Abstract
Methylene blue (MB) is commonly used as a medication and dye for various purposes and its presence in an aqueous solution may prove to be toxic and can cause various health problems. Due to this, the removal of MB dye from aqueous solutions is one of the major concerns for the scientific community. Photocatalyst is a promising material for the effective degradation of MB dye whereas traditional catalyst is not effective in the presence of visible light. In this short communication, the possibility of photocatalyst based on graphene oxide (GO) decorated with silver nanowires (AgNWs) will be explored. Graphene oxide is an insulator in nature and various oxygen containing groups are present in its structure. In order to increase its surface area and convert it from insulator to conductor, reduction is required. However, reduction using chemicals cause environmental pollution. In order to overcome this problem, electroplating method is used here for altering GO into rGO. Furthermore, in order to explore the synergetic effect of AgNWs, decoration of these has been carried out onto rGO surface. The change in the band gap of rGO in the presence of AgNWs is investigated here which is significant parameter of photocatalyst. The influence of AgNWs onto the specific surface area of rGO can also be explored. It is clearly indicated that the presence of AgNWs onto rGO can decrease the band gap and increase the specific surface area of nanocomposite, which has been required in photocatalysis application. It is expected that the synthesized nanocomposite is a promising photocatalyst for the degradation of MB dye.
Keywords
Reduced graphene oxide, Silver nanowires, Methylene blue, Nanocomposite
Short Communication
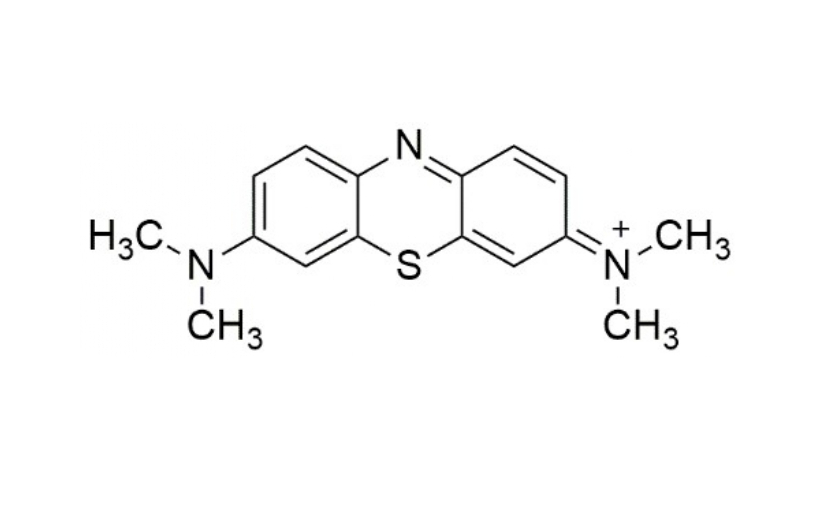
Methylene blue (MB, Figure 1) is a blue colored dye that can be used as a medication and as dye for dyeing leather and temporary hair. It is generally used in human and veterinary medicine for various therapeutic and diagnostic procedures such as a stain in bacteriology, as a redox coloring agent, as a targeting agent for melanoma, as an anti-methemoglobinemic, as an antiseptic and disinfectant, as a treatment for ifosfamide induced encephalopathy, as a treatment of vasoplegic associated with septic shock, and as a tracer in parathyroid surgery [1-3]. It has been also used for various other types of application inducing, redox indicator, peroxide generator, water testing, biological staining, and aquaculture. Moreover, the dose of MB (approximately 500 mg) in human bodies causes various types of side-effects such as headache, nausea, vomiting, chest pain, cyanosis, shortness of breath, and high blood pressure with some other side effects including hyperbilirubinemia, anemia, erythrocytic blister cells, skin discoloration, serotonin syndrome, red blood cell breakdown, and allergic reactions [4-6].
Figure 1: 2D-structure of methylene blue.
