Abstract
Recent studies have shed light on the pivotal roles of estrogen in various pathophysiological processes related to kidney diseases. However, the precise mechanisms governing the estrogen actions remain enigmatic. The downstream impacts of estrogen primarily hinge on estrogen receptors (ERs), namely ERα (NR3A1) and ERβ (NR3A2). Building upon our previous finding that ERβ participates in subcutaneous adipose tissue browning in female mice, our recent study found that ERβ functions to protect kidney from progressive renal fibrosis by inactivating transforming growth factor-β (TGF-β)/Smad3 signaling. In the normal kidney, ERβ is highly expressed by proximal tubular epithelial cells but it is lost when kidney becomes fibrotic. In contrast, activation of ERβ inhibits kidney fibrosis. Mechanistically, we uncovered that ERβ can bind to Smad3, thereby transcriptionally downregulating Smad3 and inhibiting TGF-β1/Smad3-mediated renal fibrosis. Thus, ERβ is protective in renal fibrosis and may have therapeutic potential for chronic kidney disease.
Abbreviations
ERs: Estrogen Receptors; NR3A1: Nuclear Receptor 3A1; NR3A2: Nuclear Receptor 3A2; TGF-β: Transforming Growth Factor-β; Smad3: SMAD family member 3; CKD: Chronic Kidney Disease; TNF-α: Tumor Necrosis Factor-α; DSS: Dextran Sulfate Sodium Salt; E2: Estradiol; MMP-2: Matrix Metalloproteinases-2; MMP-9: Matrix Metalloproteinases-9; IgAN: IgA Nephropathy; eGFR: Glomerular Filtration Rate; Scr: Serum creatinine; UA: Uric Acid; AQP: Aquaporin; THP: Tamm-Horsfall Protein; NCC: Sodium Chloride Cotransporter; PTECs: Proximal Tubular Epithelial Cells; UUO: Unilateral Ureteral Obstruction; 5/6Nx: 5/6 Nephrectomy; HSCs: Hepatic Stellate Cells; AMPK: AMP Kinase; PKA: Protein Kinase A; AngII: Angiotensin II; NLRP3: NOD-like Receptor thermal protein domain associated protein 3; IRI: Ischemia-Reperfusion Injury; OVX: Ovariectomized; DPN: Diarylpropionitrile
Commentary
Chronic kidney disease (CKD) has become a growing global health challenge, impacting over 800 million individuals worldwide [1]. Numerous evidences have shown that women are more resistant to CKD than men before menopause, however this beneficial effect is diminished in those with post-menopause, highlighting the pivotal role of estrogen in kidney disease [2-5]. The physiological functions of estrogen are regulated by estrogen receptors (ERs), ERα (NR3A1) and ERβ (NR3A2). These receptors, upon ligand binding, intricately modulate gene transcription by binding to specific DNA sites and interacting with co-repressors or co-activators [6]. While ERα is primarily expressed in reproductive organs such as the uterus and prostate, it is also highly expressed in metabolically active tissues like the liver and adipocytes. In contrast, ERβ exhibits a more widespread distribution. Studies reveal that ERβ has a prominent role in the nervous system [7] and immune related disease [8,9]. ERβ deficiency results in diverse behavioral abnormalities including anxiety, disrupted gut microbiota composition, and increased susceptibility to dextran sulfate sodium salt (DSS)-induced colitis. In addition, both ERα and ERβ exert an important role in energy metabolism [10,11].
