Abstract
The term glomerulonephritis (GN) describes immune-mediated disorders of the kidney’s filtration units (the glomeruli). Current classifications are based on histopathological lesion patterns. However, different underlying conditions can lead to similar tissue injury and lesions but the respective therapy depends on the underlying disease. To this end, a new classification should involve the cause of GN. The five proposed categories comprise infection-related GN, autoimmune GN, alloimmune GN, autoinflammatory GN, and monoclonal gammopathy-related GN. Making the correct diagnosis can involve numerous different avenues of diagnostics beyond or even without a kidney biopsy. Additionally, dissecting activity from chronicity in GN and considering other systemic disorders and risk factors of an individual is essential to trigger different therapies. With the assessment of now available disease activity markers immunomodulatory drugs should be used for the active phase and long-term renoprotective drugs in chronic conditions. Taken together, the proposed classification aims to improve our understanding, the diagnosis and treatment of glomerulonephritis and might pave the way to next generation patient individual precision medicine.
Keywords
Glomerulonephritis, Immunonephrology, Autoimmunity, Nephropathology
Introduction
Glomerulonephritis (GN) represents a group of immune-mediated disorders characterized by inflammation and injury of the glomeruli [1], which are the kidney's filtration units. These disorders can cause severe kidney damage comprising renal replacement therapy, thus posing a significant burden on healthcare systems [2]. Despite its historical categorization based on histopathological findings [3], the evolving understanding of the immune mechanisms underlying GN calls for a more nuanced approach to classification and treatment [1].
The glomeruli, nested within the nephron, serve as a highly specialized filtration barrier. Comprising endothelial cells, the glomerular basement membrane (GBM), and podocytes, this structure maintains the delicate balance of retaining essential proteins and cellular components while allowing the passage of water and certain solutes. Immune-mediated disruptions to this intricate barrier result in hallmark clinical features, including proteinuria, hematuria, and impaired kidney function.
