Abstract
Purpose: Spinal cord and nerves are best visualized by MRI, which is able to show structural and functional anomalies of the spine. The primary objective of this study is to identify advantages or disadvantages of the T1-weighted fluid attenuated inversion recovery (FLAIR) sequence with BLADE technique (T1W-FLAIR BLADE), with and without parallel imaging when compared with T1 Turbo Spin Echo (T1 TSE) sequence when performing MRI examination of the lumbar spine in a sagittal view.
Methods: L-spine examinations with T1W-FLAIR BLADE (with and without parallel imaging) and T1 TSE were acquired on 44 patients using a 1.5T scanner. These sequences were assessed by two radiologists a) quantitatively by comparing the signal-to-noise ratio (SNR), contrast-to-noise ratio (CNR) and relative contrast (ReCon) measurements and b) qualitatively based on different features of the images such as cerebrospinal fluid (CSF) nulling.
Results: The quantitative analysis showed that T1W-FLAIR BLADE in most tissues had better SNR values, as well as better CNR and ReCon results. SNR and CNR measurements were also better in T1W-FLAIR BLADE images with parallel imaging compared to T1 TSE. In the qualitative analysis, it was found that T1W-FLAIR BLADE sequence had superior quality of images, better contrast among tissues, and better nulling of CSF.
Conclusion: T1W-FLAIR BLADE appeared to have less motion artifacts and improved image quality, as well as improved CSF nulling. T1W-FLAIR BLADE with parallel imaging achieved the second best results out of the sequences compared. The clinicians showed a preference for T1W-FLAIR BLADE images which could be a useful addition to the standard imaging of the L-spine.
Keywords
1.5T MRI, Motion artifacts, BLADE sequences, Lumbar spine examination
Introduction
Magnetic Resonance Imaging (MRI) gives a very clear picture of the spine and it is very helpful in identifying serious spinal disorders such as cancer, infection, or nerve compression [1]. It can identify congenital abnormalities, trauma and other lesions that could be missed with other imaging tools. Currently, MRI is the gold standard for the imaging of the spinal canal and the nerves of the spine as well as imaging of injuries affecting the vertebrae and the discs (such as degeneration, hernias, etc.). MRI is also used as a tool for pre- and post-operative assessment, assessment of infections, and delineation of tumors [2].
The T1 sequence is the most important sequence for detecting bone marrow pathologies. More specifically, it provides a wide understanding of anatomical and pathological variations in the lumbar spine. In the imaging of the lumbar spine, T1W-Turbo Spin Echo (TSE) or Fast Spin Echo (FSE) images are acquired [3]. According to Melhem et al., who compared T1 FLAIR and T1 SE images, the images received from T1W-FLAIR sequence showed higher contrast around CSF, improved lesion delineation in the spinal cord and bone marrow, and showed less artifacts in comparison with the conventional T1W-SE sequences [4]. The study concluded that T1W-FLAIR images were superior to SE sequences when analyzing spinal imaging. Another study verified that T1W-FLAIR images might be an alternative approach for the imaging of L-spine instead of T1W-TSE, as it achieved better overall image quality and contrast, better signal nulling of CSF, as well as better definition of structures and lesions [5].
In most cases, patients that are to have an MRI examination of the L-spine present with severe back pain. This can be caused by many reasons, such as sciatica or even bone metastases. In these cases, it is very difficult for the patient to keep still during a long MRI examination, resulting in visible patient movement in the acquired images. Sequences that use central K-space oversampling, a technique known as PROPELLER (Periodically Rotated Overlapping Parallel Lines with Enhanced Reconstruction) can be helpful in reducing image artifacts [6]. Many studies have also shown the advantages of BLADE in T2 sequences with regards to image quality when compared to conventional sequences [6,7]. Advantages included motion elimination as well as elimination of flow and slice-overlap artifacts in MR lumbar spine imaging in both cooperative and non-cooperative patients [7]. Alternatively, there have not been many studies that show the advantages of BLADE in T1 sequences. Acquiring T1-weighted BLADE images requires a long echo train length (ETL), which may slightly increase the sequence time but a long ETL would decrease motion artifacts. A few studies on brain MR imaging have found that T1W-FLAIR BLADE presented better image quality including CSF nulling and less artifacts [6].
Parallel imaging is a technique in which TSE fills K-space more efficiently by filling multiple lines of K-space per TR. However, the difference is that these lines are acquired by certain coils that are combined together to acquire data simultaneously [8]. Parallel imaging is an important tool that can be used to improve image resolution and reduce scan times. With the appropriate software and coil configurations, it can be used with most pulse sequences. Apart from the benefits described above, parallel imaging can result in a slight loss of SNR. In addition, chemical shift may increase due to different resonant frequencies being mapped across each coil. Patient movement also causes misalignment between under-sampled data and reference scans [8].
In this study, T1W-FLAIR BLADE without parallel imaging in the sagittal plane (sag) is compared with T1W-TSE in the sagittal plane and T1W-FLAIR BLADE with parallel imaging (no fat saturation, non-contrast). The aim to show the benefits of both sequences and identify which one provides better lesion delineation and reduction of artifacts.
Methods and Materials
Subjects
This study was performed on forty-four patients (20 females and 24 males within the range of 18-82 years), who had a routine MRI examination of the L-spine between February 2014 and July 2018. All patients participating in the study signed a written consent form with all the information provided and the study was approved by the local institutional review board.