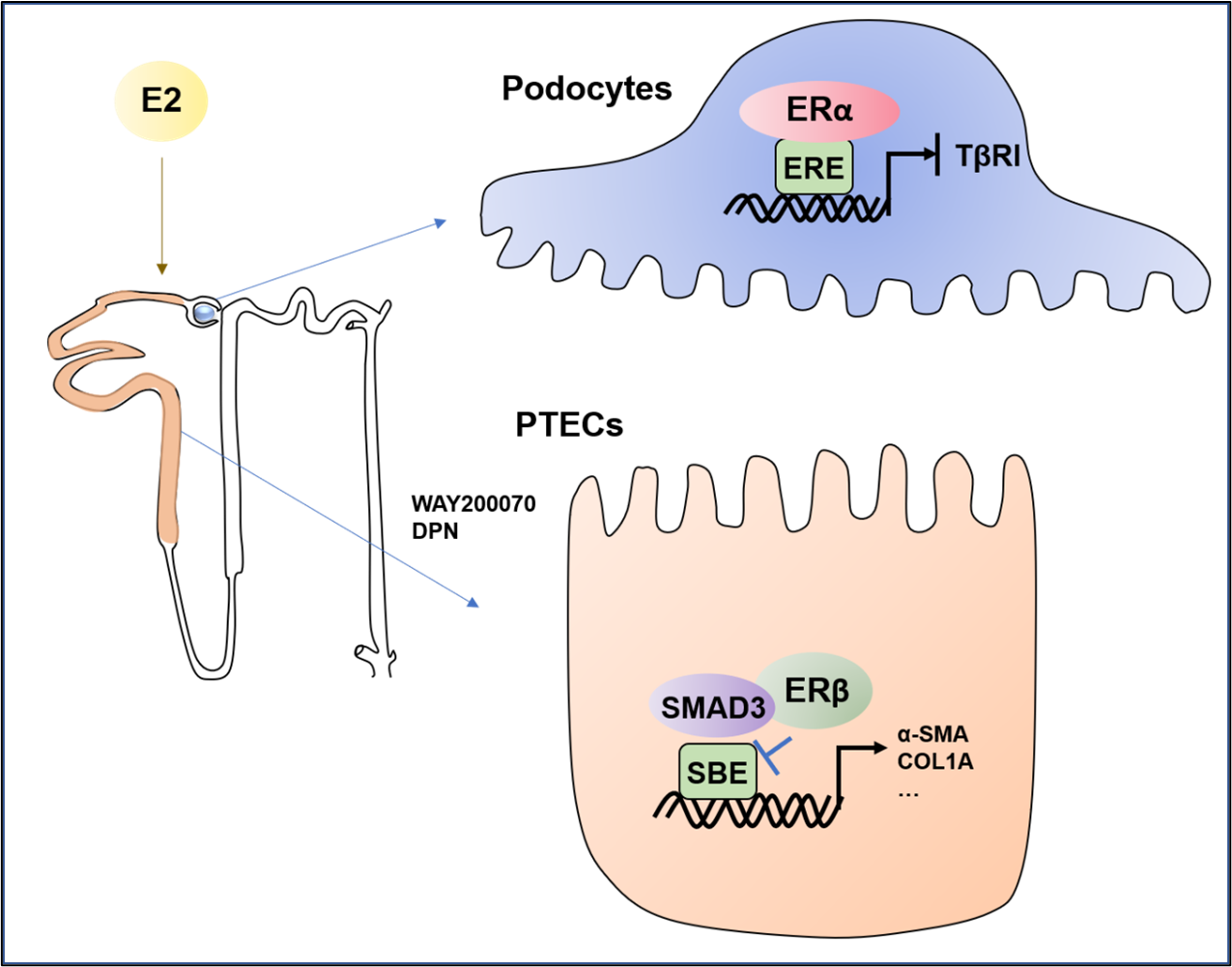
The protective role of estrogen in various kidney diseases has been reported. However, the underlying mechanisms remain inadequately elucidated [12-15]. Within the context of diabetic nephropathy, estradiol (E2) exerts a suppressive effect on albuminuria and tubulointerstitial fibrosis (TF), and glomerulosclerosis by inhibiting of the matrix metalloproteinases-2 (MMP-2) and matrix metalloproteinases-9 (MMP-9) activities [16]. It is reported that podocytes and mesangial cells are main target cells for sex steroid hormones by expressing ERα [17]. In this regard, estradiol stimulates ERα to activate signaling pathways, orchestrating gene regulation in glomerular and mesangial cells. Interestingly, while testosterone has a proapoptotic effect, 17β-estradiol demonstrates a protective effect on podocyte apoptosis preceding glomerulosclerosis in female mice. Thus, the safeguarding role of E2 on podocytes is primarily mediated by ERα (Figure 1). Notably, pretreatment of podocytes with physiological concentrations of E2 (1 nmol/l) significantly curbs apoptosis triggered by transforming growth factor- β1 (TGF- β1) and tumor necrosis factor alpha (TNF-α) in an ER-dependent manner [17]. This is further evidenced by more severe podocyte injury and glomerulosclerosis developed in ERα deficient female mice when compared with WT littermates [17]. Clinically, in patients with IgA nephropathy (IgAN), the expression of ERα is largely reduced in the glomeruli, which is also associated with declined glomerular filtration rate (eGFR), increased serum creatinine (Scr), and pathological grade [18], revealing a plausible role for ERα in IgAN.