Traditionally, GN has been categorized by lesion patterns observable in kidney biopsies [1,3]. Terms such as membranoproliferative GN, crescentic GN, and membranous nephropathy have long guided clinicians in understanding disease morphology. However, this histological approach can overlook the underlying immunological processes, leading to therapeutic ambiguity and suboptimal outcomes. For instance, two patients with similar biopsy findings might exhibit vastly different responses to the same treatment due to variations in their disease’s immunopathogenesis [1]. This discrepancy underscores the limitations of histology-centric paradigms. Recent advances in immunonephrology have illuminated the diverse mechanisms driving GN [1,4-8]. From infections that incite immune complex formation [5,9,10] to autoantibody-driven inflammation in autoimmune diseases [1,4,5,11-16], the spectrum of GN reflects the interplay of innate and adaptive immune system. Infections, autoimmune conditions, transplantation [17-22], genetic predispositions [23-31], and monoclonal gammopathies [32-34] represent distinct triggers, each with unique therapeutic implications. These insights have catalyzed the shift towards a pathogenesis-based classification [1,4,35], aligning disease categorization with precision medicine.
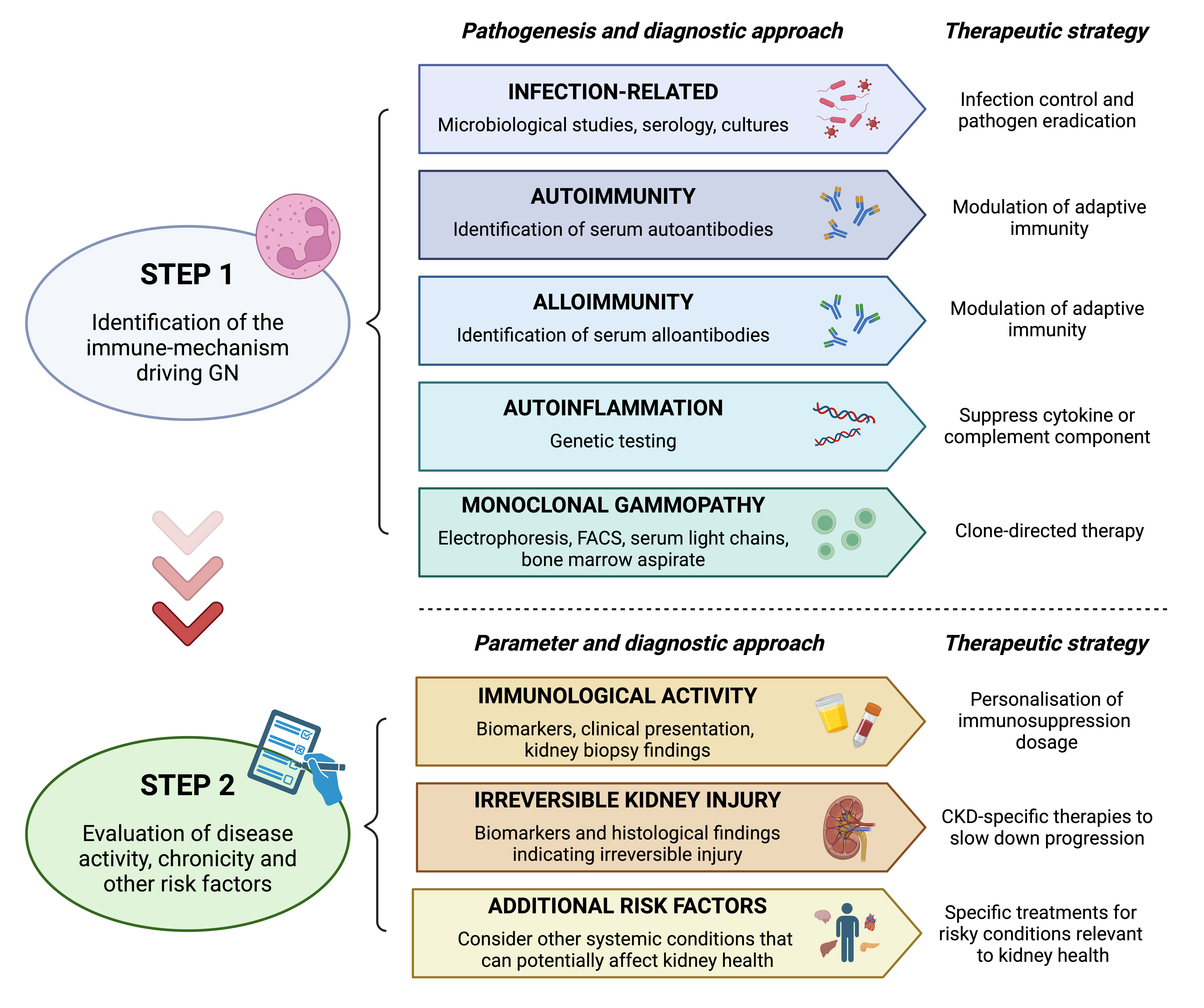
Pathogenesis-based frameworks not only enhance diagnostic accuracy but also pave the way for tailored treatments [1]. For example, infection-related GN benefits from pathogen-targeted therapies [9,10,36], while autoimmune GN responds to immune modulation strategies [14,37–50]. Similarly, the recognition of autoinflammatory GN has spurred the development of cytokine inhibitors [51], and monoclonal gammopathy-related GN requires plasma cell-directed treatments [52,53]. This review explores the proposed reclassification of GN comprising five immune-mechanism driven categories: infection-related GN, autoimmune GN, alloimmune GN, autoinflammatory GN, and monoclonal gammopathy-related GN. Additionally both current disease activity as well as chronic changes [54] should be assessed to guide treatment. Furthermore, other systemic disorders and risk factors that might affect kidney lifetime should be included in this classification. Among these, diabetes, hypertension, metabolic syndrome and lifestyle are highly relevant for patient and kidney outcome, especially if GN occurs. This classification is called GN-AC and is determined using the following algorithm (Figure 1).
Figure 1. Algorithm to classify and treat glomerulonephritis. In the first step, immune pathogenesis needs to be identified to guide treatment. In the second step, immunological activity, chronic kidney injury and additional systemic risk factors should be considered to obtain patient-individual treatment strategies.
The GN-AC Classification
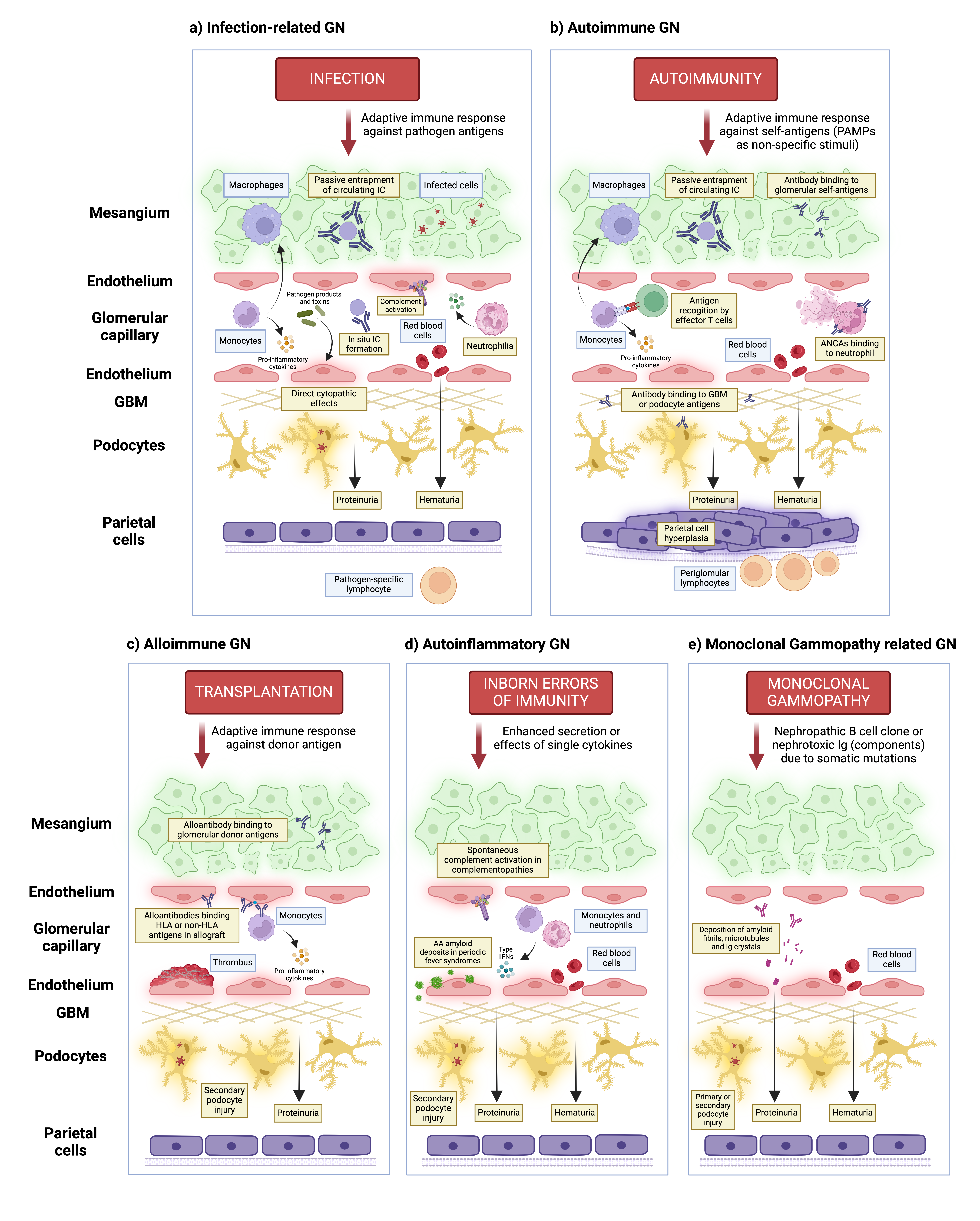
In a first step the immune mechanism driving GN is explored (Figure 2).
