Abstract
Introduction: Changes in the oxygen concentration of cellular microenvironments play a significant role in regulating cell function during muscle regeneration. Generally, most in-vitro cell culture experiments have been carried out in atmospheric conditions with 21% O2, which compared to the actual micro-environment of mature skeletal muscle of between 1% and 10% pO2 is extremely hyperoxic [1]. Culturing skeletal muscle cells in vitro within their typical physiologically hypoxic environment in situ (2-10% pO2) has been shown to increase proliferation rate, reduce apoptosis and increase multiple MRF gene expressions, compared to culturing in a normoxic environment (21% O2). However, chronic exposure (>24 hours) to a semi-severe hypoxic environment (≤ 5% O2) can lead to a decrease in cell proliferation and differentiation [2]. The effects of acute hypoxic exposure (24 hour) has limited research and could be important in understanding the effects of hypoxia on skeletal muscle during brief exposures such as those observed within intermittent hypoxic training programmes. The purpose of this work was to examine the role of acute hypoxia (24 hour) on C2C12 proliferation and relevant gene expression in 2D culture.
Methods: C2C12 mouse myoblast cells were seeded into six well plates. The cells were maintained in DMEM with 20% Foetal Bovine Serum (FBS) and 1% Penicillin/Streptomycin (Pen/Strep). C2C12 myoblasts were either exposed to 21% or 5% O2. The effects of acute hypoxic exposure (24 hour) at different time points during the proliferative phase of myogenesis, rather than chronic exposure, on cell proliferation, cellular viability and myogenic regulatory factor gene expression was examined. At 24, 48, 72, and 96 hours, RNA was extracted using the Trizol® method and mRNA expression of myogenic regulatory factors, myoD, myf5 and myogenin were detected using the 2-ΔΔCT method. Cell counts and cell viability were also quantified.
Results: No significant difference was found between cells cultured in normoxic conditions (21%) and those that were exposed to low oxygen conditions for 24 hours at various time points over a 96 hour culture period, with regards to proliferation rate, cell viability, and myogenic regulatory gene expression (Myf5, MyoD and Myogenin).
Discussion: The effect of acute low oxygen exposure lasting 24 hours appears to not be insufficient in having an effect on the proliferation rate, viability or transcription factor expression of C2C12 cells during the proliferative phase of myogenesis.
Introduction
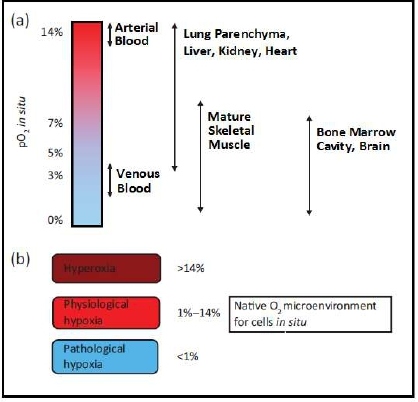
Within human tissue, oxygen concentrations are considerably less than the 21% O2 concentrations found in ambient air and is termed physiological hypoxia. Typically, the partial pressure of oxygen (pO2) of human tissue lies between 1% and 14% [3], with considerable local and regional variations (Figure 1). Within the tissues’ own microenvironment, changes in oxygen concentrations play a significant role in regulating cell function and can have effects including maintenance of stem cell pluripotency, angiogenesis, stem cell differentiation, ATP regulation, and pH regulation [3-5].
Satellite cells are muscle-resident stem cells found below the basal lamina adhered to the muscle fibres [6]. Activation of the quiescent satellite cells is brought about in order to regenerate or repair muscle tissue, which is generally due to a stress induced change in their micro-environment, caused by either weight bearing exercise or injury [7-9]. Once activated the stem cells undergo proliferation at which point, they are known as adult myoblasts. These then differentiate and fuse into multinucleated skeletal muscle fibres [10.11]. This process, known as myogenesis, is dependent on several transcription factors known as myogenic regulatory factors (MRFs); MyoD, Myf5, Myogenin and MRF4 [2,12-14]. Prior to activation the quiescent satellite cells express the transcription factor PAX7 [10]. Once activated within 12 hours, there is a rapid up-regulation of MyoD and Myf5, along with PAX7 and all possess distinct functional roles [2]. Myf5 is a regulator for muscle progenitor proliferation, whilst MyoD is necessary for the consequent differentiation of these precursors [2]. During the proliferation phase of myogenesis, there is co-expression of both MyoD and Myf5 [15].
