Keywords
Drought resistance, Innate immunity, Signaling, Receptor kinase, Rice
Commentary
Microbial infection can cause cell damage in both plants and animals, as well as triggering stress responses commonly induced by environmental (abiotic) cues. While significant progress has been made in understanding how host immunity restricts pathogen growth, little is known about the role of the immune system in controlling abiotic stress responses associated with pathogen attack. The immune receptor chitin elicitor receptor kinase 1 (CERK1), which recognizes the fungal cell wall component chitin [1], also plays a role in salt stress signaling. This novel function is thought to be mediated by the detection of elevated Na+ ion levels caused by fungal infection [2].
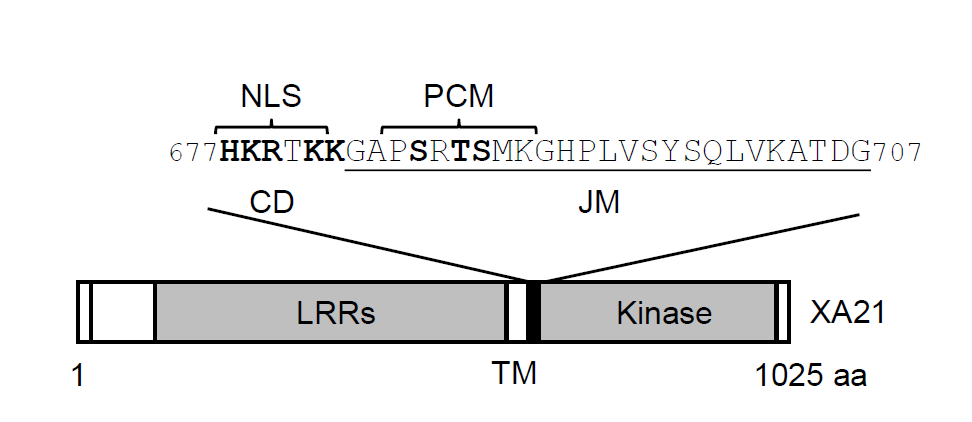
The rice (Oryza sativa) gene Xa21 provides immunity against a broad range of strains of the Gram-negative bacterium Xanthomonas oryzae pv. oryzae (Xoo), which causes the vascular disease bacterial leaf blight [3,4]. Xa21 encodes a receptor-like kinase (RLK) consisting of an extracellular leucine-rich repeat (LRR) domain, a single transmembrane helix, and an intracellular region composed of a charged domain, a juxtamembrane (JM) motif, and a serine/threonine kinase domain (Figure 1) [3]. XA21 acts as an immune receptor that recognizes a sulfated peptide from the Xoo protein RaxX (Required for activation of XA21-mediated immunity X) [5-8]. Notably, XA21 shares structural similarity with the immune receptors discovered in flies, mice, and humans [8].
Figure 1. Schematic representation of XA21. The domains of XA21 were described previously [3]. The positively charged residues in the NLS and the autophosphorylated residues in the JM domain are shown in bold. LRRs: Leucine-Rich Repeats; TM: Transmembrane domain; CD: Charged Domain; JM: Juxtamembrane domain; NLS: Nuclear Localization Signal; PCM: Putative proteolytic Cleavage Motif.
The intracellular portion of XA21 can undergo autophosphorylation on multiple serines and threonines in vitro, including the Ser-686, Thr-688, and Ser-689 residues in the JM domain, which also possesses a putative proteolytic cleavage motif (PCM) (Figure 1) [9,10]. Immunoblot analysis of wild-type XA21 and its autophosphorylation variants in transgenic rice plants revealed that the immune receptor can be cleaved at a site(s) near the transmembrane domain by an unidentified protease, although the precise location of the cleavage site(s) remains to be determined [10-12]. Mutations of the three autophosphorylated amino acids in the JM domain or Lys-736, a well-conserved residue located inside the XA21 kinase domain that is essential for its kinase activity, led to markedly reduced steady-state accumulation of XA21 in rice and increased sensitivity to cleavage. These findings suggest that XA21 is stabilized by autophosphorylation. Given that the over-accumulation of immune receptors might lead to heightened immune responses, which could have detrimental effects on plants [13,14], we hypothesized that proteolytic cleavage might play a role in regulating XA21 levels to maintain homeostasis in rice [10]. Accumulating evidence indicates that, besides XA21 in rice, three well-characterized Arabidopsis thaliana receptor kinases/RLKs (SYMBIOSIS RECEPTOR-LIKE KINASE [SYMRK], CERK1, and BRASSINOSTEROID-INSENSITIVE 1-ASSOCIATED RECEPTOR KINASE 1 [BAK1]) can be proteolytically cleaved [15-18]. Notably, the corresponding proteases and the physiological significance of the cleavages remain to be identified.