Hence, to lessen its drawbacks and for the protection of environment, the elimination of MB dye from aqueous solution is of the major concern for the scientist community. For this, a number of techniques, such as, membrane filtration [7], oxidation [8], reduction [9], flocculation [10], chemical precipitation [11], adsorption [12] and sonochemical [13] are available to tackle such type of pollutants. Among all these methods, photocatalysis is a significant method for the removal of organic pollutants and variety of semiconductor photocatalysts with different wavelengths are available in literature [14]. In solar energy, more than 43% of energy falls in the visible region and traditional photocatalysts, such as TiO2 have a large band gap (3.2 eV) and they require Ultraviolet light for photocatalysis [15]. Thus, these semiconductor photocatalysts cannot utilize the solar energy (visible light) in the catalysis process and have motivated the scientist community to develop efficient photocatalysts which can utilize the visible light of solar energy.
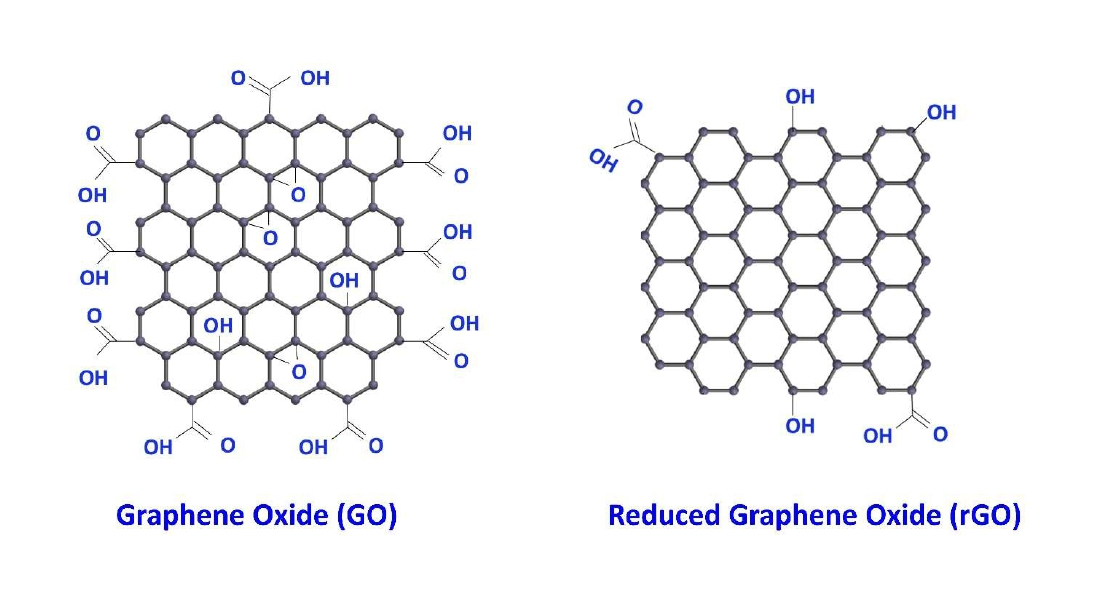
In modern era, nanotechnology, an emerging science; has fascinated scientists due to its novel properties. Plasmonic based photo catalyst is a promising agent for the reduction of synthetic dyes [16]. Graphene consists of a single layer of sp2 hybridized carbon atoms forming a two-dimensional (2D) hexagonal lattice and it possesses excellent properties such as high electron mobility, high young’s modulus, large specific surface area and excellent thermal conductivity [17,18]. Graphene oxide (GO) as shown in Figure 2, a monolayer sheet is in oxidized form and decorated by the oxygen-containing polar functionalities of graphite which can enhance its surface functionality and it can be widely used in a number of applications such as sensor, electronic, drug delivery, solar desalination, catalyst, biomedicine antibacterial coatings and water treatment [19-22].
Figure 2: Graphene Oxide and reduced Graphene oxide.