Figure 2. Detailed molecular mechanisms of the different immunophenotypes of glomerulonephritis. GN: Glomerulonephritis; IC: Immune Complex; HLA: Human Leucocyte Antigen.
Infection-related GN
Infection-related GN is triggered by acute, subacute or persistent infections of bacterial, viral, fungal or parasitic origin [5,9,10,55,56]. Common GN causing pathogens are e.g. beta-hemolytic streptococci [9] or HIV [55]. These infections lead to an activation of the host defense. Through a deposition of circulating immune complexes in the glomeruli, the activation of complement, or direct cytotoxic effects of the pathogens, immune cells are attracted to the glomeruli leading to further inflammation and damage. Therapeutic approaches for this GN entity should rely primarily on pathogen eradication. Immunosuppressive treatment should be avoided unless the infection is well controlled [10,36].
Autoimmune GN
Autoimmune GN is caused by a loss of immune tolerance leading to autoantibody production, as seen e.g. in systemic lupus erythematosus [57,58] or anti-PLA2R-related membranous GN [59]. Both the adaptive as well as the innate immune system contribute to this GN entity. Well-established immuno-serological assays can nowadays detect pathogenic antibodies (anti-dsDNA, MPO-ANCA, PR3-ANCA, anti-PLA2R and probably anti-slit diaphragm like anti-Nephrin [60] that drive autoimmune GN. Besides antibody producing B cells and long-living plasma cells, autoreactive T cells [61], and neutrophils play a crucial role [62]. Therapeutic approaches aim to target these immune components. Unspecific immune-modulating agents include glucocorticoids and calcineurin inhibitors. Antibody producing cells like B-cells or plasma cells can be specifically targeted using Rituximab, Obinutuzumab [43,44] or emerging CAR-T cell therapies [63]. The latest scientific evidence is reassuring that with these cell-targeted therapies a sustainable suppression of autoimmunity and inflammation is possible.
Alloimmune GN
Alloimmune GN develops in transplant recipients due to immune responses of the host against donor antigens [17,18]. Often CD8+ T cells, NK cells, complement system and donor-specific antibodies are involved. Whereas T-cell mediated rejection mainly leads to tubulointerstitial inflammation, antibody-mediated rejection additionally can lead to an inflammation of the kidneys’ glomeruli. Current therapeutic approaches focus on immunosuppressive regimens that suppress adaptive immune response to induce immunological tolerance and prevent graft rejection [64].
Autoinflammatory GN
Autoinflammatory GN results from genetic defects causing dysregulation in innate immune pathways [1,4,65]. These comprise activation of complementary system or cytokine overproduction. These rare diseases can be diagnosed with molecular genetic testing or specific assays (e.g. phagocytosis assay for chronic granulomatous disease, complement diagnostic for complement-mediated GN). To treat this entity a targeted blockade of specific inflammatory pathways like anti-IL-1, anti-TNF, anti-interferon, or complement inhibitors is favored. Broad immunosuppression should be avoided if possible due to side-effects.
Monoclonal gammopathy-related GN
Monoclonal gammopathy-related GN is caused by nephrotoxic monoclonal immunoglobulins [32,33] or their components that are produced by clonal B cells or plasma cells [33]. For diagnosis immuno-serological examinations like electrophoresis and immune fixation are used, whereas kidney biopsy workup shows monoclonal immunoglobulin deposition in the glomeruli. Here, therapeutic approaches should focus on the elimination of the pathogenic clone through treatment agents derived from multiple myeloma therapy protocols.
In a second step the current activity of the GN should be evaluated and compared to chronic changes. Furthermore, we propose to consider other systemic preconditions and risk factors that are affecting kidney lifetime.
Activity
Activity (A) defines the current immunological activity of a disease. Ranging from absent, low, moderate or high based on biomarkers, clinical presentation, and biopsy findings, therapy guidance can be achieved. For example, high activity would typically necessitate intensive immunosuppressive therapy to prevent further acute injury [52]. Low activity would question potential harmful immunosuppressive treatments, and therapy would rely on treating e.g. chronicity.
Chronicity
Chronicity (C) reflects the duration and extent of irreversible kidney damage [66-69]. A categorization into absent, early chronic kidney disease (CKD), advanced CKD, or kidney failure, seems appropriate. Treatment of chronicity is possible using CKD-specific therapies, such as renin-angiotensin system inhibitors, sodium-glucose cotransporter-2 inhibitors, or mineralocorticoids. With these drugs the progression of renal dysfunction can be mitigated.
Additional risk factors
Additionally, we propose considering risk factors for future kidney health comprising systemic or other relevant conditions of a patient. Besides metabolic disorders and hypertension, kidney life limiting conditions like heart failure, or liver cirrhosis should be considered. Treatment of these diseases is crucial to prevent further damage to the kidney. As the kidney is a central organ of the body, dependent on the function of other organs, a holistic treatment approach is needed. In the case of GN treating these risk factors is crucial as without it, patients might not benefit from highly specific and costly GN treatments.