MR Imaging techniques
All the lumbar spine (L-spine) MRI examinations in this study were acquired on a 1.5T scanner (Magnetom, Avanto, Siemens Healthcare Sector, Erlangen, Germany) and a Synergy body phased-array surface coil. The parameters of each sequence are presented in (Table 1).
|
Sequences |
T1 TSE Sag |
T1 Sag Blade Flair |
T1 Sag Blade Flair PI |
|
TR (msec) |
591 |
2000 |
2000 |
|
TE (msec) |
11 |
47 |
45 |
|
TI (msec) |
|
860 |
860 |
|
Matrix (Phase/Freq) |
384/288 |
256/256 |
256/256 |
|
BW (Hz/pix) |
161 |
362 |
362 |
|
A.A (min) |
2:43 |
4:06 |
2:25 |
|
ET |
2 |
49 |
49 |
|
Thickness (mm) |
4 mm |
4 mm |
4 mm |
|
Space (mm) |
10% |
10% |
10% |
|
FOV (mm) |
280 |
280 |
280 |
|
NSA |
2 |
1 |
1 |
Turbo Spin Echo (TSE) or Fast Spin Echo (FSE) pulse sequence is the main sequence used in almost all MR imaging nowadays [9]. TSE is basically a spin echo sequence with the advantage of a shorter acquisition time. While in a SE sequence a 90° excitation pulse is followed by a 180° rephasing pulse, filling only one line of K-space per TR, in the TSE sequence several 180° rephasing pulses produce an echo train, thus producing more than one phase encoding steps and subsequently filling more than one line of K-space per TR [8]. This allows the TSE sequence to be acquired in much less time than the conventional spin echo (CSE) without compromising spatial resolution, image weighting or signal-to-noise ratio (SNR) [8]. To improve spatial resolution, a greater number of phase-encoding steps can be used. Last but not least, TSE provides a better image when metal objects are present due to less signal loss from susceptibility artifacts [9].
Generally speaking, although TSE and CSE are two of the main sequences that are used in most MR imaging examinations, TSE has largely replaced CSE in certain examinations, such as musculoskeletal scans, especially in T2 weighting. However, an issue that can occur during the acquisition of TSE images is blurring at the edges of tissues with different T2 decay values. This is because each line of K-space filled during an echo train contains data from echoes with a different TE. When using long echo trains, late echoes that have low signal amplitude, contribute to the resolution of K-space. If these echoes are negligible, then the resolution is lost from the image and blurring occurs. This loss of resolution, however, may be reduced by decreasing the spacing between echoes and/or the turbo factor [10].
Inversion recovery (IR) was initially developed to provide good T1 contrast but nowadays, in combination with FSE, produces images in few minutes. IR is generally used to suppress the signal from certain tissues in conjunction with long TE, and T2 weighting [8]. The IR sequence begins with an 180° inverting pulse. When the inverting pulse is removed, the magnetic vector of the tissue’s nuclei begins to relax back to the initial state. Then, a 90° excitation pulse is applied at a specific inversion time depending on the contrast that wants to be achieved [8]. Fluid attenuated inversion recovery (FLAIR) is a variation of IR. In FLAIR, the signal from CSF is nulled as described, thus making it the sequence of choice in brain and spine imaging for the diagnosis of periventricular and cord lesions. For spinal imaging, a STIR sequence, which is another variation of IR, can follow after FLAIR as it provides better image contrast as it achieves fat suppression and visualizes lesions better.
Important factors for eliminating motion artifacts are the echo train length (ETL) and the bandwidth (BW) values which is basically the number of frequencies that can be transmitted or received during a certain time. Although BLADE takes almost twice the time of conventional sequences to acquire, it has a larger ETL and BW, thus, reducing artifacts. Originally, the technique of the PROPELLER was introduced by Pipe and colleagues [11-14]. During this acquisition, in order to achieve artifact reduction, multiple ETL of a TSE are acquired in a rotating and partially overlapping manner which represent “blades”. Then, on each dataset acquired from the blades, phase correction is performed to remove phase inconsistencies from motion artifacts. In a similar way, with the BLADE technique which is the equivalent of PROPELLER for SIEMENS Medical System (Erlangen, Germany), the acquired blades are rotated around the center of K-space, achieving central K-space oversampling, which is key for image artifact reduction [6].
Parallel imaging is a technique that contributes to shorter acquisition times in MRI scans. Parallel imaging relies on the use of multiple coils to receive information to fill K-space. Although, this information can be acquired more quickly when compared to conventional methods, there is a reduced amount of data, leading to a slight loss of signal, without that affecting though the visualization of the lesion and the surrounding tissues. To solve this problem in parallel imaging, a special algorithm is used which combines all the information received from the coils to make a complete image. The faster acquisition time that is provided with parallel imaging is very beneficial in cases where involuntary patient motion is expected as well as in imaging where breath hold is needed [15,16].
Results
Quantitative analysis
The results of the quantitative analysis are presented in (Table 2). The findings show that T1W-FLAIR BLADE sequences have improved SNR in most of the tissues. In further detail, the results showed that T1W-FLAIR BLADE sequence without parallel imaging had statistically significant difference in the imaging of bone marrow (p<0.001), spinal cord (p<0.01), fat tissue (p<0.01) and most importantly in nulling CSF (p<0.001). In many of the cases, T1W-FLAIR BLADE with parallel imaging had the second-best results, followed by T1W-TSE. With regards to CNR measurements, in all of the cases T1W-FLAIR BLADE without parallel imaging had the better results. In detail, the results showed significant statistical difference between bone marrow/disc (p<0.001), CSF/spinal cord (p<0.01), nerve root/fat tissue (p<0.005), CSF/bone marrow (p<0.001), CSF/disc (p<0.001) and disc/fat tissue (p<0.005). T1W-FLAIR BLADE with parallel imaging had in all cases the second-best results, followed by T1W-TSE. The results for relative contrast showed that the T1W-FLAIR BLADE sequence without parallel imaging had again statistically significant difference in most of the cases. In detail, between bone marrow/disc (p<0.005), CSF/spinal cord (p<0.001), nerve root/ fat tissue (p<0.001), CSF/bone marrow (p<0.001) and CSF/disc (p<0/001). Although, in most cases T1W-FLAIR BLADE showed superior results than T1W-FLAIR BLADE with parallel imaging, the results for the imaging between disc and fat tissue showed that T1W-FLAIR BLADE with parallel imaging was significantly better (p<0.005). T1W-TSE had the lowest results amongst all three sequences.