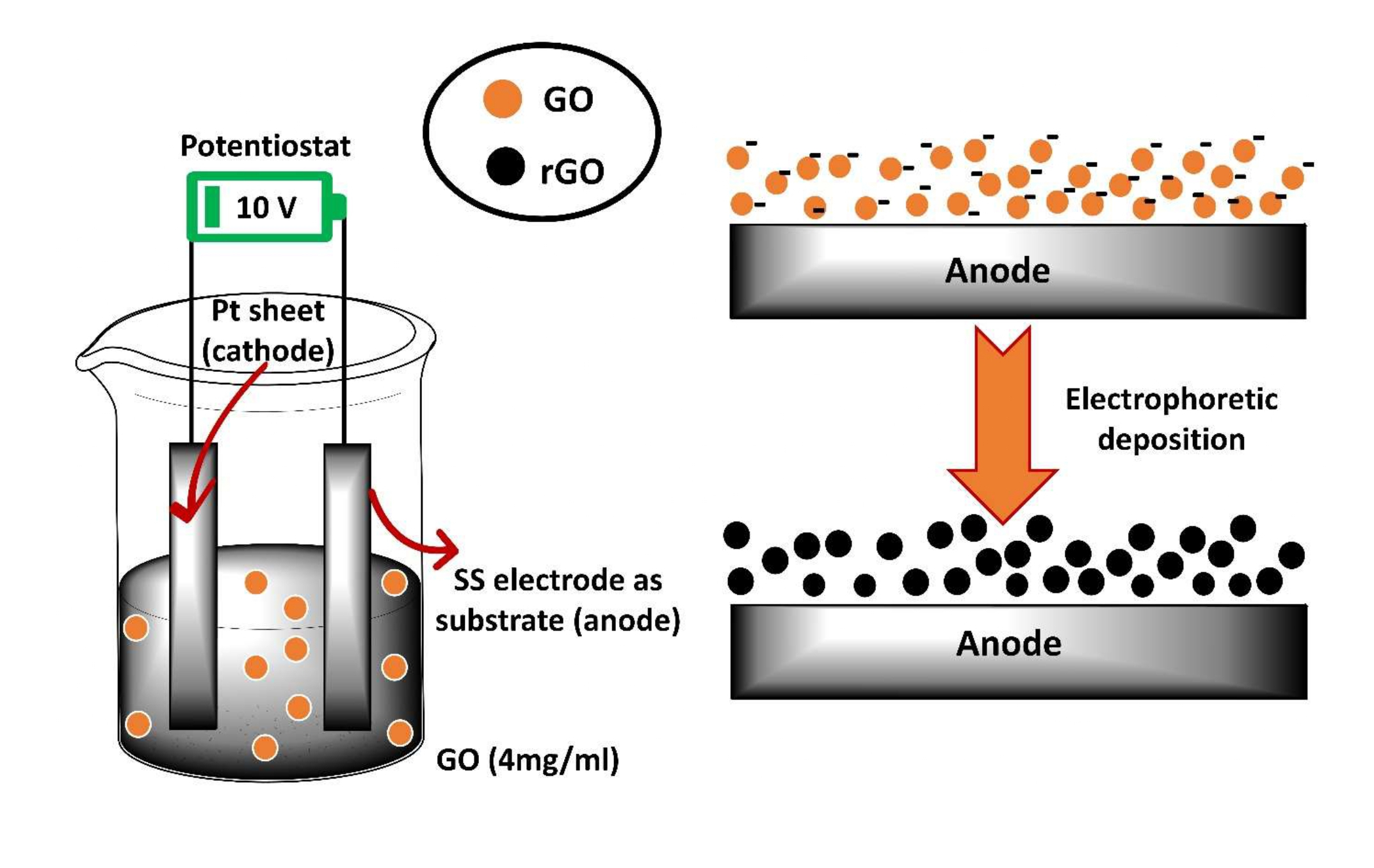
In order to enhance the surface area of GO, reduction of oxygen containing groups is required. The reduction of these oxygen containing groups, i.e., GO into rGO can be carried out by using various chemicals such as hydrazine, hydroquinone, sodium borohydride etc. as a simple method [23,24]. But these chemicals are toxic and hazardous and also cause the secondary environment pollution [25]. Hence, the need of environmental protection has led to enhance the interest in eco-friendly method for the reduction of GO into rGO. Keeping this thing in mind, electroplating method has been used for the reduction of GO into rGO. Figure 3 describes the synthesis of rGO on stainless steel by using potentiostat techniques [26]. The GO possess negative charge so it is attracted towards anode and get reduced and finally becomes rGO. In this method no chemical is required, by using simple techniques rGO is deposited successfully on the stainless steel.
Figure 3: Synthesis of reduced graphene oxide on stainless steel.
