-associated oxidative stress is detectable by metabonomics. Molecular BioSystems. 2017;13(11):2202-17.
89. Geraghty P, Hardigan AA, Wallace AM, Mirochnitchenko O, Thankachen J, Arellanos L, et al. The Glutathione Peroxidase 1–Protein Tyrosine Phosphatase 1B–Protein Phosphatase 2A Axis. A Key Determinant of Airway Inflammation and Alveolar Destruction. American Journal of Respiratory Cell and Molecular Biology. 2013 Nov;49(5):721-30.
90. Foronjy RF, Mirochnitchenko O, Propokenko O, Lemaitre V, Jia Y, Inouye M, et al. Superoxide dismutase expression attenuates cigarette smoke–or elastase-generated emphysema in mice. American Journal of Respiratory and Critical Care Medicine. 2006 Mar 15;173(6):623-31.
91. Hubbard RC, Ogushi F, Fells GA, Cantin AM, Jallat S, Courtney M, et al. Oxidants spontaneously released by alveolar macrophages of cigarette smokers can inactivate the active site of alpha 1-antitrypsin, rendering it ineffective as an inhibitor of neutrophil elastase. The Journal of Clinical Investigation. 1987 Nov 1;80(5):1289-95.
92. Kirkham PA, Barnes PJ. Oxidative stress in COPD. Chest. 2013 Jul 1;144(1):266-73.
93. Saberi P, Phengrasamy T, Nguyen DP. Inhaled corticosteroid use in HIV‐positive individuals taking protease inhibitors: a review of pharmacokinetics, case reports and clinical management. HIV Medicine. 2013 Oct;14(9):519-29.
94. Drummond MB, Kirk GD. HIV-associated obstructive lung diseases: insights and implications for the clinician. The Lancet Respiratory Medicine. 2014 Jul 1;2(7):583-92.
95. DerSarkissian M, Bhak RH, Oglesby A, Priest J, Gao E, Macheca M, et al. Retrospective analysis of comorbidities and treatment burden among patients with HIV infection in a US Medicaid population. Current Medical Research and Opinion. 2020 May 3;36(5):781-8.
96. Hall HI, Song R, Tang T, An Q, Prejean J, Dietz P, et al. HIV trends in the United States: diagnoses and estimated incidence. JMIR Public Health and Surveillance. 2017;3(1):e8.
97. MacDonald DM, Melzer AC, Collins G, Avihingsanon A, Crothers K, Ingraham NE, et al. Smoking and accelerated lung function decline in HIV-positive individuals: a secondary analysis of the START pulmonary substudy. Journal of Acquired Immune Deficiency Syndromes (1999). 2018 Nov 1;79(3):e85.
98. Sutradhar I, Gupta RD, Hasan M, Wazib A, Sarker M. Prevalence and risk factors of chronic obstructive pulmonary disease in Bangladesh: a systematic review. Cureus. 2019 Jan;11(1).
99. DeMarco RF, Gallagher D, Bradley-Springer L, Jones SG, Visk J. Recommendations and reality: Perceived patient, provider, and policy barriers to implementing routine HIV screening and proposed solutions. Nursing Outlook. 2012 Mar 1;60(2):72-80.
100. North CM, Kakuhikire B, Vořechovská D, Hausammann-Kigozi S, McDonough AQ, Downey J, Christiani DC, Tsai AC, Siedner MJ. Prevalence and correlates of chronic obstructive pulmonary disease and chronic respiratory symptoms in rural southwestern Uganda: a cross-sectional, population-based study. Journal of Global Health. 2019 Jun;9(1).
101. Thuppal SV, Jun S, Cowan A, Bailey RL. The nutritional status of HIV-infected US adults. Current Developments in Nutrition. 2017 Oct 1;1(10):e001636.
102. Drummond MB, Kirk GD. HIV-associated obstructive lung diseases: insights and implications for the clinician. The Lancet Respiratory Medicine. 2014 Jul 1;2(7):583-92.