Practical Implications
This new GN-AC classification is empowered by multidimensional immunophenotyping, including serological biomarkers, genetic testing, kidney biopsy, and non-invasive methods. Research efforts of the last decades identified autoantibodies (e.g. anti-PLA2R, MPO-ANCA, PR3-ANCA, anti-GBM) and free light chains to monitor disease activity. Especially for autoinflammatory GN genetic testing is crucial [51,62,70] and now widely available. Kidney biopsy workups, being the gold-standard of GN diagnosis, can now be complemented by systemic immune evaluations. Additionally, light microscopy, immunohistochemistry and electron microscopy might be supplemented with spatial transcriptomics in the future for a better identification of disease-causing cells in the different GN entities. With the emerging biomarkers, diagnostic precision and patient outcomes will be improved and might abrogate in more and more cases the necessity to perform invasive and potential harmful kidney biopsies.
We believe that a pathogenesis-based categorization has profound implications for treatment (Table 1). Infections require direct control, obviating the need for immunosuppression unless pathogen clearance is achieved. Autoimmune and alloimmune forms benefit from adaptive immunity-targeted therapies, while autoinflammatory types respond to specific cytokine or complement inhibition. Furthermore, monoclonal gammopathies necessitate clonal eradication as the cornerstone of management. Besides the treatment of active disease, chronic injury should be targeted as well (Table 2).
|
Classification criteria |
Report |
Therapy |
|
GN: Glomerulonephritis defined by immunophenotyping |
Infection-related: specify etiology and chronicity |
Infection control, assess immunotherapy |
|
Autoimmunity: specify autoantigen (e.g. gd-IgA1, PLA2R, MPO, PR3, exostosin 1-2) and systemic disorder (SLE, AAV) |
Immunotherapy (targeted or broad spectrum) |
|
|
Alloimmunity: specify type of graft and alloantigen (HLA class I or II, non-HLA antibodies) |
Immunosuppression, B-targeted therapy, PLEX, complement blockade, plasma-cell targeted therapy |
|
|
Autoinflammation |
Specific pathway blockade |
|
|
Monoclonal gammopathy |
Clone-directed therapy |
|
|
A: Activity (immunological) |
0: Absent |
Observation |
|
1: Low (serum biomarkers and/or nephrotic syndrome and/or little active injury on kidney biopsy) |
Observation or specific therapy |
|
|
2: Moderate (serum biomarkers and/or moderate active injury on kidney biopsy) |
Specific therapy |
|
|
3: High (serum biomarkers and/or nephrotic syndrome and/or high active injury on kidney biopsy) |
Intense specific therapy, consider bail-out therapies if refractory |
|
|
C: Chronicity |
0: Absent (no CKD or no glomerulosclerosis/fibrosis on biopsy) |
Observation |
|
1: Early CKD (G1/A1-A2) and/or few glomerulosclerosis/fibrosis on biopsy |
Observation or CKD therapy |
|
|
2: Advanced CKD (G2-4/A1-3) and/or significant glomerulosclerosis/fibrosis on kidney biopsy |
CKD therapy |
|
|
3: Kidney failure (G5, G5D), severe glomerulosclerosis/fibrosis or atrophy in kidney biopsy |
CKD therapy, consider kidney replacement therapies |
|
|
Additional risk factors |
Lifestyle and clinical history (e.g. sedentarism, tobacco use, therapeutic adherence, past or future pregnancies) |
Assess clinical lifestyle changes, planned pregnancies, warning signs |
|
Previous organ specific diseases (e.g. previous AKI, nephrectomy, heart failure, cirrhosis, dementia) |
Control of comorbidities, rehabilitation if possible |
|
|
Systemic diseases / Immunodeficiencies (e.g. type 2 diabetes mellitus, SLE, APS, AIDS) |
Control of comorbidities, referral to other specialists if necessary |
|
|
Serologic background (CMV, EBV, HIV) and microbiological hazards |
Prophylaxis, chronic ART, if necessary, referral to other specialists if necessary |
|
|
Genetic background (COL4A3, ADPKD, APOL1) |
Specific treatment if possible |
|
|
PLA2R: Phospholipase-A2-receptor, MPO: Myeloperoxidase; PR3: Proteinase 3; SLE: Systemic Lupus Erythematosus; AAV: ANCA-Associated Vasculitis; HLA: Human Leucocyte Antigen; CKD: Chronic Kidney Disease; APS: Anti-Phospholipid Syndrome; AIDS: Acquired Immunodeficiency Syndrome; CMV: Cytomegalovirus; EBV: Epstein-Barr Virus; HIV: Human Immunodeficiency Virus; ART: Anti-Retroviral Therapy; COL4A3: Collagen Type IV Alpha 3; ADPKD: Autosomal Dominant Polycystic Kidney Disease; APOL1: Apolipoprotein L1. |
||
|
Glomerulonephritis subtype |
Activity treatment |
Chronicity treatment |
|
Infection-related |
Infection control, e.g., antibiotics |
CKD treatment, e.g., including ACEi and SGLT2i |
|
Autoimmunity |
Immunosuppressive or clone-targeted therapy |
CKD treatment, e.g., including ACEi and SGLT2i |
|
Alloimmunity |
Immunosuppression |
Modification of immunosuppression and CKD treatment |
|
Autoinflammation |
Selective immunotherapy like blockade of a specific cytokine |
CKD treatment, e.g., including ACEi and SGLT2i |
|
Monoclonal Gammopathy |
Chemotherapeutic agents depending on the nature of the clone |
CKD treatment, e.g., including ACEi and SGLT2i |
|
ACEi: Angiotensin Converting Enzyme inhibitor. SGLT2i: Sodium Glucose Cotransporter 2 inhibitor. |
||