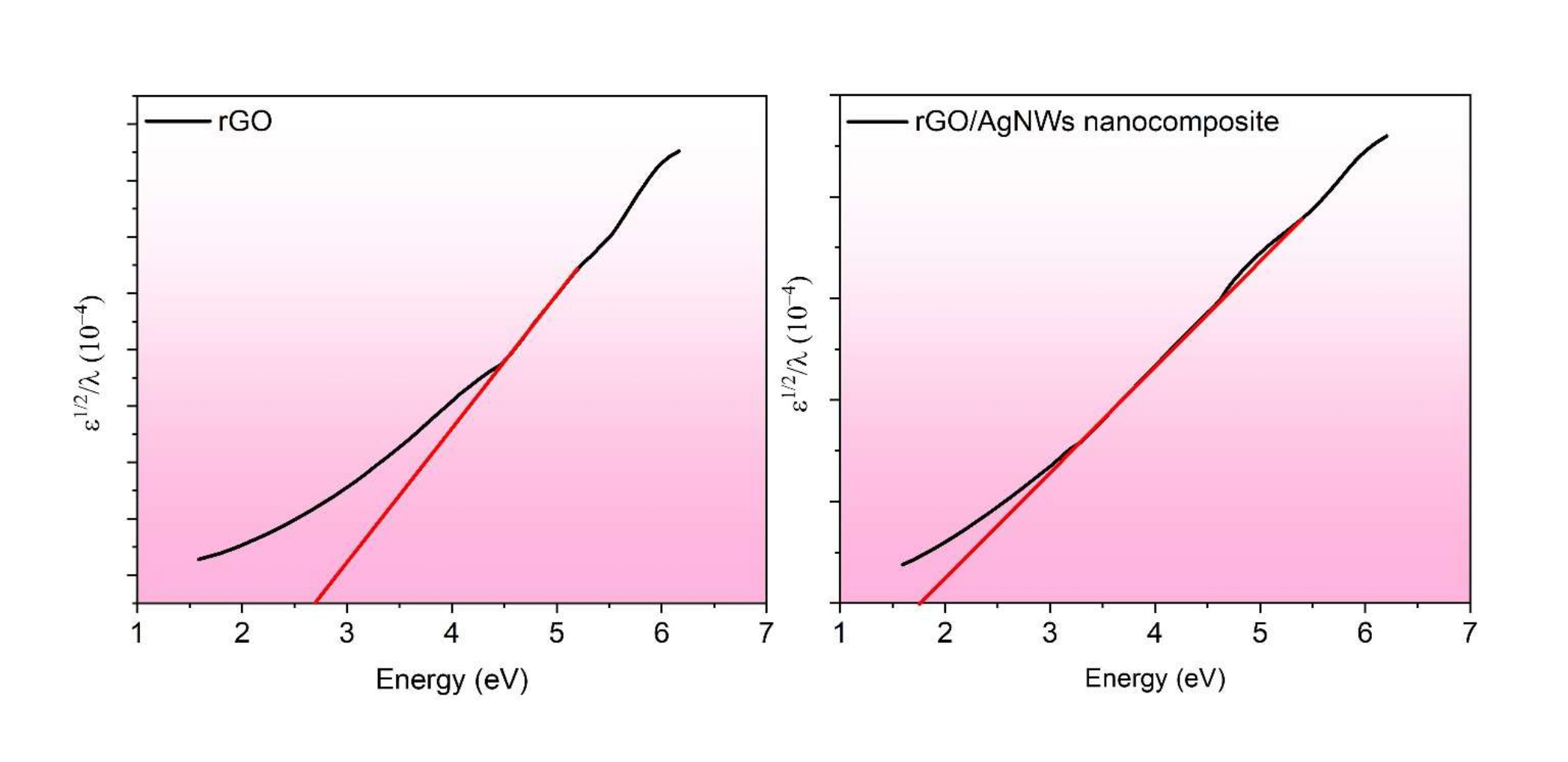
The required mass of rGO onto stainless steel can be altered by changing the synthesis procedure such as concentration of GO, electroplating time duration and applied voltage. Furthermore, the metal nanostructure has a suitable band position, Surface Plasmonic Resonance effect, photonic stability and its biocompatibility, which make them distinctive and acts as a substrate in the electron transfer reaction, which is necessary for the reduction of any reactant. The decoration of AgNWs onto the surface of rGO layer has been carried out in order to enhances its surface area. Also, the presence of AgNWs onto rGO is responsible for the tunning of band gap of material which has been confirmed from the Tauc plot. The optical band gap of rGO and rGO/AgNWs has been calculated from the UV-Vis absorption spectra by using the Tauc's equation [27]. Tauc plot of the bandgap of synthesized rGO and rGO/AgNWs nanocomposite is shown in Figure 4.