Figure 1: The possible role of estrogen signaling in kidney disease [17,19,20,26]. In the kidney, ERα is primarily expressed in podocytes and exerts a protective effect against podocyte apoptosis and renal injury in diabetic nephropathy by binding to the promoter region of TGF-βRI and then inactivating TGF-β/smad3 signaling; ERβ is primarily expressed in renal proximal tubular epithelial cells (PTECs) and inhibits renal fibrosis by binding to SMAD3 and inhibiting Smad3 transcription activity.
In comparison to ERα, the role of ERβ in kidney diseases remains unclear. Recent work by Liu et al. found that ERβ is expressed by renal tubular epithelial cells but not by glomerular cells in normal human kidneys, which is significantly reduced in patients with lupus nephritis [19]. Notably, the expression of ERβ in renal tubular epithelial cells is also negatively correlated with serum uric acid (UA) levels and glomerulosclerosis [19]. By employing specific antibodies against markers such as aquaporin 1 (AQP1, a proximal tubule marker), aquaporin 2 (AQP2, a collecting duct marker), Tamm-Horsfall protein (THP, an ascending thick limb of Henle's loop marker), sodium chloride cotransporter (NCC, a distal tubule marker) and nephrin (a glomerular marker), we uncovered that ERβ is distinctly expressed in renal proximal tubular epithelial cells (PTECs) across species [20]. Importantly, we also detected that decreased ERβ expression in PTECs is correlated closely with the severity of progressive renal fibrosis in patients with IgAN, which is also observed mouse models with progressive renal fibrosis induced by unilateral ureteral obstruction (UUO) or 5/6 nephrectomy (5/6Nx) as well as in vitro cultured tubular cells [20]. Thus, like ERα, ERβ is also protective in renal fibrosis and the substantial loss of ERβ is associated with progressive renal fibrosis (Figure 1).
The anti-fibrotic role of ERβ have been explored in cardiac fibroblast [21-23] and hepatic stellate cells (HSCs) [24,25]. In the context of cardiac fibrosis, ERβ activation triggers AMP kinase (AMPK) and protein kinase A (PKA), impeding angiotensin II (AngII)-induced de-phosphorylation of Rho, thereby activating Rho kinase A21. This cascade curtails Rho kinase-mediated suppression of TGF-β expression [21]. In rat primary HSCs, E2 can inhibit the proliferation and transformation of HSCs through the ERβ-dependent mechanism [25]. In contrast, TGF-β induces liver fibrosis by downregulating ERβ expression. Furthermore, inhibition of ERβ also exacerbates NOD-like receptor thermal protein domain associated protein 3 (NLRP3) activation and inflammation in HSCs [24], which is reversed by overexpressing ERβ [25]. The anti-fibrotic role of ERs is also observed in kidney disease in which female rats exhibit resistant to ischemia-reperfusion injury (IRI) induced acute kidney injury and fibrosis, which is further improved by E2 supplementation but is exacerbated by ovariectomized (OVX) [26]. By using ERβ deficient mice, we also found that mice lacking ERβ develop more severe renal fibrosis after UUO or 5/6 nephrectomy, which is blocked by giving the ERβ selective agonists such as diarylpropionitrile (DPN) or WAY200070 [20].
