Abstract
In Iowa there has been an upward trend in the number of cases of Lyme disease. Due to this increase, it is important to understand the transmission pattern and the factors that play a role in the spread of this disease. Lyme disease is caused by the bacterium, Borrelia burgdorferi, and is transmitted by Ixodes scapularis, within the United States. I. scapularis has a two-year life cycle that includes three life stages: larva, nymph, and adult, and requires a blood meal between each stage. Ticks can pick up B. burgdorferi through a blood meal at any stage. It is the nymph stage that is the most active and most likely to infect humans. The final blood meal, taken as an adult, usually occurs upon the reproductive host, Odocoileus virginianus, (white-tailed deer). Since white-tailed deer play such an important role in the life cycle of I. scapularis, it is essential to get a better understanding of this relationship. 111 ticks were collected from 60 harvested deer, during the first shotgun season of hunting in December 2018, from Northeast Iowa. The deer were from 11 different counties throughout Iowa, 5 of these counties having deer with infested adult I. scapularis. 96 of the total ticks tested positive for the presence of B. burgdorferi. Of the counties with I. scapularis collected, all had at least one tick positive for B. burgdorferi.
Keywords
Borrelia burgdorferi, harvested deer, Iowa, Ixodes scapularis, Lyme disease, Odocoileus virginianus, PCR, tick, tick-borne
Introduction
The number of cases within the United States of tick-borne diseases is increasing at startling rates, having more than doubled in the past 13 years. A high percentage (82%) of these cases is due to the most commonly reported vector-borne illness in the United States, Lyme disease [1]. These high rates are primarily reported in the eastern part of the United States, particularly along the Pacific Coast, and in regions of the upper Midwest, particularly in Wisconsin and Minnesota, but increasing numbers continue to spread to other regions as well [2]. Throughout Iowa there has been an incline in the number of cases of Lyme disease, and is of public health concern [3,4]. Because of this increase, it is important to get a better understanding of the different factors that control the presence of ticks, and the Lyme disease pathogen in Iowa.
Lyme disease is caused by the spirochete bacterium, Borrelia burgdorferi, that is transmitted to humans primarily through the vector, Ixodes scapularis, commonly known as the blacklegged tick or deer tick [5,6]. Lyme disease has become fairly prevalent in areas that support the system through which the B. burgdorferi is transmitted. Many small mammals, primarily rodents, act as reservoir hosts of B. burgdorferi, and are common blood-sources for ticks, where a tick can pick up B. burgdorferi during the larval stage. Ticks then enter the nymph stage and can attach to other animals, including humans and pets, for their next blood meal where an infected tick can transfer B. burgdorferi to the new host [7,8]. Most individuals that are infected in the Midwest, with this Lyme disease pathogen, are infected during the summer months of May through August, when the nymph stage of the tick is most active [9]. Typically, Lyme disease will thrive in areas that are suburban and located close to forested areas, allowing for small mammals from the forest to thrive off of the abundance of resources provided from the people of the suburban area. The small mammal species that are able to adapt to environments relatively easily has allowed for, not only their survival but, the survival and success of B. burgdorferi. This contributes to why the most common cases of Lyme disease that are seen today occur in the Midwest and Northeast, areas that were both at one point dense forests but have changed due to human influences [10,11].
White-tailed deer (Odocoileusvirginianus), which serves as a primary host of I. scapularis, also plays a role in the successful populations of both I. scapularis and B. burgdorferi [12-15]. Though white-tailed deer are incompetent as reservoirs of B. burgdorferi [16,17], they are the primary source of the final bloodmeal for I. scapularis females, and the primary site for tick mating [18,19]. During the winter of 2018, ticks were collected from harvested deer within Northeast Iowa. From the collected ticks, total DNA was isolated and tested for the presence of the Borrelia burgdorferi specific gene, OspA, which indicates the presence of the pathogen. With this information, we will have a stronger understanding of the relationship between white-tailed deer, I. scapularis, and B. burgdorferi in Northeast Iowa.
Material and Methods
Two days were dedicated to the collection of ticks from harvested deer, December 1st and 2nd, 2018. The location was a site within Northeast Iowa. As deer were being brought to this location, individuals were asked where they shot the deer. The deer then went through initial processing, including the removal of antlers or heads if so desired from the hunter. The deer’s coat was then searched for ticks using forceps and fingers [20]. Searching was focused towards the dorsal surface of the deer on the head and neck region, as this is where the majority of I. scapularis attach [21]. Time spent for each deer varied on different factors including the number of ticks present, amount of head and neck region remaining, and individual researchers. However, it is estimated that roughly 3-5 minutes was spent searching for ticks per each deer.