Figure 4: Tauc plot of the bandgap of rGO and rGO/AgNWs nanocomposite.
The bandgap of the rGO is found to be decreased from 2.69 eV to 1.74 eV in the presence of AgNWs. It is also confirmed that the bandgap decreases non-linearly by virtue of the presence of AgNWs. The results reveal that the presence of AgNWs onto rGO will be responsible for the decline in the wide-bandgap and enhancement in photocatalytic reduction activity. Hence, the synthesized GO/AgNWs nanocomposite has suitable band position, Surface Plasmonic Resonance effect, photonic stability and its biocompatibility.
The specific surface area is an important property of nanocomposite and play an important role in catalysis application.
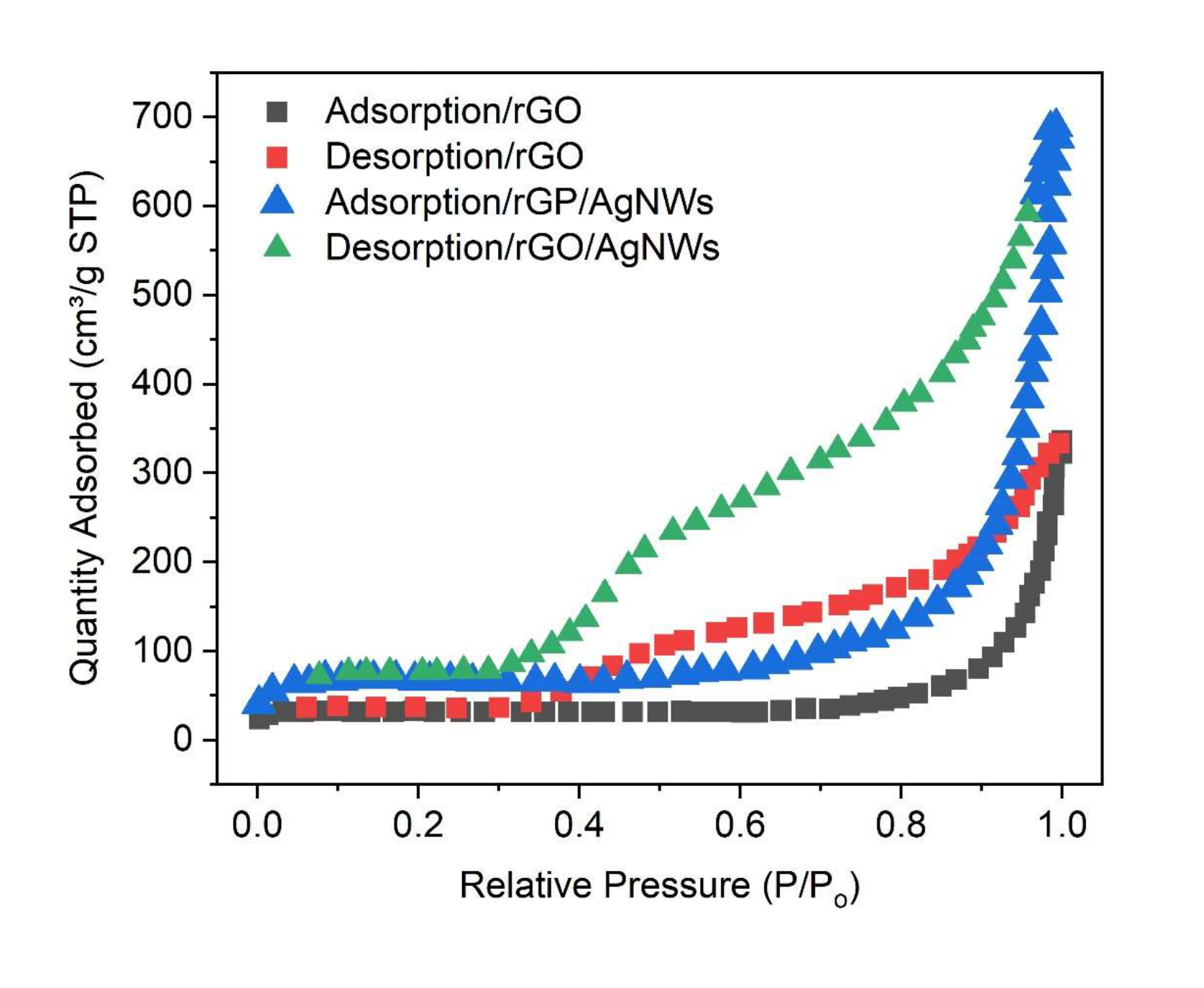
In order to explore the Brunauer–Emmett–Teller (BET) surface area, N2 adsorption and desorption has been performed and displayed in Figure 5. The isotherm curve of both rGO and rGO/AgNWs reveal that both the samples are porous in nature with a hysteresis loop at high partial pressures. The total active specific surface area of rGO is about 150.123 m2/g. However, the value of active specific surface area in case of rGO/AgNWs is increased as compared to rGO, which also confirms the porous nature of rGO/AgNWs nanocomposite as confirmed from FE-SEM micrographs.
Figure 5: BET Surface area of rGO and rGO/AgNWs nanocomposite.
Conclusion
It can be concluded that the problem related to deletion of methylene blue dye from aqueous solution has been resolved by using the photocatalyst based on reduced graphene oxide decorated with AgNWs. The modification of GO has been carried out by the reduction of GO into rGO through electroplating method without the usage of any toxic and hazardous chemicals. Moreover, the silver nanowires have been also decorated onto surface of rGO which increase its surface area, porosity and conductivity of the sample which is essential for photocatalyst in catalysis application. The rGO/AgNWs is efficient photocatalyst with tuneable band gap and higher surface area for the degradation of MB dye in aqueous solution. Furthermore, it is expected that this short note may provide the knowledge of promising photocatalyst based on rGO/AgNWs for effective photocatalytic degradation of methylene blue from the aqueous solution.