XA21 is constitutively expressed in rice leaves [10,11]. The protein localizes to the plasma membrane and endoplasmic reticulum of plant cells [11,19] and forms complexes with six partners comprising XA21 binding protein 3 (XB3), XB24, Luminal-binding protein 3, XB25, OsSERK2, and Paladin, as revealed by co-immunoprecipitation experiments using rice protein extracts [11,12,20-23]. These findings support the idea that, once activated, XA21 might be capable of initiating multiple signaling pathways.
After infection, Xoo primarily accumulates in the xylem vessels of rice leaves causing disease symptoms that resemble those of drought-stressed plants [24]. We previously demonstrated that, in addition to immunity against Xoo, XA21 confers resistance to drought stress [25]. A question raised by this study was how drought conditions are perceived by the immune receptor to activate downstream signaling. We cannot exclude the possibility that water-deficit stress induces the production of a yet-to-be-discovered rice protein/peptide that is recognized by XA21, possibly through its LRR domain. This hypothesis would imply that XA21 can recognize two distinct ligands: one for pathogen defense and one for the water-deficit stress response. Indeed, in Arabidopsis, the damage-associated elicitor PEPTIDE 1 (PEP1) binds to the LRR-receptor kinase PEP1 RECEPTOR 1 (AtPEPR1) and activates immune responses [26,27]. However, XA21 was shown to be a highly selective receptor that specifically recognizes the bacterial ligand RaxX, but not plant PSY (plant peptide containing sulfated tyrosine) peptides, which shares similarities with RaxX [28].
An alternative possibility is that water-deficit stress induces the production of a protease that catalyzes the proteolysis of XA21. Given that the charged domain (CD) in the intracellular region of XA21 harbors a functional nuclear localization signal (NLS) (Figure 1) [12], the proteolytic cleavage of XA21 might trigger the nuclear translocation of the intracellular part of this protein together with its binding proteins. In mammals, membrane-localized receptors (e.g., Notch) can be processed by multiple proteases for maturation as well as activation [29]. The activation of immune receptors in the absence of pathogens would represent a non- canonical, ligand-independent avenue to stimulate the immune system. Such a mechanism might provide the flexibility required for the immune system to function in complex environmental and possibly developmental contexts.
Here, we examined the potential interplay between XA21-mediated drought resistance and Xoo immunity. To this end, we inoculated 2-week-old seedlings of the previously characterized Myc-XA21-FLAG rice line B7-12 and the empty vector control line A36 with the incompatible Xoo strain PXO99A. Following inoculation, we cultured the seedlings in water or 13% (w/v) polyethylene glycol (PEG), a nonionic water-soluble polymer used to simulate drought in plants [30]. Ten days later, B7- 12 seedlings cultured in water displayed normal resistance to Xoo, as evidenced by the reduction in lesion lengths and bacterial growth (Figure 2). However, PEG treatment suppressed XA21-mediated resistance to Xoo. Therefore, distinct defense signaling pathways, i.e., drought signaling and disease resistance signaling, are likely initiated by XA21 after Xoo infection and water-deficit treatments, respectively; and there might be a trade-off between the PEG-induced dehydration response and bacterial immunity.
Figure 2. PEG treatment suppresses XA21-mediated resistance to incompatible Xoo strain PXO99A. Two-week-old seedlings of the indicated lines were inoculated with incompatible Xoo strain PXO99A using the leaf-clipping method. After inoculation, the seedlings were cultured in water or 13% (w/v) PEG in a growth chamber (27ºC, under fluorescent light with a 16-h-light/8-h-dark photoperiod) for the indicated time. Lesion length in diseased leaves (n = 15 per line) at 10-days post inoculation (dpi) (a, b) and the growth of PXO99A in inoculated seedlings (c) were measured. Asterisks indicate statistically significant differences from the controls (**, p<0.01; *, p<0.05).