|
SNR |
T1-TSE |
T1-Blade-Flair |
T1-Blade-Flair-PI |
p |
|
BM |
67.6 ± 35.2 |
102.4 ± 38.8 |
72.5 ± 33.2 |
5.0E-04 |
|
DS |
36.4 ± 22.1 |
41.4 ± 17.6 |
30.4 ± 14.7 |
4.8E-02 |
|
RT |
43.5 ± 23.7 |
45.9 ± 34.0 |
36.8 ± 29.9 |
2.4E-01 |
|
SC |
42.3 ± 14.9 |
52.7 ± 17.6 |
39.8 ± 15.2 |
1.2E-02 |
|
CSF |
20.0 ± 9.1 |
7.8 ± 4.2 |
8.3 ± 3.6 |
0.0E+00 |
|
FTS |
150.9 ± 66.1 |
205.0 ± 66.2 |
177.9 ± 62.2 |
5.2E-03 |
|
CNR |
T1-TSE |
T1-Blade-Flair |
T1-Blade-Flair-PI |
p |
|
BM/DS |
31.2 ± 16.3 |
61.0 ± 28.1 |
42.1 ± 22.8 |
0.0E+00 |
|
CSF/SC |
22.3 ± 9.1 |
44.9 ± 15.3 |
31.5 ± 13.8 |
0.0E+00 |
|
RT/FT |
108.0 ± 57.0 |
159.1 ± 62.6 |
141.1 ± 56.1 |
3.2E-03 |
|
CSF/BM |
47.6 ± 27.0 |
94.6 ± 35.8 |
64.2 ± 31.6 |
0.0E+00 |
|
CSF/DS |
16.4 ± 14.8 |
33.7 ± 15.8 |
22.1 ± 13.7 |
0.0E+00 |
|
DS/RT |
15.7 ± 21.3 |
19.1 ± 26.0 |
16.1 ± 23.6 |
6.5E-01 |
|
DS/FT |
114.6 ± 47.8 |
163.5 ± 54.8 |
147.5 ± 52.7 |
1.4E-03 |
|
ReCNR |
T1-TSE |
T1-Blade-Flair |
T1-Blade-Flair-PI |
p |
|
BM/DS |
31.7 ± 10.3 |
42.6 ± 10.8 |
40.0 ± 11.3 |
1.3E-03 |
|
CSF/SC |
35.9 ± 12.6 |
74.6 ± 8.5 |
64.1 ± 12.1 |
0.0E+00 |
|
RT/FT |
54.9 ± 14.5 |
66.2 ± 18.7 |
68.1 ± 18.9 |
8.0E-04 |
|
CSF/BM |
52.9 ± 7.0 |
86.0 ± 4.5 |
77.7 ± 9.4 |
0.0E+00 |
|
CSF/DS |
24.9 ± 13.4 |
68.3 ± 9.5 |
55.0 ± 16.6 |
0.0E+00 |
|
DS/RT |
17.8 ± 15.2 |
24.3 ± 22.8 |
24.0 ± 24.7 |
8.4E-01 |
|
DS/FT |
62.4 ± 8.4 |
66.6 ± 7.7 |
70.8 ± 7.9 |
1.5E-03 |
Qualitative analysis
According to the findings of the qualitative analysis, both radiologists agreed that CSF nulling was significantly better for T1W-FLAIR BLADE images without parallel imaging than T1W-TSE images (p<0.001). The inter-observer agreement was perfect for T1W-FLAIR BLADE imaging, almost perfect for T1W-FLAIR BLADE with parallel imaging and moderate for T1W-TSE. T1W-FLAIR BLADE provided better contrast for bone marrow, spinal cord, fat tissue and most importantly CSF. The contrast between the spinal cord, the cauda equina and CSF was also significantly better for T1W-FLAIR BLADE images than T1W-TSE images. T1W-FLAIR BLADE with parallel imaging had better results than T1W-TSE but worse than T1W-FLAIR BLADE without parallel imaging. Both of the observers found that the contrast between bone marrow-disc, nerve root-fat tissue, CSF-bone marrow, CSF-disc and disc-fat tissue was better for T1W-FLAIR BLADE images without parallel imaging, immediately followed by T1W-FLAIR BLADE images with parallel imaging and finally T1 TSE, demonstrating substantial inter-observer agreement.
Discussion
Although there have been many technical advances in MRI since it was first established, MRI of the L-spine is still challenging [14]. Unfortunately, there is a high chance of artifacts that can decrease the image quality, such as flow artifacts or motion artifacts, along with ringing artifacts, even in cooperative patients. However, if the patient is not cooperative there is a further risk of physical motion which can add motion artifacts, thus compromising image quality even more [17]. It is worth mentioning that in our experience, there is no significant improvement with BLADE in image quality in the presence of metal artifacts. So far, the successful application of BLADE in MR imaging of the brain to control motion artifacts (Figure 1) has been seen in pediatric or non-cooperative patients. BLADE is also seen to reduce flow artifacts after contrast administration. Some advantages have also been reported from BLADE in abdominal imaging but there are not sufficient data for the application of BLADE in spinal imaging [18].
Figure 1: (A) T1 TSE sagittal, (B) T1 FLAIR BLADE sagittal, (C) T2 TSE sagittal, (D) T2 FLAIR BLADE sagittal. These images illustrate the ability of FLAIR BLADE to eliminate motion artifacts in both T1 and T2 weighted images.