Collected ticks were placed into a vial containing 70% ethanol until they were able to be identified to species and stage using published keys [22] and then processed for molecular studies. Total DNA was isolated from the ticks by the following methods: ticks were removed from the ethanol and allowed to dry completely and placed individually into a 1.5mL tube. 5 µl of 0.7M ammonium hydroxide was added and the tube was then placed into liquid nitrogen. The ticks were ground with a micro pestle. 95 µl of 0.7M ammonium hydroxide was added and placed into 100°C for 15 minutes, with the lid open to allow for some evaporation. Tubes were then centrifuged for 10 minutes at 10,000g. Finally, the liquid was transferred to a new tube and stored at -20ºC until PCR (Polymerase Chain Reaction) was carried out.
Two different PCR tests were carried out for each sample. The first was a PCR of the 18S rRNA gene to verify successful DNA isolation. And second, PCR was carried out to determine the presence of the B. burgdorferi specific gene, OspA. The 18S rRNA primers were: Forward primer 5’GTGCCAGCAGCCGCGGTA3’ and Reverse primer 5’GTGGTGCCCTTCCGTCAATTC3’. The following PCR conditions were used for the 18S rRNA amplification: Initial denaturation: 95ºC for 3 minutes, 40 cycles of: 95ºC for 30 seconds (denaturation), 50ºC for 30 seconds (annealing) and 68ºC for 1 minutes (ex tension), and a final extension at 68ºC for 10 minutes. The OspA primers were: Forward primer 5’CTTTAAGCTCAAGCTTGTCTACTGT3’ and Reverse primer 5’TTATGAAAAAATATTTATTGGGAAT3’. The following PCR conditions were used for the OspA amplification: Initial denaturation: 95ºC for 3 minutes, 45 cycles of: 95ºC for 30 seconds, 50ºC for 30 seconds and 68ºC for 30 seconds, and a final extension at 68ºC for 10 minutes. PCRs products were then run out on a 2% agarose gel to determine the presence of gene amplification. Control PCRs were carried out as well. Water served as a negativecontrol and DNA isolated from B. burgdorferi (ATCC, Inc.) as the positive control.
Results
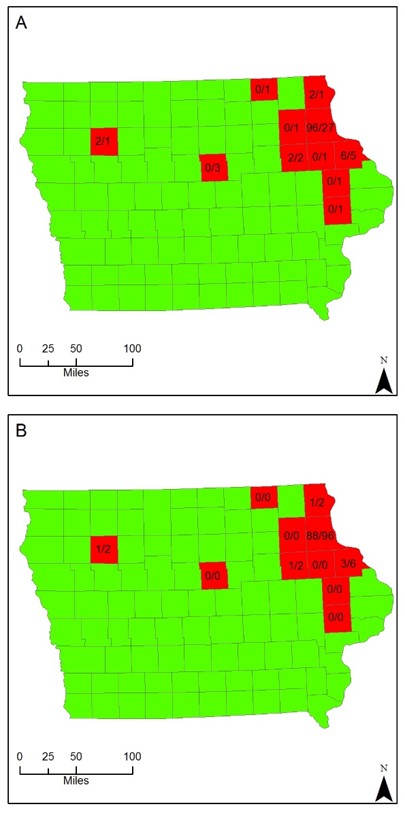
60 white-tailed deer, which were killed during the first shotgun hunting season of 2018, were examined at a location in Northeast Iowa. The 60 deer were from 11 counties throughout Iowa, most being located within Northeast and Eastern Iowa. Three of the 60 deer were from an undisclosed location, however, within the state of Iowa. The majority of the deer that were examined were from Clayton county (45%), then Dubuque county (8.3%), Hardin county (5%), Buchanan county (3.3%), and the remaining counties with 1 deer (1.7%): Allamakee, Buena Vista, Cedar, Delaware, Fayette, Howard, Jones, and 5% from undisclosed locations (Figure 1).
Figure 1. County locations (red) of sampled Odocoileus virginianus with the number of Ixodes scapularis collected per total deer for each county (A) and the number of ticks testing positive for Borrelia burgdorferi of the total ticks collected for each county (B).