Figure 1: (a) Values of micro-environmental O2 found in tissues in situ (b) O2 micro-environment for cells residing within different tissues in mammalian body. Image adapted from Stamati et al. [3]; values taken from Warkerntin [16], Das et al. [17] & Li et al. [18].
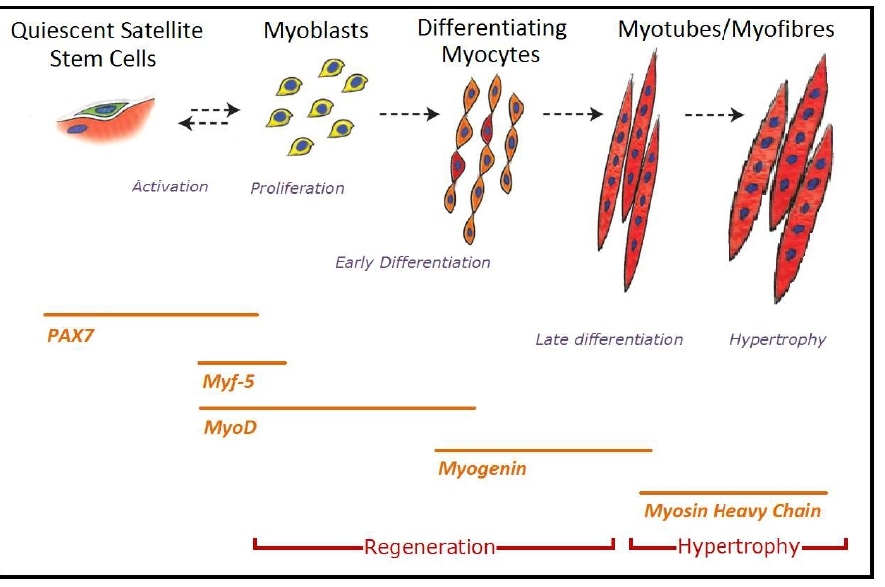
With the co-existence of MyoD and Myf5, the genes activate the upregulation of Myogenin. MyoD and Myogenin stimulate the myoblasts to undergo terminal differentiation and fusion into myofibers through the activation of genes in mature muscle such as the Myosin heavy chain [11,14]. Subsequently P21, a cell cycle arrest protein, is activated, causing an irreversible exit of the myoblast from the cell cycle. It is also known that MRF4 is actively expressed during the differentiation phase and continues to be released once newly regenerated myofibers have been fused, indicating that MRF4 has a role in myofiber maturation [19] (Figure 2).
Figure 2: (a) Schematic of the stages of myogenesis from quiescent satellite cells to myotube formation and hypertrophy, along with an indication of the time periods at which various genes are expressed. Image adapted from Bentzinger et al. [20].
The local microenvironment, otherwise known as the stem cell niche, within the skeletal muscle that accommodates quiescent satellite cells is generally between 1% and 10% pO2 [1]. Stress induced changes of the oxygen concentration caused by trauma or weight bearing exercise is believed to be one of the main physiological regulators of satellite cell activity and activation, indicating manipulation of pO2 exposure could be an effective means of improving muscle regeneration [21]. There is some physiological data of local interstitial pO2 which has been evaluated using a number of techniques, including oxygen microelectrodes, microspectrophotometry, proton magnetic resonance spectroscopy and phosphorescence quenching [22,23]. However, the exact systemic environment of satellite cells that precisely regulates quiescence or activation, and self-renewal or differentiation, is largely unknown [21].