Furthermore, recent years have brought substantial advances in the treatment of glomerulonephritis, particularly in lupus nephritis. Obinutuzumab, a type II anti-CD20 monoclonal antibody, has emerged as a promising agent. In the REGENCY trial, the addition of obinutuzumab to standard therapy significantly improved renal outcomes in patients with lupus nephritis compared to standard therapy alone [71]. Moreover, a recent head-to-head comparison of obinutuzumab versus rituximab in patients with membranous nephropathy suggested superior efficacy and a favorable pharmacodynamic profile for obinutuzumab [72]. A further innovative therapeutic avenue involves CAR-T cell therapy targeting CD19-positive B cells. A 2024 case series demonstrated that CD19-directed CAR-T cell therapy induced remission in refractory autoimmune diseases, including lupus nephritis. This treatment was associated with sustained B-cell depletion, reduced disease activity, and preservation of kidney function. Although still under clinical investigation, these findings indicate a potential paradigm shift in the treatment of patients who do not respond to conventional immunosuppression [73]. In the field of IgA nephropathy (IgAN), novel therapies targeting the complement system are being developed. Iptacopan, an oral alternative complement pathway inhibitor that selectively binds factor B, has demonstrated promising results. In the phase 3 APPLAUSE-IgAN trial, iptacopan significantly reduced proteinuria compared to placebo in patients at high risk of disease progression. In August 2024, the FDA granted accelerated approval for iptacopan to reduce proteinuria in adults with primary IgAN [74]. For C3 glomerulopathy (C3G), a rare complement-mediated kidney disease, iptacopan also showed efficacy. In a phase 2 study, iptacopan reduced proteinuria by approximately 45% in patients with C3G, leading to its FDA approval in March 2025 for the treatment of adults with C3G to reduce proteinuria [75]. Another complement inhibitor, pegcetacoplan, targeting C3 itself, has also demonstrated substantial benefit in patients with C3G and immune complex-mediated membranoproliferative glomerulonephritis, as shown in the VALIANT trial [76]. To support clinical implementation, the most recent KDIGO Clinical Practice Guidelines provide comprehensive, evidence-based recommendations [77,78].
The proposed GN-AC model fosters advancements in research. It encourages the exploration of immunopathogenesis of GN. With the discovery of novel biomarkers (e.g. anti-Nephrin [79], anti-Podocin [80] therapeutic innovations become possible beyond ancient histological paradigms. The educational simplification provides a coherent framework for understanding GN, making it accessible to researchers and clinicians across disciplines. Furthermore, for the design of clinical trials the selection of participants can now be based on immunological activity rather than non-specific markers like proteinuria. This might enhance trial efficacy and relevance.
Conclusion
The GN-AC classification represents a transformative approach to GN diagnosis and management. By aligning disease categorization with immunopathogenesis, the framework bridges the gap between disease mechanisms and treatment. It overcomes limitations of traditional histopathological methods, providing clearer guidance for treatment and fosters a deeper understanding of GN. With advancements in biomarker development and non-invasive diagnostics, the holistic GN-AC system has the potential to significantly improve patient outcomes and streamline GN-related research and education.
Conflicts of Interest
The authors declare no conflicts of interest on the topic of the review.
Funding
M.K. is supported by the Deutsche Forschungsgemeinschaft (DFG TRR322, project A7). M.P.-L. is supported by the Spanish Society of Nephrology.
References
2. GBD Chronic Kidney Disease Collaboration. Global, regional, and national burden of chronic kidney disease, 1990-2017: a systematic analysis for the Global Burden of Disease Study 2017. Lancet. 2020 Feb 29;395(10225):709-33.
3. Rovin BH, Adler SG, Barratt J, Bridoux F, Burdge KA, Chan TM, et al. KDIGO 2021 Clinical Practice Guideline for the Management of Glomerular Diseases. Kidney Int. 2021 Oct;100(4):S1-S276.
4. Romagnani P, Kitching AR, Leung N, Anders HJ. The five types of glomerulonephritis classified by pathogenesis, activity and chronicity (GN-AC). Nephrol Dial Transplant. 2023 Nov 8;38(Supplement_2): ii3-ii10.
5. Couser WG, Johnson RJ. The etiology of glomerulonephritis: roles of infection and autoimmunity. Kidney Int. 2014 Nov;86(5):905-14.
6. Vivarelli M, Barratt J, Beck LH, Fakhouri F, Gale DP, Goicoechea de Jorge E, et al. The role of complement in kidney disease: conclusions from a Kidney Disease: Improving Global Outcomes (KDIGO) Controversies Conference. Kidney Int. 2024 Sep;106(3):369-91.
7. Reiser J, Ingelfinger JR. Kidney Disease and Antinephrin Antibodies. N Engl J Med. 2024 Aug 1;391(5):463–467.
8. Foresto-Neto O, Menezes-Silva L, Leite JA, Andrade-Silva M, Câmara NOS. Immunology of Kidney Disease. Annu Rev Immunol. 2024 Jun;42(1):207-33.
9. Nasr SH, Radhakrishnan J, D’Agati VD. Bacterial infection-related glomerulonephritis in adults. Kidney Int. 2013 May;83(5):792-803.
10. Satoskar AA, Parikh S V, Nadasdy T. Epidemiology, pathogenesis, treatment and outcomes of infection-associated glomerulonephritis. Nat Rev Nephrol. 2020 Jan;16(1):32-50.
11. Floege J, Amann K. Primary glomerulonephritides. Lancet. 2016 May 14;387(10032):2036-48.
12. Bajema IM, Wilhelmus S, Alpers CE, Bruijn JA, Colvin RB, Cook HT, et al. Revision of the International Society of Nephrology/Renal Pathology Society classification for lupus nephritis: clarification of definitions, and modified National Institutes of Health activity and chronicity indices. Kidney Int. 2018 Apr;93(4):789-96.
13. Farinha F, Barreira S, Couto M, Cunha M, Fonseca D, Freitas R, et al. Risk of chronic kidney disease in 260 patients with lupus nephritis: analysis of a nationwide multicenter cohort with up to 35 years of follow-up. Rheumatology (Oxford). 2025 Mar 1;64(3):1201-9.