111 adult I. scapularis were collected from 23 of the 60 examined deer, representing 5 of the 11 counties. The mean number of I. scapularis from infested deer was 5 per deer, with the median of 2.11 of the 23 deer only had one tick located within the area of sampling (47.8%). Two of the infested deer, both from Clayton County, had a high number of ticks when compared to the other infested deer, 30 and 32 ticks (56% of the total ticks collected) (Figure 1).
Total DNA was extracted from the ticks and tested for the presence of a B. burgdorferi specific gene by PCR. 96 of the 111 ticks tested positive (86.5%). Of the counties with collected ticks, four of them had a 50% positive rate for B. burgdorferi (Allamakee, Buchanan, Buena Vista, and Dubuque). Clayton County had the highest percentage of positive carriers, 91.7%, and of the three ticks collected from undisclosed locations, 66.7% were positive for B. burgdorferi (Figure 1).
Discussion
This study was carried out to gain a better understanding of the relationship between three factors of Lyme disease, within Northeastern Iowa. In the relationship between white-tailed deer and ticks, the deer serve as the primary host, the site for mating and the final blood meal for ticks. There is a high amount of deer within Iowa, with a deer herd estimated to be about 400,000 with 108,398 harvested in 2018 due to hunting. The county with the highest amount of deer harvested during hunting season was Clayton County, within Northeast Iowa (Iowa DNR). This is also the same county with the highest representation within our study. We looked through 60 deer from 11 different counties, out of the 99 total within Iowa.
Of the 60 deer sampled, 111 I. scapularis were collected with the mean of 5 ticks per deer. Ticks were found on deer from 5 different counties, all counties where ticks have previously been categorized as reported or established [23]. The number of ticks collected from each infested deer ranged from 1-32. These numbers likely do not reflect the total number of ticks per infested deer for multiple reasons. The amount of time dedicated to searching for and collecting ticks from each deer varied, due to the different researchers inspecting the deer and the rate at which the deer were arriving and needed to be processed. Another factor was the condition of the deer as it arrived. Some hunters decided to keep the head or antlers, removing a majority of the animal that the search was focused on. Finally, one other factor could be the amount of time from when the deer had died to when the animal was brought to us, as the ticks may have fallen off before processing and searching for ticks.
Conclusion
Of the collected ticks, a total of 86.5% were positive for the presence of B. burgdorferi, all from counties where cases of Lyme disease have been reported. Lyme disease has become fairly prevalent in areas that support the system through which the bacterium B. burgdorferi is transmitted. Future work needs to focus on other factors that play a role in the prevalence levels of B. burgdorferi. Future work also needs to be done to increase the number of deer sampled and to increase the number of counties represented across Iowa.
Acknowledgements
We would like to thank fellow members of the Grussendorf lab, especially Thomas Scroggs and Emily Less, for their assistance with this project. We would like to thank David Koch Ph.D for his assistance in data collection. This work was funded by the Women in Science Fellowship which is supported by the Monticello College Foundation.
Author Contributions
All of the authors of this manuscript have read and approved the final version and have made substantive contributions to the work described. All of the authors have no conflicts of interest.
Funding
This work was funded by the Women in Science Fellowship which is supported by the Monticello College Foundation.
References
2. Walter KS, Carpi G, Caccone A, Diuk-Wasser MA. Genomic insights into the ancient spread of Lyme disease across North America. Nature Ecology & Evolution. 2017 Oct;1(10):1569-76.
3. Iowa Department of Natural Resources. 2018. Deer and Turkey Harvest Report, https://gooutdoorsiowa.com/RealTimeHarvestReport.aspx. Accessed October 2019.
4. Iowa Department of Public Health – Center for Acute Disease Epidemiology. 2018. Historical Case Count Report, http://idph.iowa.gov/cade. Accessed October 2019.
5. Burgdorfer W, Barbour AG, Hayes SF, Benach JL, Grunwaldt E, Davis JP. Lyme disease-a tick-borne spirochetosis?. Science. 1982 Jun 18;216(4552):1317-9.
6. Johnson RC, Schmid GP, Hyde FW, Steigerwalt AG, Brenner DJ. Borrelia burgdorferi sp. nov.: etiologic agent of Lyme disease. International Journal of Systematic and Evolutionary Microbiology. 1984 Oct 1;34(4):496-7.