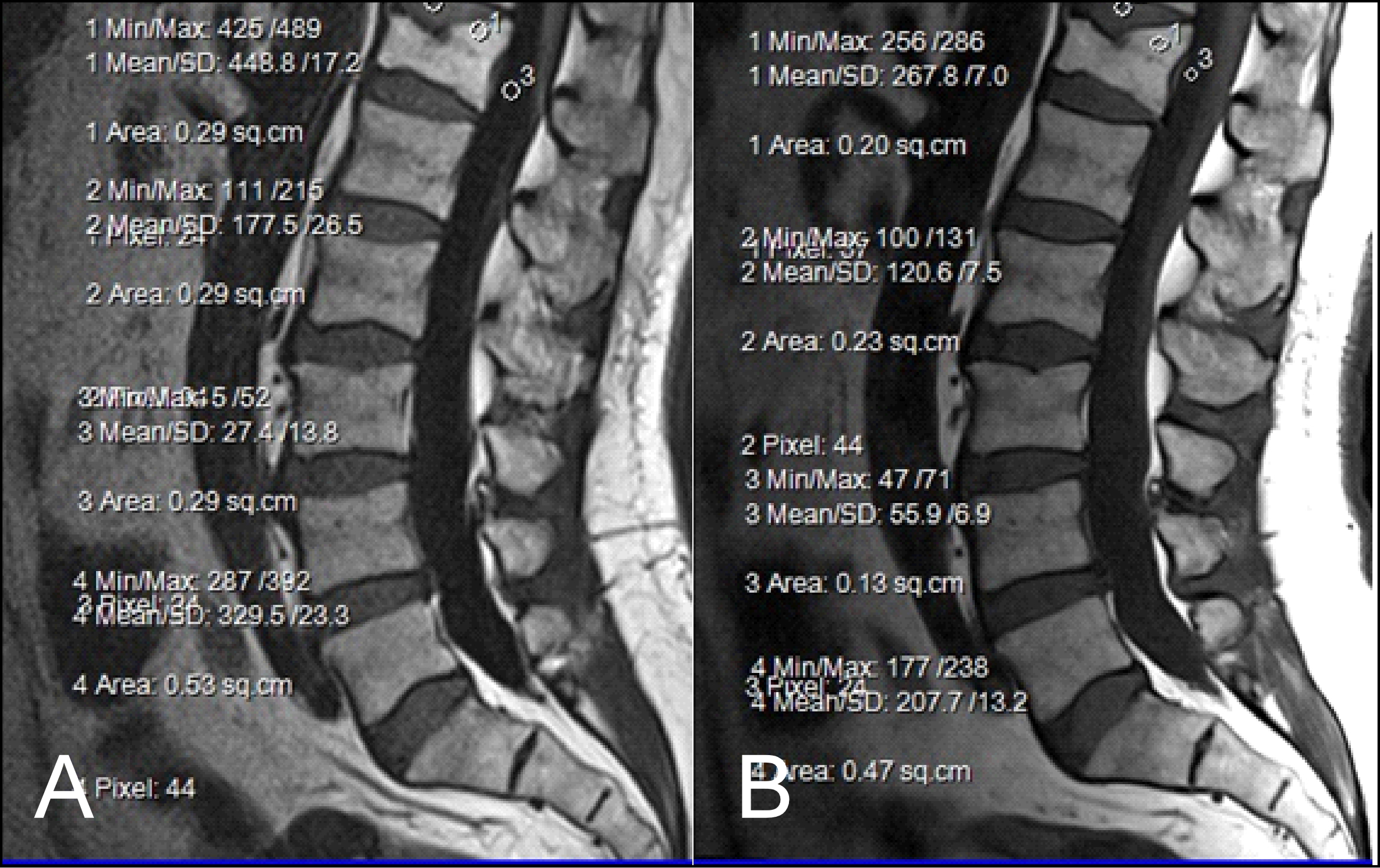
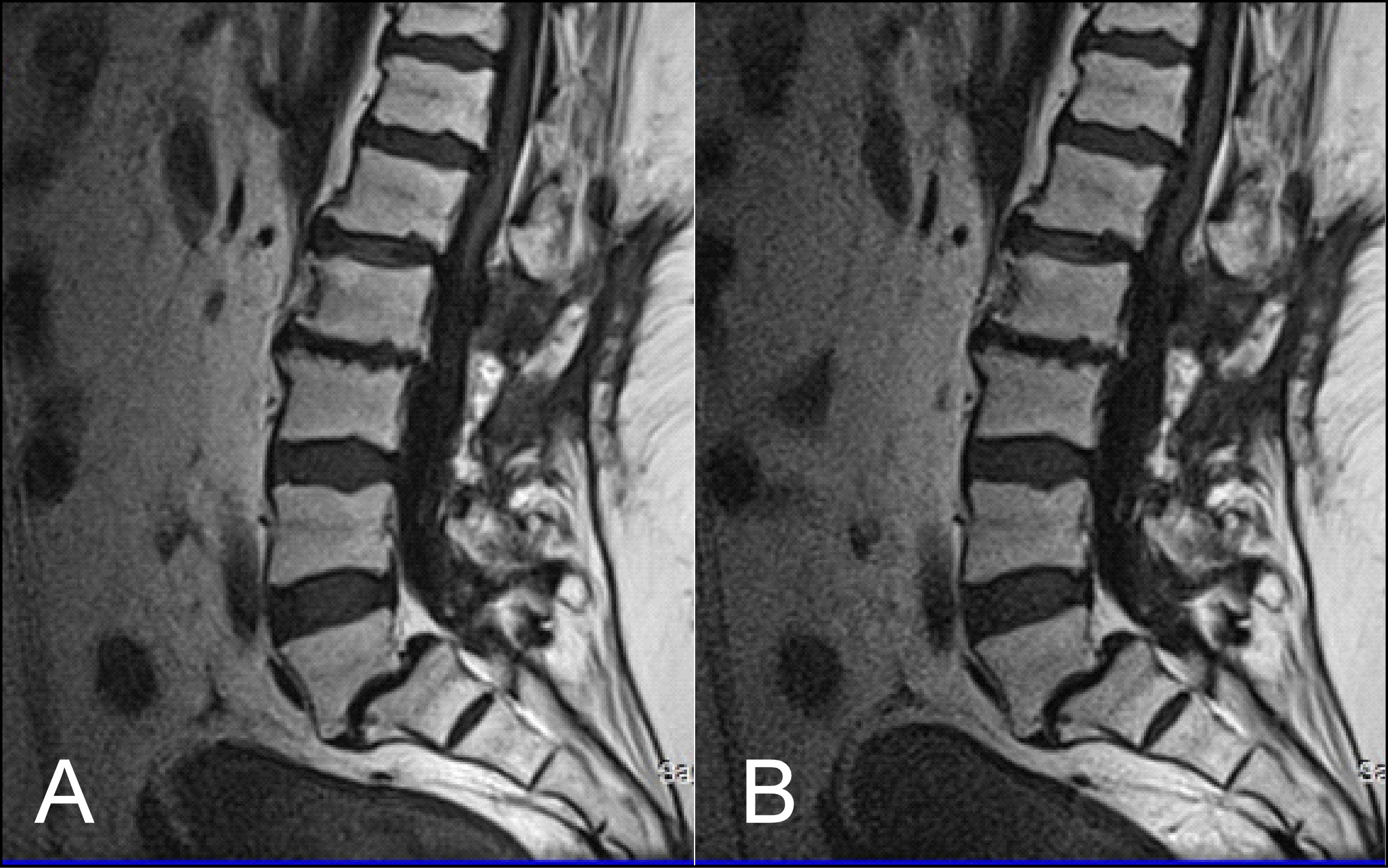
The results obtained from the quantitative analysis of this study showed that the SNR and CNR results were significantly greater for T1W-FLAIR BLADE. More specifically, T1W-FLAIR BLADE sequence achieved better CSF nulling and better contrast between spongy bone and intervertebral disc as well as between spongy bone and intervertebral disc with CSF (Figure 2). A study by Alibek et al., has also demonstrated the superiority in image contrast of T1W-FLAIR images showing that they achieve better lesion to background and grey to white matter CNR [19]. The inherent restrictions of parallel imaging, i.e., reduction of SNR and increased possibility for reconstruction artifacts, were confirmed in our findings where the images without parallel imaging had better SNR (Figure 3).
Figure 2: (A) T1 FLAIR BLADE, (B) T1 TSE. The results obtained from the quantitative analysis showed that the FLAIR BLADE sequences have better SNR in most tissues. T1 FLAIR BLADE sequence achieved better CSF nulling and better contrast between spongy bone and intervertebral disc. The contrast was also better when comparing spongy bone and intervertebral disc with CSF.
Figure 3: (A) T1 FLAIR BLADE without parallel imaging, (B) T1 FLAIR BLADE with parallel imaging. T1 FLAIR BLADE images without parallel imaging were found to have better quality than the T1 FLAIR BLADE images with parallel imaging. Although they both eliminated motion artifacts, the images without parallel imaging have better SNR as well as better contrast between tissues.
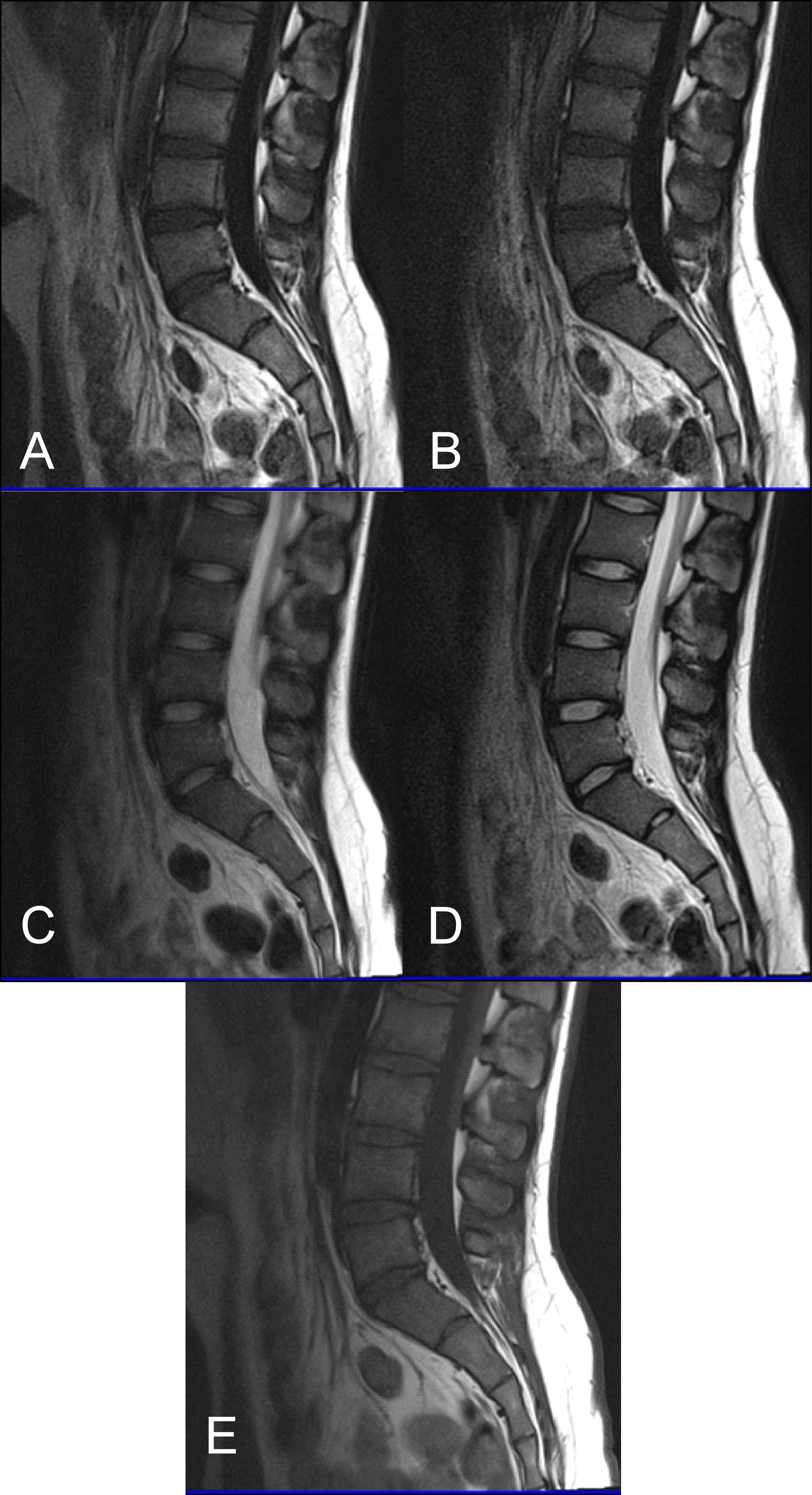