A major technical barrier that has long hindered the reliable detection of molecular events associated with XA21 signaling under physiological conditions is the molecular events associated with XA21 signaling under physiological conditions is the difficulty in synchronizing signaling due to the slow spread of Xoo in the xylem following inoculation using the leaf-clipping method [31]. We overcame this obstacle in our newly developed XA21-mediated drought resistance system. Drought treatment exerts intense stress throughout the plant to generate signals for XA21 activation. Our method should facilitate the identification of additional components involved in XA21 signaling, such as the hypothesized proteases that can cleave the immune receptor, using currently available molecular tools.
Acknowledgments
We thank the NSF (no. 2114833) and USDA-NIFA (no. 2015-67013-22910) for support. XZ was supported by a joint scholarship from the Northeast Agricultural University and China Scholarship Council. No conflict of interest declared.
References
2. Espinoza C, Liang Y, Stacey G. Chitin receptor CERK 1 links salt stress and chitin?triggered innate immunity in Arabidopsis. The Plant Journal. 2017 Mar;89(5):984-95.
3. Song WY, Wang GL, Chen LL, Kim HS, Pi LY, Holsten T, et al. A receptor kinase-like protein encoded by the rice disease resistance gene, Xa21. science. 1995 Dec 15;270(5243):1804-6.
4. Wang GL, Song WY, Ruan DL, Sideris S, Ronald PC. The cloned gene, Xa21, confers resistance to multiple Xanthomonas oryzae pv. oryzae isolates in transgenic plants. Molecular Plant-Microbe Interactions: MPMI. 1996 Dec 1;9(9):850-5.
5. Pruitt RN, Schwessinger B, Joe A, Thomas N, Liu F, Albert M, et al. The rice immune receptor XA21 recognizes a tyrosine-sulfated protein from a Gram-negative bacterium. Science Advances. 2015 Jul 24;1(6):e1500245.
6. Wei T, Chen TC, Ho YT, Ronald PC. Mutation of the rice XA21 predicted nuclear localization sequence does not affect resistance to Xanthomonas oryzae pv. oryzae. PeerJ. 2016 Oct 5;4:e2507.
7. Luu DD, Joe A, Chen Y, Parys K, Bahar O, Pruitt R, et al. Biosynthesis and secretion of the microbial sulfated peptide RaxX and binding to the rice XA21 immune receptor. Proceedings of the National Academy of Sciences. 2019 Apr 23;116(17):8525-34.
8. Ercoli MF, Luu DD, Rim EY, Shigenaga A, Teixeira de Araujo Jr A, Chern M, et al. Plant immunity: Rice XA21-mediated resistance to bacterial infection. Proceedings of the National Academy of Sciences. 2022 Feb 22;119(8):e2121568119.
9. Liu GZ, Pi LY, Walker JC, Ronald PC, Song WY. Biochemical characterization of the kinase domain of the rice disease resistance receptor-like kinase XA21. Journal of Biological Chemistry. 2002 Jun 7;277(23):20264-9.
10. Xu WH, Wang YS, Liu GZ, Chen X, Tinjuangjun P, Pi LY, et al. The autophosphorylated Ser686, Thr688, and Ser689 residues in the intracellular juxtamembrane domain of XA21 are implicated in stability control of rice receptor?like kinase. The Plant Journal. 2006 Mar;45(5):740-51.
11. Wang YS, Pi LY, Chen X, Chakrabarty PK, Jiang J, De Leon AL, et al. Rice XA21 binding protein 3 is a ubiquitin ligase required for full Xa21-mediated disease resistance. The Plant Cell. 2006 Dec;18(12):3635-46.
12. Park CJ, Ronald PC. Cleavage and nuclear localization of the rice XA21 immune receptor. Nature communications. 2012 Jun 26;3(1):1-6.
13. Huot B, Yao J, Montgomery BL, He SY. Growth–defense tradeoffs in plants: a balancing act to optimize fitness. Molecular Plant. 2014 Aug 1;7(8):1267-87.
14. He Z, Webster S, He SY. Growth–defense trade-offs in plants. Current Biology. 2022 Jun 20;32(12):R634-9.
15. Antolín-Llovera M, Ried MK, Parniske M. Cleavage of the SYMBIOSIS RECEPTOR-LIKE KINASE ectodomain promotes complex formation with Nod factor receptor 5. Current Biology. 2014 Feb 17;24(4):422-7.