7. Dennis DT, Nekomoto TS, Victor JC, Paul WS, Piesman J. Reported distribution of Ixodes scapularis and Ixodes pacificus (Acari: Ixodidae) in the United States. Journal of Medical Entomology. 1998 Sep 1;35(5):629-38.
8. Radolf JD, Caimano MJ, Stevenson B, Hu LT. Of ticks, mice and men: understanding the dual-host lifestyle of Lyme disease spirochaetes. Nature Reviews Microbiology. 2012 Feb;10(2):87-99.
9. Stafford KC, Cartter ML, Magnarelli LA, Ertel SH, Mshar PA. Temporal correlations between tick abundance and prevalence of ticks infected with Borrelia burgdorferi and increasing incidence of Lyme disease. Journal of Clinical Microbiology. 1998 May 1;36(5):1240-4.
10. Guerra M, Walker E, Jones C, Paskewitz S, Cortinas MR, Stancil A, et al. Predicting the risk of Lyme disease: habitat suitability for Ixodes scapularis in the north central United States. Emerging Infectious Diseases. 2002 Mar;8(3):289.
11. Ostfeld RS, Cepeda OM, Hazler KR, Miller MC. Ecology of Lyme disease: habitat associations of ticks (Ixodes scapularis) in a rural landscape. Ecological Applications. 1995 May;5(2):353-61.
12. Nguyen A, Mahaffy J, Vaidya NK. Modeling transmission dynamics of lyme disease: Multiple vectors, seasonality, and vector mobility. Infectious Disease Modelling. 2019 Jan 1;4:28-43.
13. Raizman EA, Holland JD, Shukle JT. White-tailed deer (Odocoileus virginianus) as a potential sentinel for human Lyme disease in Indiana. Zoonoses and Public Health. 2013 May;60(3):227-33.
14. Rand PW, Lubelczyk C, Lavigne GR, Elias S, Holman MS, Lacombe EH, et al. Deer density and the abundance of Ixodes scapularis (Acari: Ixodidae). Journal of Medical Entomology. 2003 Mar 1;40(2):179-84.
15. Stafford III KC, Denicola AJ, Kilpatrick HJ. Reduced abundance of Ixodes scapularis (Acari: Ixodidae) and the tick parasitoid Ixodiphagus hookeri (Hymenoptera: Encyrtidae) with reduction of white-tailed deer. Journal of Medical Entomology. 2003 Sep 1;40(5):642-52.
16. Jaenson TG, Tälleklint L. Incompetence of roe deer as reservoirs of the Lyme Borreliosis spirochete. Journal of Medical Entomology. 1992 Sep 1;29(5):813-7.
17. Telford III SR, Mather TN, Moore SI, Wilson ML, Spielman A. Incompetence of deer as reservoirs of the Lyme disease spirochete. The American Journal of Tropical Medicine and Hygiene. 1988 Jul 1;39(1):105-9.
18. Kilpatrick HJ, Labonte AM, Stafford III KC. The relationship between deer density, tick abundance, and human cases of Lyme disease in a residential community. Journal of Medical Entomology. 2014 Jul 1;51(4):777-84.
19. Spielman A, Wilson ML, Levine JF, Piesman J. Ecology of Ixodes dammini-borne human babesiosis and Lyme disease. Annual Review of Entomology. 1985 Jan;30(1):439-60.
20. Davis JP, Schell WL, Amundson TE, Godsey Jr MS, Spielman A, Burgdorfer W, et al. Lyme disease in Wisconsin: epidemiologic, clinical, serologic, and entomologic findings. The Yale Journal of Biology and Medicine. 1984 Jul;57(4):685.
21. Schmidtmann ET, Carroll JF, Watson DW. Attachment-site patterns of adult blacklegged ticks (Acari: Ixodidae) on white-tailed deer and horses. Journal of Medical Entomology. 1998 Jan 1;35(1):59-63.
22. Keirans JE, Clifford CM. The genus Ixodes in the United States: a scanning electron microscope study and key to the adults. Journal of Medical Entomology. 1978 Jul 1;15(suppl_2):1-38.
23. Oliver JD, Bennett SW, Beati L, Bartholomay LC. Range expansion and increasing Borrelia burgdorferi infection of the tick Ixodes scapularis (Acari: Ixodidae) in Iowa, 1990–2013. Journal of Medical Entomology. 2017 Nov 7;54(6):1727-34.