As stated earlier, sagittal T1 weighted images play an integral role in the MR imaging of the L-spine. One of the reasons is that they provide great information about anatomical structures. In order to assess the importance of the BLADE sequence compared to a TSE, both a qualitative and quantitative analysis were performed by evaluating contrast in between anatomical structures and the SNR, CNR and ReCon measurement respectively [18]. Although T1W-TSE is a faster sequence, T1W-FLAIR BLADE scored better in quantitative and qualitative analyses. Pathologies and abnormalities were better visualized in the latter one. Furthermore, in T1W-FLAIR BLADE there was improved image contrast as well as greater depiction of anatomical structures and either degeneration or metastatic lesions. Both the quantitative and qualitative analysis showed that T1W-FLAIR BLADE achieved better results than T1W-TSE. In addition to that, T1W-FLAIR BLADE images without parallel imaging were found to have better quality than the T1W-FLAIR BLADE images with parallel imaging. Although they both eliminated motion artifacts, the images without parallel imaging have better SNR as well as better contrast between tissues (Figure 3). Moreover, a study showed that T1W-FLAIR images can successfully eliminate chemical shift artifacts compared to T1W-TSE due to greater receiver bandwidth [8]. This statement was confirmed by our findings (Figure 4).
Figure 4: (A) T1 FLAIR BLADE, (B) T1 FLAIR BLADE with parallel imaging, (C) T2 TSE, (D) T2 BLADE TSE, (E) T1 TSE. T1 FLAIR BLADE achieves better CSF nulling, better elimination of motion artifacts as well as better contrast between tissues. In T1 FLAIR BLADE there is also less chemical shift due to the fact that a greater bandwidth (1) is used. T2 BLADE appears to have better image quality compared to T2 TSE.