14. Kitching AR, Anders HJ, Basu N, Brouwer E, Gordon J, Jayne DR, et al. ANCA-associated vasculitis. Nature reviews Disease primers. 2020 Aug 27;6(1):71.
15. Binda V, Moroni G, Messa P. ANCA-associated vasculitis with renal involvement. J Nephrol. 2018;31(2):197-208.
16. Bobart SA, Han H, Tehranian S, De Vriese AS, Roman JCL, Sethi S, et al. Noninvasive Diagnosis of PLA2R-Associated Membranous Nephropathy: A Validation Study. Clin J Am Soc Nephrol. 2021 Dec;16(12):1833-9.
17. Bestard O, Thaunat O, Bellini MI, Böhmig GA, Budde K, Claas F, et al. Alloimmune Risk Stratification for Kidney Transplant Rejection. Transpl Int. 2022; 35:10138.
18. Meneghini M, Crespo E, Niemann M, Torija A, Lloberas N, Pernin V, et al. Donor/Recipient HLA Molecular Mismatch Scores Predict Primary Humoral and Cellular Alloimmunity in Kidney Transplantation. Front Immunol. 2020;11:623276.
19. Chutani A, Guevara-Pineda D, Lerner GB, Menon MC. Re-Evaluating the Transplant Glomerulopathy Lesion-Beyond Donor-Specific Antibodies. Transpl Int. 2024; 37:13365.
20. Cosio FG, Gloor JM, Sethi S, Stegall MD. Transplant glomerulopathy. Am J Transplant. 2008 Mar;8(3):492-6.
21. Voora S, Adey DB. Management of Kidney Transplant Recipients by General Nephrologists: Core Curriculum 2019. Am J Kidney Dis. 2019 Jun;73(6):866-79.
22. Kidney Disease: Improving Global Outcomes (KDIGO) Transplant Work Group. KDIGO clinical practice guideline for the care of kidney transplant recipients. Am J Transplant. 2009 Nov;9 Suppl 3: S1-155.
23. Noris M, Caprioli J, Bresin E, Mossali C, Pianetti G, Gamba S, et al. Relative Role of Genetic Complement Abnormalities in Sporadic and Familial aHUS and Their Impact on Clinical Phenotype. Clinical Journal of the American Society of Nephrology. 2010 Oct;5(10):1844-59.
24. Timmermans SAMEG, Damoiseaux JGMC, Werion A, Reutelingsperger CP, Morelle J, van Paassen P. Functional and Genetic Landscape of Complement Dysregulation Along the Spectrum of Thrombotic Microangiopathy and its Potential Implications on Clinical Outcomes. Kidney Int Rep. 2021 Apr;6(4):1099-109.
25. Cornec-Le Gall E, Alam A, Perrone RD. Autosomal dominant polycystic kidney disease. Lancet. 2019 Mar 2;393(10174):91-35.
26. Savige J, Lipska-Zietkiewicz BS, Watson E, Hertz JM, Deltas C, Mari F, et al. Guidelines for Genetic Testing and Management of Alport Syndrome. Clin J Am Soc Nephrol. 2022 Jan;17(1):143-54.
27. Wang M, Chun J, Genovese G, Knob AU, Benjamin A, Wilkins MS, et al. Contributions of Rare Gene Variants to Familial and Sporadic FSGS. J Am Soc Nephrol. 2019 Sep;30(9):1625-40.
28. Domingo-Gallego A, Pybus M, Bullich G, Furlano M, Ejarque-Vila L, Lorente-Grandoso L, et al. Clinical utility of genetic testing in early-onset kidney disease: seven genes are the main players. Nephrol Dial Transplant. 2022 Mar 25;37(4):687-96.
29. Gbadegesin RA, Ulasi I, Ajayi S, Raji Y, Olanrewaju T, Osafo C, et al. H3Africa Kidney Disease Research Network. APOL1 Bi- and Monoallelic Variants and Chronic Kidney Disease in West Africans. N Engl J Med. 2025 Jan 16;392(3):228-38.
30. Barbour TD, Pickering MC, Terence Cook H. Dense deposit disease and C3 glomerulopathy. Semin Nephrol. 2013 Nov 1;33(6):493-507.
31. Caravaca-Fontán F, Lucientes L, Cavero T, Praga M. Update on C3 Glomerulopathy: A Complement-Mediated Disease. Nephron. 2020;144(6):272-80.
32. Leung N, Bridoux F, Nasr SH. Monoclonal Gammopathy of Renal Significance. N Engl J Med. 2021 May 20;384(20):1931-41.
33. Leung N, Bridoux F, Batuman V, Chaidos A, Cockwell P, D’Agati VD, et al. The evaluation of monoclonal gammopathy of renal significance: a consensus report of the International Kidney and Monoclonal Gammopathy Research Group. Nat Rev Nephrol. 2019 Jan;15(1):45-59.
34. Leung N, Bridoux F, Hutchison CA, Nasr SH, Cockwell P, Fermand JP, et al. Monoclonal gammopathy of renal significance: when MGUS is no longer undetermined or insignificant. Blood. 2012 Nov 22;120(22):4292-5.
35. Nozal P, López-Trascasa M. Autoantibodies against alternative complement pathway proteins in renal pathologies. Nefrología (English Edition). 2016 Sep 1;36(5):489-95.
36. Arivazhagan S, Lamech TM, Myvizhiselvi M, Arumugam V, Alavudeen SS, Dakshinamoorthy S, et al. Efficacy of Corticosteroids in Infection-Related Glomerulonephritis-A Randomized Controlled Trial. Kidney Int Rep. 2022 Oct;7(10):2160–2165.