The recent progress in understanding of estrogen signaling includes finding that ERα can bind to the promoter region of TGF-βRI to inactivating TGF-β/Smad signaling [26]. Thus, E2 exerts its protective effect on acute kidney injury via the ERα-mediated transcriptional inhibition of TGF-βRI expression [26]. Furthermore, we also found that ERβ can also bind Smad3 directly to transcriptionally inhibit TGF-β/Smad3 signaling [20]. We summarized the current understanding for estrogen signaling in kidney disease in Figure 1. Thus, deletion or pharmacological inhibition of Smad3 prevents the loss of ERβ and progressive renal fibrosis. Indeed, since activation of TGF-β/Smad3 signaling is a well-recognized mechanism related to renal fibrosis [27], ERβ can competitively inhibit the association of Smad3 with the Smad-binding element, thereby downregulating the transcription of the fibrosis-related genes. Thus, ERβ may represent as a promising therapeutic agent for renal fibrosis and may be a hormone replacement therapy for CKD patients although more clinical evidence is needed.
Author Contributions
WS: funding acquisition and writing original draft; RC: writing the draft; QW: revised the draft; HL: conceptualization and edited manuscript.
Acknowledgements
This work was supported by the Shenzhen Science and Technology Project (JCYJ20210324094213037), the Basic Research Foundation of Guangdong province (2022A1515012595) and Shenzhen University 2035 Program for Excellent Research (86901-00000214).
Conflict of Interest
The authors declare no-conflict of interest.
References
2. Neugarten J, Acharya A, Silbiger SR. Effect of gender on the progression of nondiabetic renal disease: a meta-analysis. Journal of the American Society of Nephrology. 2000 Feb 1;11(2):319-29.
3. Elliot SJ, Karl M, Berho M, Potier M, Zheng F, Leclercq B, et al. Estrogen deficiency accelerates progression of glomerulosclerosis in susceptible mice. The American Journal of Pathology. 2003 May 1;162(5):1441-8.
4. Shen H, Holliday M, Sheikh-Hamad D, Li Q, Tong Q, Hamad CD, et al. Sirtuin-3 mediates sex differences in kidney ischemia-reperfusion injury. Translational Research. 2021 Sep 1;235:15-31.
5. Yu M, Ryu DR, Kim SJ, Choi KB, Kang DH. Clinical implication of metabolic syndrome on chronic kidney disease depends on gender and menopausal status: results from the Korean National Health and Nutrition Examination Survey. Nephrology Dialysis Transplantation. 2010 Feb 1;25(2):469-77.
6. Enmark E, Pelto-Huikko M, Grandien KA, Lagercrantz S, Lagercrantz J, Fried G, et al. Human estrogen receptor β-gene structure, chromosomal localization, and expression pattern. The Journal of Clinical Endocrinology & Metabolism. 1997 Dec 1;82(12):4258-65.
7. Wang L, Andersson S, Warner M, Gustafsson JÅ. Estrogen receptor (ER) β knockout mice reveal a role for ERβ in migration of cortical neurons in the developing brain. Proceedings of the National Academy of Sciences. 2003 Jan 21;100(2):703-8.
8. Kovats S. Estrogen receptors regulate innate immune cells and signaling pathways. Cellular Immunology. 2015 Apr 1;294(2):63-9.
9. Hill L, Jeganathan V, Chinnasamy P, Grimaldi C, Diamond B. Differential roles of estrogen receptors α and β in control of B-cell maturation and selection. Molecular Medicine. 2011 Mar;17(3):211-20.
10. Martınez de Morentin PB, González-Garcıa I, Martins L, Lage R, Fernández-Mallo D, Martınez-Sánchez N, et al. Estradiol regulates brown adipose tissue thermogenesis via hypothalamic AMPK. Cell Metabolism. 2014;20(1):41-53.
11. Savva C, González-Granillo M, Li X, Angelin B, Korach-André M. Sex-specific metabolic changes during estrogen receptor beta activation in obesity. Atherosclerosis. 2020 Dec 1;315:e87.