Conflicts of Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this short communication.
References
2. Kasozi DM, Gromer S, Adler H, Zocher K, Rahlfs S, Wittlin S, et al. The bacterial redox signaller pyocyanin as an antiplasmodial agent: comparisons with its thioanalog methylene blue. Redox Report. 2011 Jul 1;16(4):154-65.
3. Dorman N. Citations. BioTechniques 2012; 53:2, 69.
4. Singh J, Dhaliwal AS. Plasmon-induced photocatalytic degradation of methylene blue dye using biosynthesized silver nanoparticles as photocatalyst. Environmental Technology. 2018 Nov 4.
5. Aadil KR, Mussatto SI, Jha H. Synthesis and characterization of silver nanoparticles loaded poly (vinyl alcohol)-lignin electrospun nanofibers and their antimicrobial activity. International Journal of Biological Macromolecules. 2018 Dec 1;120:763-7.
6. Verma S, Rao BT, Singh R, Kaul R. Photocatalytic degradation kinetics of cationic and anionic dyes using Au–ZnO nanorods: Role of pH for selective and simultaneous degradation of binary dye mixtures. Ceramics International. 2021 Sep 3.
7. Singh J, Thakur S, Sehgal R, Dhaliwal AS, Kumar V. Surface Engineering of Nanofiber Membranes via Electrospinning-Embedded Nanoparticles for Wastewater Treatment. In: Electrospun Nanofibers 2021; 251-283.
8. Ullah AA, Kibria AF, Akter M, Khan MN, Tareq AR, Firoz SH. Oxidative degradation of methylene blue using Mn 3 O 4 nanoparticles. Water Conservation Science and Engineering. 2017 Mar 1;1(4):249-56.
9. Siong VL, Lee KM, Juan JC, Lai CW, Tai XH, Khe CS. Removal of methylene blue dye by solvothermally reduced graphene oxide: a metal-free Adsorption and Photodegradation Method. RSC Advances. 2019;9(64):37686-95.
10. Mahmoud MS, Farah JY, Farrag TE. Enhanced removal of Methylene Blue by electrocoagulation using iron electrodes. Egyptian Journal of Petroleum. 2013 Jun 1;22(1):211-6.