T1W-FLAIR BLADE also had higher values in all the relative contrast measurements. T1W-FLAIR BLADE showed better results when comparing CSF with spongy bone, CSF with intervertebral disc and intervertebral disc with spongy bone. These results from the quantitative analysis meant better conspicuity between the following anatomical structures: intervertebral disc-CSF, intervertebral disc-spinal cord, vertebral body-CSF and spinal cord-CSF. Both radiologists found that T1W-FLAIR images achieved better CSF nulling compared to T1W-TSE. It was also found that T1W-FLAIR BLADE was significantly superior to T1W-TSE when reviewing the contrast at the spinal cord and cauda equina interface, demonstrating substantial inter-observer agreement. Due to CSF nulling that is achieved in FLAIR sequences, T1W-FLAIR sequence is a very useful tool to distinguish cysts within the spinal cord, tumors and demyelinating disease. Another study showed that T1W-FLAIR images had also a higher CNR over T1W-TSE images in 16 metastatic lesions in the vertebrae, which helped diagnose the extent of the lesion in the spinal cord. Moreover, the findings of this study are in line with the results of other studies, which show that the T1W-FLAIR sequence could be more beneficial for L-spine imaging than T1W-TSE, due to CSF nulling, higher image contrast and definition of anatomical structures, degeneration, or metastatic lesions (Figure 5) [11]. The results of our study also support the above, i.e., T1W-FLAIR BLADE being superior to T1W-TSE for the aforementioned reasons.