37. Kidney Disease: Improving Global Outcomes (KDIGO) Lupus Nephritis Work Group. KDIGO 2024 Clinical Practice Guideline for the management of LUPUS NEPHRITIS. Kidney Int. 2024 Jan;105(1S): S1-S69.
38. Farinha F, Pepper RJ, Oliveira DG, McDonnell T, Isenberg DA, Rahman A. Outcomes of membranous and proliferative lupus nephritis - analysis of a single-centre cohort with more than 30 years of follow-up. Rheumatology (Oxford). 2020 Nov 1;59(11):3314-23.
39. Rojas-Rivera JE, García-Carro C, Ávila AI, Espino M, Espinosa M, Fernández-Juárez G, et al. Consensus document of the Spanish Group for the Study of the Glomerular Diseases (GLOSEN) for the diagnosis and treatment of lupus nephritis. Nefrología (English Edition). 2023 Jan;43(1):6-47.
40. Furie R, Rovin BH, Houssiau F, Contreras G, Teng YKO, Curtis P, et al. Safety and Efficacy of Belimumab in Patients with Lupus Nephritis: Open-Label Extension of BLISS-LN Study. Clin J Am Soc Nephrol. 2022 Nov;17(11):1620-30.
41. Jayne D, Rovin B, Mysler E, Furie R, Houssiau F, Trasieva T, et al. Anifrolumab in lupus nephritis: results from second-year extension of a randomised phase II trial. Lupus Sci Med. 2023 Aug;10(2): e000910.
42. Walsh M, Merkel PA, Peh CA, Szpirt WM, Puéchal X, Fujimoto S, et al. Plasma Exchange and Glucocorticoids in Severe ANCA-Associated Vasculitis. New England Journal of Medicine. 2020;382(7):622-31.
43. Hu X, Zhang M, Xu J, Gao C, Yu X, Li X, et al. Comparison of Obinutuzumab and Rituximab for Treating Primary Membranous Nephropathy. Clin J Am Soc Nephrol. 2024 Dec 1;19(12):1594-602.
44. Rovin BH, Furie RA, Ross Terres JA, Giang S, Schindler T, Turchetta A, et al. Kidney Outcomes and Preservation of Kidney Function With Obinutuzumab in Patients With Lupus Nephritis: A Post Hoc Analysis of the NOBILITY Trial. Arthritis Rheumatol. 2024 Feb;76(2):247-54.
45. Cattran DC, Appel GB, Hebert LA, Hunsicker LG, Pohl MA, Hoy WE, et al. A randomized trial of cyclosporine in patients with steroid-resistant focal segmental glomerulosclerosis. North America Nephrotic Syndrome Study Group. Kidney Int. 1999 Dec;56(6):2220-6.
46. Caravaca-Fontán F, Fernández-Juárez GM, Floege J, Goumenos D, Kronbichler A, Turkmen K, et al. The management of membranous nephropathy-an update. Nephrol Dial Transplant. 2022 May 25;37(6):1033-42.
47. Lafayette R, Kristensen J, Stone A, Floege J, Tesař V, Trimarchi H, et al. Efficacy and safety of a targeted-release formulation of budesonide in patients with primary IgA nephropathy (NefIgArd): 2-year results from a randomised phase 3 trial. Lancet. 2023 Sep 9;402(10405):859-70.
48. Walsh M, Merkel PA, Peh CA, Szpirt WM, Puéchal X, Fujimoto S, et al. Plasma Exchange and Glucocorticoids in Severe ANCA-Associated Vasculitis. N Engl J Med. 2020 Feb 13;382(7):622-31.
49. Jayne DRW, Merkel PA, Schall TJ, Bekker P, ADVOCATE Study Group. Avacopan for the Treatment of ANCA-Associated Vasculitis. N Engl J Med. 2021 Feb 18;384(7):599-609.
50. Rovin BH, Teng YKO, Ginzler EM, Arriens C, Caster DJ, Romero-Diaz J, et al. Efficacy and safety of voclosporin versus placebo for lupus nephritis (AURORA 1): a double-blind, randomised, multicentre, placebo-controlled, phase 3 trial. Lancet. 2021 May 29;397(10289):2070-80.
51. Hegazy MT, Fayed A, Nuzzolese R, Sota J, Ragab G. Autoinflammatory diseases and the kidney. Immunol Res. 2023 Aug;71(4):578-87.
52. Zand L, Rajkumar SV, Leung N, Sethi S, El Ters M, Fervenza FC. Safety and Efficacy of Daratumumab in Patients with Proliferative GN with Monoclonal Immunoglobulin Deposits. J Am Soc Nephrol. 2021 May 3;32(5):1163-73.
53. Dimopoulos MA, Merlini G, Bridoux F, Leung N, Mikhael J, Harrison SJ, et al. Management of multiple myeloma-related renal impairment: recommendations from the International Myeloma Working Group. Lancet Oncol. 2023 Jul;24(7): e293-311.
54. Kidney Disease: Improving Global Outcomes (KDIGO) CKD Work Group. KDIGO 2024 Clinical Practice Guideline for the Evaluation and Management of Chronic Kidney Disease. Kidney Int. 2024 Apr;105(4S):S117-S314.
55. Muehlig AK, Gies S, Huber TB, Braun F. Collapsing Focal Segmental Glomerulosclerosis in Viral Infections. Front Immunol. 2021;12:800074.
56. Moudgil A, Nast CC, Bagga A, Wei L, Nurmamet A, Cohen AH, et al. Association of parvovirus B19 infection with idiopathic collapsing glomerulopathy. Kidney Int. 2001 Jun;59(6):2126-33.
57. Anders HJ, Saxena R, Zhao MH, Parodis I, Salmon JE, Mohan C. Lupus nephritis. Nat Rev Dis Primers. 2020 Jan 23;6(1):7.