11. Shokoohi R, Khazaei M, Godini K, Azarian G, Latifi Z, Javadimanesh L, et al. Degradation and mineralization of methylene blue dye by peroxymonosulfate/Mn3O4 nanoparticles using central composite design: Kinetic study. Inorganic Chemistry Communications. 2021 Mar 2:108501.
12. Singh J, Dhaliwal AS. Effective Removal of Methylene Blue Dye Using Silver Nanoparticles Containing Grafted Polymer of Guar Gum/Acrylic Acid as Novel Adsorbent. Journal of Polymers and the Environment. 2021 Jan;29(1):71-88.
13. Roosta M, Ghaedi M, Daneshfar A, Sahraei R, Asghari A. Optimization of the ultrasonic assisted removal of methylene blue by gold nanoparticles loaded on activated carbon using experimental design methodology. Ultrasonics Sonochemistry. 2014 Jan 1;21(1):242-52.
14. Singh J, Dhaliwal AS. Novel green synthesis and characterization of the antioxidant activity of silver nanoparticles prepared from Nepeta Leucophylla root extract. Analytical Letters. 2019 Jan 22;52(2):213-30.
15. Xie W, Li R, Xu Q. Enhanced photocatalytic activity of Se-doped TiO2 under visible light irradiation. Scientific Reports. 2018 Jun 8;8(1):1-0.
16. Singh J, Kumar S, Dhaliwal AS. Green synthesis of silver nanoparticles using Ocimum tenuiflorum leaf extract: Characterization, antioxidant and catalytic activity. In: AIP Conference Proceedings, AIP Publishing LLC. 2021 Aug 5; 2352(1): 040017.
17. Papageorgiou DG, Kinloch IA, Young RJ. Mechanical properties of graphene and graphene-based nanocomposites. Progress in Materials Science. 2017 Oct 1;90:75-127.
18. Xu M, Liang T, Shi M, Chen H. Graphene-like two-dimensional materials. Chemical Reviews. 2013 May 8;113(5):3766-98.
19. Kumar YR, Deshmukh K, Sadasivuni KK, Pasha SK. Graphene quantum dot based materials for sensing, bio-imaging and energy storage applications: a review. RSC Advances. 2020;10(40):23861-98.
20. Homaeigohar S, Elbahri M. Graphene membranes for water desalination. NPG Asia Mater 9: e427.
21. Singh J, Dhaliwal AS. Water retention and controlled release of KCl by using microwave-assisted green synthesis of xanthan gum-cl-poly (acrylic acid)/AgNPs hydrogel nanocomposite. Polymer Bulletin. 2020 Sep;77(9):4867-93.
22. Singh J, Kumar S, Dhaliwal AS. Controlled release of amoxicillin and antioxidant potential of gold nanoparticles-xanthan gum/poly (Acrylic acid) biodegradable nanocomposite. Journal of Drug Delivery Science and Technology. 2020 Feb 1;55:101384.
23. Yu W, Sisi L, Haiyan Y, Jie L. Progress in the functional modification of graphene/graphene oxide: A review. RSC Advances. 2020;10(26):15328-45.
24. Khojasteh H, Salavati-Niasari M, Safajou H, Safardoust-Hojaghan H. Facile reduction of graphene using urea in solid phase and surface modification by N-doped graphene quantum dots for adsorption of organic dyes. Diamond and Related Materials. 2017 Oct 1;79:133-44.
25. Singh J, Dhaliwal AS. Synthesis, characterization and swelling behavior of silver nanoparticles containing superabsorbent based on grafted copolymer of polyacrylic acid/Guar gum. Vacuum. 2018 Nov 1;157:51-60.
26. Singh J, Dhaliwal AS. Electrochemical and photocatalytic degradation of methylene blue by using rGO/AgNWs nanocomposite synthesized by electroplating on stainless steel. Journal of Physics and Chemistry of Solids. 2022 Jan 1;160:110358.
27. Makuła P, Pacia M, Macyk W. How to correctly determine the band gap energy of modified semiconductor photocatalysts based on UV–Vis Spectra. Journal of Physical Chemistry Letters. 2018; 9(23):6814-6817.