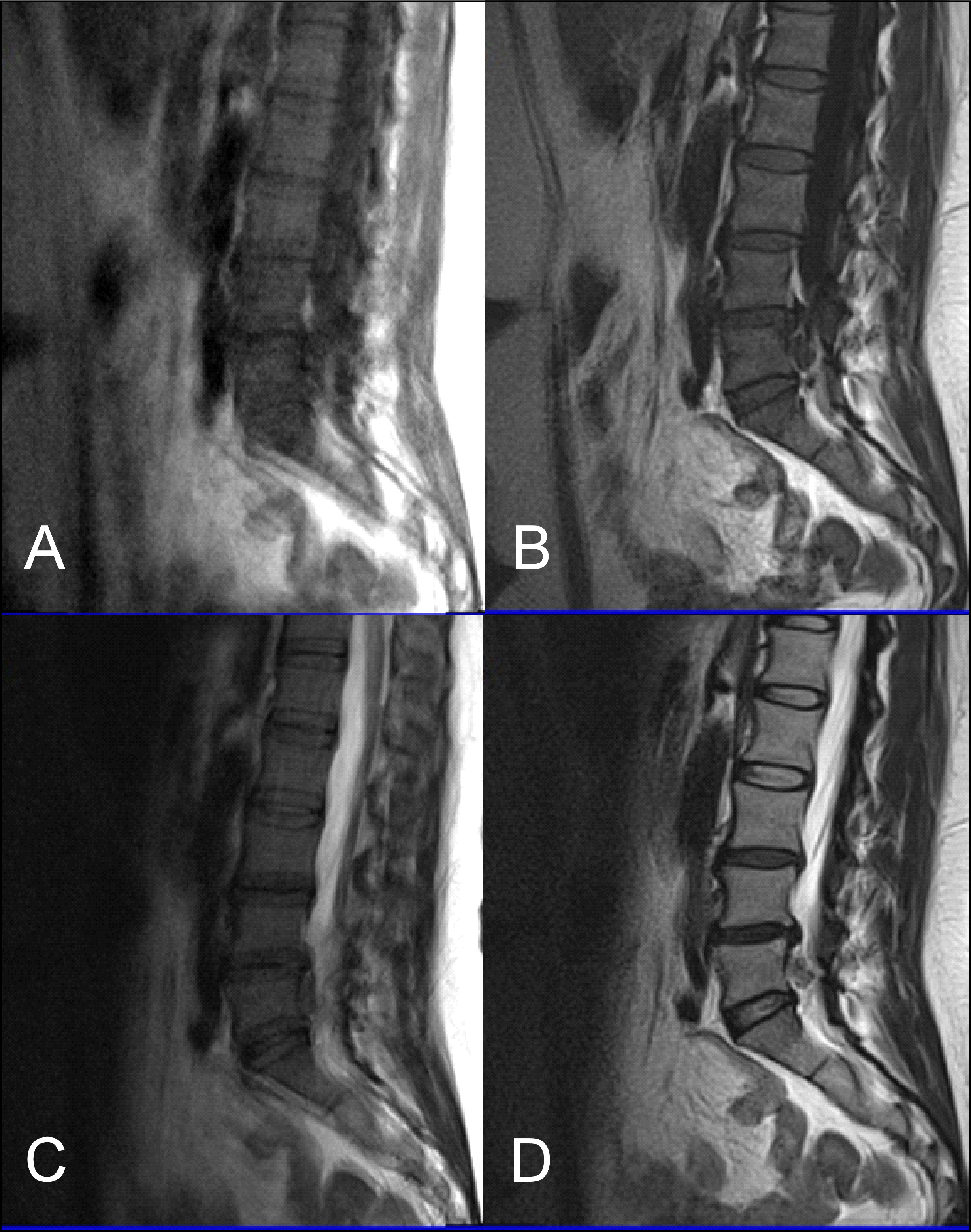
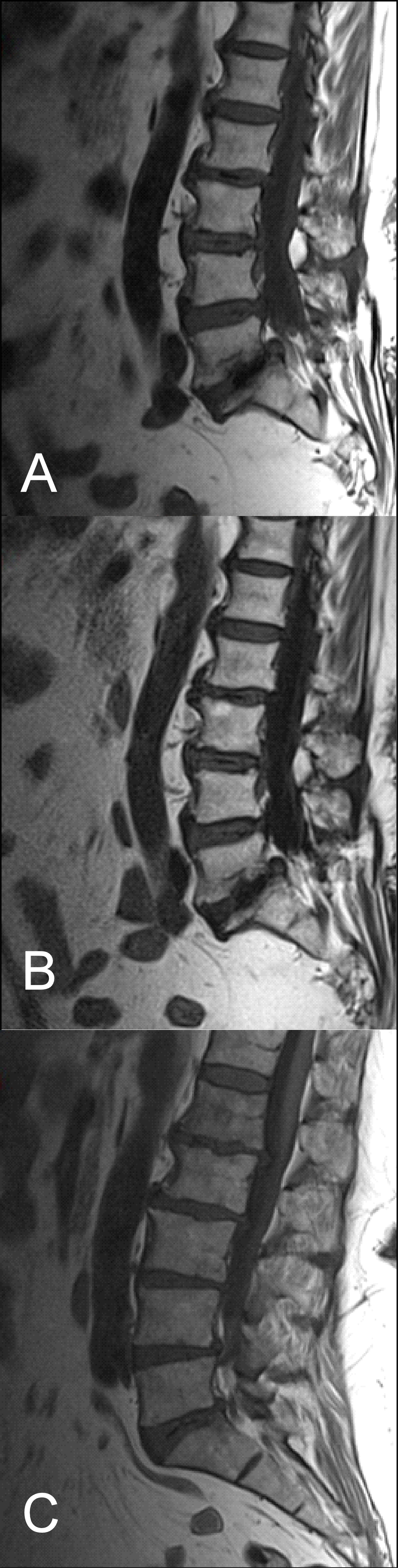
Figure 5: (A) T1 FLAIR BLADE, (B) T1 FLAIR BLADE with parallel imaging, (C) T1 TSE. T1 FLAIR BLADE shows better image contrast. In images A and B, a lesion is depicted behind L3-L5 which is not depicted in the TSE in image C. In T1 FLAIR BLADE we can achieve better CSF nulling, and also better lesion delineation. The lesion is better visualized in the image with parallel imaging as parallel imaging eliminates artifacts.