58. Almaani S, Meara A, Rovin BH. Update on Lupus Nephritis. Clin J Am Soc Nephrol. 2017 May 8;12(5):825-35.
59. Ronco P, Beck L, Debiec H, Fervenza FC, Hou FF, Jha V, et al. Membranous nephropathy. Nat Rev Dis Primers. 2021 Sep 30;7(1):69.
60. Watts AJB, Keller KH, Lerner G, Rosales I, Collins AB, Sekulic M, et al. Discovery of Autoantibodies Targeting Nephrin in Minimal Change Disease Supports a Novel Autoimmune Etiology. J Am Soc Nephrol. 2022 Jan;33(1):238-52.
61. Ohmes J, Comdühr S, Akbarzadeh R, Riemekasten G, Humrich JY. Dysregulation and chronicity of pathogenic T cell responses in the pre-diseased stage of lupus. Front Immunol. 2022 Oct 28; 13:1007078.
62. Wigerblad G, Kaplan MJ. Neutrophil extracellular traps in systemic autoimmune and autoinflammatory diseases. Nat Rev Immunol. 2023 May;23(5):274-88.
63. Müller F, Taubmann J, Bucci L, Wilhelm A, Bergmann C, Völkl S, et al. CD19 CAR T-Cell Therapy in Autoimmune Disease — A Case Series with Follow-up. New England Journal of Medicine. 2024 Feb 22;390(8):687-700.
64. Ekberg H, Tedesco-Silva H, Demirbas A, Vítko Š, Nashan B, Gürkan A, et al. Reduced Exposure to Calcineurin Inhibitors in Renal Transplantation. New England Journal of Medicine. 2007 Dec 20;357(25):2562-75.
65. Ricklin D, Reis ES, Lambris JD. Complement in disease: a defence system turning offensive. Nature Reviews Nephrology. 2016 Jul;12(7):383-401.
66. Romagnani P, Remuzzi G, Glassock R, Levin A, Jager KJ, Tonelli M, et al. Chronic kidney disease. Nat Rev Dis Primers. 2017 Nov 23; 3:17088.
67. Casal Moura M, Fervenza FC, Specks U, Sethi S. Kidney biopsy chronicity grading in antineutrophil cytoplasmic antibody-associated vasculitis. Nephrol Dial Transplant. 2022 Aug 22;37(9):1710-21.
68. Lichtnekert J, Anders HJ. Lupus nephritis-related chronic kidney disease. Nat Rev Rheumatol. 2024 Nov;20(11):699–711.
69. Rojas-Rivera JE, Bakkaloglu SA, Bolignano D, Nistor I, Sarafidis PA, Stoumpos S, Cozzolino MG, Ortiz A. Chronic kidney disease: the missing concept in the 2019 EULAR/ERA-EDTA recommendations for lupus nephritis. Nephrol Dial Transplant. 2023 Dec 20;39(1):151-8.
70. Noris M, Remuzzi G. Genetics of immune-mediated glomerular diseases: focus on complement. Seminars in Nephrology. 2017 Sep 1;37(5):447-63.
71. Furie RA, Rovin BH, Garg JP, Santiago MB, Aroca-Martínez G, Zuta Santillán AE, et al; REGENCY Trial Investigators. Efficacy and Safety of Obinutuzumab in Active Lupus Nephritis. N Engl J Med. 2025 Feb 7.
72. Hu X, Zhang M, Xu J, Gao C, Yu X, Li X, et al. Comparison of Obinutuzumab and Rituximab for Treating Primary Membranous Nephropathy. Clin J Am Soc Nephrol. 2024 Dec 1;19(12):1594-602.
73. Müller F, Taubmann J, Bucci L, Wilhelm A, Bergmann C, Völkl S, et al. CD19 CAR T-Cell Therapy in Autoimmune Disease - A Case Series with Follow-up. N Engl J Med. 2024 Feb 22;390(8):687-700.
74. Vivarelli M, Barratt J, Beck LH Jr, Fakhouri F, Gale DP, Goicoechea de Jorge E, et al. for Conference Participants. The role of complement in kidney disease: conclusions from a Kidney Disease: Improving Global Outcomes (KDIGO) Controversies Conference. Kidney Int. 2024 Sep;106(3):369-91.
75. Wong E, Nester C, Cavero T, Karras A, Le Quintrec M, Lightstone L, et al. Efficacy and Safety of Iptacopan in Patients With C3 Glomerulopathy. Kidney Int Rep. 2023 Sep 22;8(12):2754-64.
76. ClinicalTrials.gov. VALIANT: Pegcetacoplan in C3G and IC-MPGN. [Available from: https://clinicaltrials.gov/ct2/show/NCT05067127]
77. Kidney Disease: Improving Global Outcomes (KDIGO) Glomerular Diseases Work Group. KDIGO 2021 Clinical Practice Guideline for the Management of Glomerular Diseases. Kidney Int. 2021 Oct;100(4S):S1-S276.
78. Kidney Disease: Improving Global Outcomes (KDIGO) Lupus Nephritis Work Group. KDIGO 2024 Clinical Practice Guideline for the management of LUPUS NEPHRITIS. Kidney Int. 2024 Jan;105(1S): S1-S69.
79. Hengel FE, Dehde S, Lassé M, Zahner G, Seifert L, Schnarre A, et al; International Society of Glomerular Disease. Autoantibodies Targeting Nephrin in Podocytopathies. N Engl J Med. 2024 Aug 1;391(5):422-33.
80. Raglianti V, Angelotti ML, De Chiara L, Allinovi M, Cirillo L, Manonelles A, et al. Anti-slit Antibodies against Podocin and Kirrel1 in Pediatric and Adult Podocytopathies. J Am Soc Nephrol. 2025 Apr 1;36(4):702-5.