12. Gross ML, Adamczak M, Rabe T, Harbi NA, Krtil J, Koch A, et al. Beneficial effects of estrogens on indices of renal damage in uninephrectomized SHRsp rats. Journal of the American Society of Nephrology. 2004 Feb 1;15(2):348-58.
13. Mankhey RW, Bhatti F, Maric C. 17β-Estradiol replacement improves renal function and pathology associated with diabetic nephropathy. American Journal of Physiology-Renal Physiology. 2005 Feb;288(2):F399-405.
14. Lemos CC, Mandarim-de-Lacerda CA, Dorigo D, Coimbra TM, Bregman R. Chronic renal failure in male and female rats. Journal of Nephrology. 2005 Jul 1;18(4):368-73.
15. Wu CC, Chang CY, Chang ST, Chen SH. 17β-estradiol accelerated renal tubule regeneration in male rats after ischemia/reperfusion-induced acute kidney injury. Shock. 2016 Aug 1;46(2):158-63.
16. Inada A, Inada O, Fujii NL, Nagafuchi S, Katsuta H, Yasunami Y, et al. Adjusting the 17β–Estradiol-to-Androgen Ratio Ameliorates Diabetic Nephropathy. Journal of the American Society of Nephrology: JASN. 2016 Oct;27(10):3035-50.
17. Doublier S, Lupia E, Catanuto P, Periera-Simon S, Xia X, Korach K, et al. Testosterone and 17β-estradiol have opposite effects on podocyte apoptosis that precedes glomerulosclerosis in female estrogen receptor knockout mice. Kidney International. 2011 Feb 2;79(4):404-13.
18. Yu W, Zhao B, Zhong H, Yao G. Estrogen receptor alpha expression in renal tissue and its relationship with prognosis in immunoglobulin A nephropathy. International Journal of Clinical and Experimental Pathology. 2020;13(9):2319-25.
19. Liu H, Peng L, Ma J, He L, Long K, Ouyang X, et al. Low expression of estrogen receptor β in renal tubular epithelial cells may cause hyperuricemia in premenopausal patients with systemic lupus erythematosus. Lupus. 2021 Apr;30(4):560-7.
20. Cao R, Su W, Sheng J, Guo Y, Su J, Zhang C, et al. Estrogen receptor β attenuates renal fibrosis by suppressing the transcriptional activity of Smad3. Biochimica et Biophysica Acta (BBA)-Molecular Basis of Disease. 2023 Aug 1;1869(6):166755.
21. Pedram A, Razandi M, Narayanan R, Levin ER. Estrogen receptor beta signals to inhibition of cardiac fibrosis. Molecular and Cellular Endocrinology. 2016 Oct 15;434:57-68.
22. Pedram A, Razandi M, O'Mahony F, Lubahn D, Levin ER. Estrogen receptor-β prevents cardiac fibrosis. Molecular Endocrinology. 2010 Nov 1;24(11):2152-65.
23. Iorga A, Umar S, Ruffenach G, Aryan L, Li J, Sharma S, et al. Estrogen rescues heart failure through estrogen receptor Beta activation. Biology of Sex Differences. 2018 Dec;9(1):48.
24. Lin L, Zhou M, Que R, Chen Y, Liu X, Zhang K, et al. Saikosaponin-d protects against liver fibrosis by regulating the estrogen receptor-β/NLRP3 inflammasome pathway. Biochemistry and Cell Biology. 2021;99(5):666-74.
25. Zhou Y, Shimizu I, Lu G, Itonaga M, Okamura Y, Shono M, et al. Hepatic stellate cells contain the functional estrogen receptor β but not the estrogen receptor α in male and female rats. Biochemical and Biophysical Research Communications. 2001 Sep 7;286(5):1059-65.
26. Ren L, Li F, Di Z, Xiong Y, Zhang S, Ma Q, et al. Estradiol ameliorates acute kidney ischemia-reperfusion injury by inhibiting the TGF-βRI-SMAD pathway. Frontiers in Immunology. 2022 Feb 24;13:822604.