An interesting implementation of T1W-FLAIR BLADE images would be in 3T MRI. Theoretically, 3T MRI is expected to have an SNR that is twice as good as the SNR received at 1.5T. However, there is a slight increase in susceptibility and chemical shift artifacts that T1W-FLAIR BLADE has been shown to eliminate successfully [9,11]. Another issue that T1W-FLAIR BLADE could help overcome in 3T MRIs is the nulling of CSF. At 3T MRI relaxation times are increased so the contrast for T1 images is reduced. This may result in CSF appearing less dark which T1W-FLAIR BLADE could help resolve effectively as it achieves great CSF nulling [5].
To conclude, T1W-FLAIR BLADE without parallel imaging achieved the highest scores from all the sequences. T1W-FLAIR BLADE with parallel imaging was the second-best sequence in our study with T1 TSE following. T1W-FLAIR BLADE was found to have better CSF nulling and better SNR measurements for the spinal cord. T1W-FLAIR BLADE also had better CNR measurements for spinal cord-CSF with better imaging of disc-cord and cord-CSF interfaces. Moreover, it showed better overall image quality than T1W-TSE and greater reduction of artifacts. T1W-FLAIR BLADE with or without parallel imaging shows great potential for 3T MRI as it reduces susceptibility and chemical shift errors as well as improved CSF nulling. Last but not least, T1W-FLAIR BLADE was the preferred choice of the clinicians for the imaging of the L-spine.
Ethical Approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Conflict of Interest
The authors declare that they have no conflict of interest.
References
2. Radiology Info. [Online] Radiological Society of North America, Inc. (RSNA).
3. Wilmink JT. Lumbar spinal imaging in radicular pain and related conditions: understanding diagnostic images in a clinical context. Springer Science & Business Media; 2010 Feb 8.
4. Melhem ER, Israel DA, Eustace S, Jara H. MR of the spine with a fast T1-weighted fluid-attenuated inversion recovery sequence. American Journal of Neuroradiology. 1997 Mar 1;18(3):447-54.
5. Lavdas E, Vlychou M, Arikidis N, Kapsalaki E, Roka V, Fezoulidis IV. Comparison of T1-weighted fast spin-echo and T1-weighted fluid-attenuated inversion recovery images of the lumbar spine at 3.0 Tesla. Acta Radiologica. 2010 Jan 1;51(3):290-5.
6. Mavroidis P, Giankou E, Tsikrika A, Kapsalaki E, Chatzigeorgiou V, Batsikas G, et al. Brain imaging: comparison of T1W FLAIR BLADE with conventional T1W SE. Magnetic Resonance Imaging. 2017 Apr 1;37:234-42.
7. Lavdas E, Mavroidis P, Kostopoulos S, Glotsos D, Roka V, Koutsiaris AG, et al. Elimination of motion, pulsatile flow and cross-talk artifacts using blade sequences in lumbar spine MR imaging. Magnetic Resonance Imaging. 2013 Jul 1;31(6):882-90.
8. Westbrook C, Roth CK, Talbot J. MRI in Practice Fourth Edition. Wiley-Blackwell, A John Wiley & Sons, Ltd., Publication, 2011.
9. Lavdas E, Mavroidis P, Vassiou K, Roka V, Fezoulidis IV, Vlychou M. Elimination of chemical shift artifacts of thoracic spine with contrast-enhanced FLAIR imaging with fat suppression at 3.0 T. Magnetic Resonance Imaging. 2010 Dec 1;28(10):1535-40.
10. Hirokawa Y, Isoda H, Maetani YS, Arizono S, Shimada K, Togashi K. Evaluation of motion correction effect and image quality with the periodically rotated overlapping parallel lines with enhanced reconstruction (PROPELLER)(BLADE) and parallel imaging acquisition technique in the upper abdomen. Journal of Magnetic Resonance Imaging: An Official Journal of the International Society for Magnetic Resonance in Medicine. 2008 Oct;28(4):957-62.
11. Forbes KP, Pipe JG, Bird CR, Heiserman JE. PROPELLER MRI: clinical testing of a novel technique for quantification and compensation of head motion. Journal of Magnetic Resonance Imaging: An Official Journal of the International Society for Magnetic Resonance in Medicine. 2001 Sep;14(3):215-22.
12. Hennig J, Nauerth A, Friedburg HR. RARE imaging: a fast imaging method for clinical MR. Magnetic Resonance in Medicine. 1986 Dec;3(6):823-33.
13. Walker MT, Partovi S, Karis JP, Fram FK. Fast, versatile, and cost-effective FSE MR imaging: technical considerations and clinical applications. Barrow Quarterly. 2000;16:1-5.
14. Pipe JG. Motion correction with PROPELLER MRI: application to head motion and free‐breathing cardiac imaging. Magnetic Resonance in Medicine: An Official Journal of the International Society for Magnetic Resonance in Medicine. 1999 Nov;42(5):963-9.
15. Deshmane A, Gulani V, Griswold MA, Seiberlich N. Parallel MR imaging. Journal of Magnetic Resonance Imaging. 2012 Jul;36(1):55-72.
16. Fellner C, Menzel C, Fellner FA, Ginthoer C, Zorger N, Schreyer A, et al. BLADE in sagittal T2-weighted MR imaging of the cervical spine. American Journal of Neuroradiology. 2010 Apr 1;31(4):674-81.
17. Bushberg JT, Boone JM. The essential physics of medical imaging. Lippincott Williams & Wilkins; 2011 Dec 20.
18. Glockner JF, Hu HH, Stanley DW, Angelos L, King K. Parallel MR imaging: a user’s guide. Radiographics. 2005 Sep;25(5):1279-97.
19. Alibek S, Adamietz B, Cavallaro A, Stemmer A, Anders K, Kramer M, et al. Contrast-enhanced T1-weighted fluid-attenuated inversion-recovery BLADE magnetic resonance imaging of the brain: an alternative to spin-echo technique for detection of brain lesions in the unsedated pediatric patient? Academic Radiology. 2008 Aug 1;15(8):986-95.