16. Petutschnig EK, Stolze M, Lipka U, Kopischke M, Horlacher J, Valerius O, et al. A novel A rabidopsis CHITIN ELICITOR RECEPTOR KINASE 1 (CERK1) mutant with enhanced pathogen?induced cell death and altered receptor processing. New Phytologist. 2014 Dec;204(4):955-67.
17. Domínguez-Ferreras A, Kiss-Papp M, Jehle AK, Felix G, Chinchilla D. An overdose of the Arabidopsis coreceptor Brassinosteroid insensitive1-associated receptor kinase1 or its ectodomain causes autoimmunity in a suppressor of bir1-1-dependent manner. Plant Physiology. 2015 Jul;168(3):1106-21.
18. Zhou J, Wang P, Claus LA, Savatin DV, Xu G, Wu S, et al. Proteolytic processing of SERK3/BAK1 regulates plant immunity, development, and cell death. Plant physiology. 2019 May;180(1):543-58.
19. Chen F, Gao MJ, Miao YS, Yuan YX, Wang MY, Li Q, et al. Plasma membrane localization and potential endocytosis of constitutively expressed XA21 proteins in transgenic rice. Molecular Plant. 2010 Sep 1;3(5):917-26.
20. Chen X, Chern M, Canlas PE, Ruan D, Jiang C, Ronald PC. An ATPase promotes autophosphorylation of the pattern recognition receptor XA21 and inhibits XA21-mediated immunity. Proceedings of the National Academy of Sciences. 2010 Apr 27;107(17):8029-34.
21. Park CJ, Bart R, Chern M, Canlas PE, Bai W, Ronald PC. Overexpression of the endoplasmic reticulum chaperone BiP3 regulates XA21-mediated innate immunity in rice. PLoS One. 2010 Feb 17;5(2):e9262.
22. Chen X, Zuo S, Schwessinger B, Chern M, Canlas PE, Ruan D, et al. An XA21-associated kinase (OsSERK2) regulates immunity mediated by the XA21 and XA3 immune receptors. Molecular Plant. 2014 May 1;7(5):874-92.
23. Chen TC, Chern M, Steinwand M, Ruan D, Wang Y, Isharani A, et al. Paladin, a tyrosine phosphatase-like protein, is required for XA21-mediated immunity in rice. Plant Communications. 2021 Jul 12;2(4):100215.
24. NIÑO‐LIU DO, Ronald PC, Bogdanove AJ. Xanthomonas oryzae pathovars: model pathogens of a model crop. Molecular Plant Pathology. 2006 Sep;7(5):303-24.
25. Shamsunnaher, Chen X, Zhang X, Wu XX, Huang X, Song WY. Rice immune sensor XA21 differentially enhances plant growth and survival under distinct levels of drought. Sci Rep. 2020 Oct 9;10(1):16938.
26. Huffaker A, Pearce G, Ryan CA. An endogenous peptide signal in Arabidopsis activates components of the innate immune response. Proceedings of the National Academy of Sciences. 2006 Jun 27;103(26):10098-103.
27. Yamaguchi Y, Pearce G, Ryan CA. The cell surface leucine-rich repeat receptor for At Pep1, an endogenous peptide elicitor in Arabidopsis, is functional in transgenic tobacco cells. Proceedings of the National Academy of Sciences. 2006 Jun 27;103(26):10104-9.
28. Pruitt RN, Joe A, Zhang W, Feng W, Stewart V, Schwessinger B, et al. A microbially derived tyrosine?sulfated peptide mimics a plant peptide hormone. New Phytologist. 2017 Jul;215(2):725-36.
29. Kopan R, Ilagan MX. The canonical Notch signaling pathway: unfolding the activation mechanism. Cell. 2009 Apr 17;137(2):216-33.
30. Lagerwerff JV, Ogata G, Eagle HE. Control of osmotic pressure of culture solutions with polyethylene glycol. Science. 1961 May 12;133(3463):1486-7.
31. Kauffman HE. An improved technique for evaluating resistance of rice varieties to Xanthomonas oryzae. Plant Dis. Rep. 1973;57:537-41