Generally, most in vitro cell culture experiments have been carried out in atmospheric conditions with 21% O2, which compared to the actual micro-environment of mature skeletal muscle of between 1% and 10% pO2 is extremely hyperoxic [1]. To develop a better understanding of the molecular mechanisms behind myogenesis and the cell cycle in-vitro, it has been suggested that experiments should be performed in environmentally low conditions that mimic the normal physiological environments. Research in this area has shown that satellite cells cultured in physiologically hypoxic conditions (2-10% O2) result in a significant increase in proliferation rate, improved survival of mature fibres, decreased satellite cell adipogenesis, promoted myogenesis and larger myotube formations, compared to cultures grown in 21% pO2 [24]. It is also noted that the signalling pathways responsible for regulating stem cell proliferation and differentiation are impacted by the O2 concentration of the surrounding micro-environment. With regards to myogenesis and MRF expression, during the proliferation phase there is a significant up-regulation of MyoD, Myf5 and Myogenin when C2C12 murine cells are cultured in 6% O2 conditions compared to 20% [25].
When the pO2 is dropped below 2% within the microenvironment, also known as pathological hypoxia, the expression of differentiation markers (MyoD, Myf5, and Myogenin) begin to be inhibited, along with the inhibition of quiescent satellite cell activation and of multinucleated myotube formation [26]. Furthermore, there is a notable reduction in satellite cell proliferation and increased apoptosis [27,28]. The inhibition of myogenic differentiation is found to be through accelerated MyoD degradation by the ubiquitin-proteasome pathway [28].
In general the atmospheric conditions used in traditional muscle cells culture in vitro is largely ignored, and recent experiments have found culturing cells in more physiologically atmospheric conditions may be more beneficial for myogenesis and lead to a better understanding of the molecular processes behind skeletal muscle repair and regeneration [29]. Whether acute exposure of low oxygen on muscular regeneration is as beneficial as chronic exposure is yet to be examined, and may play a vital role in using the manipulation of O2 concentrations in improving muscle self-renewal, damage repair and treating regenerative disease [1].
The effects of changes in intramyocellular oxygen pressure caused by resistance training on muscle morphology is limited. However growing research suggests that a moderately hypoxic muscle environment may partially contribute to in vivo muscle hypertrophic responses to blood flow restriction training and resistance training under hypoxic conditions [30-32]. In Vitro studies have also found that culturing cells in a moderately hypoxic environment (6-15% pO2) promotes muscle differentiation and induces skeletal muscle hypertrophy [25,33]. However, a number of differences exist between in vivo and in vitro study environments with regards to the length of exposure to the hypoxic environment. In vitro the hypoxic exposure is continuous throughout the culture process, while the intramyocellular hypoxic environment created by resistance training under hypoxic conditions and blood flow restriction studies is generally acute. The effect of acute hypoxic exposure (24 hours) on muscle differentiation and hypertrophy in vitro is unknown.
The aim of the following study is to further support the ever-growing research that culturing cells in a physiologically hypoxic environment is optimal for myogenesis compared to an ambient atmosphere. Specifically, the following study aims to establish the effects of 24 hours of acute physiological hypoxia (5% pO2) during the proliferative phase of myogenesis, including proliferation rate, cellular viability and myogenic regulatory factor gene expression of proliferating C2C12 myoblasts.
Method
Cell culture
C2C12 murine myoblasts were provided by the Health Protection Agency Culture Collections (HPA Cultures, Salisbury, UK). Three separate lines of C2C12’s within 1.8 ml vials were removed from liquid nitrogen and quickly defrosted. The cells were plated at 12,500 cells/cm2 in T80 culture flasks (80 cm2 Flasks, NUNC, Denmark) and suspended in 20 ml of growth medium (GM) consisting of high glucose Dulbecco’s Modified Eagle’s Medium (DMEM) (Sigma-Aldrich, Haverhill, UK), 20% Foetal Bovine Serum (FBS) (PAA, Paisley, UK) , and 1% penicillin/streptomycin (pen/strep) (Sigma-Aldrich, Haverhill, UK). Once suspended the cells were kept within a humidified incubator (Heracell 240i CO2 Incubator, Thermo Fisher Scientific Inc, USA) where conditions were kept constant at 37°C in a normoxic environment (21% O2, 5% CO2, 74 % N2). Growth medium was replaced every 48 hours, and once cells had reached 80% confluence, cells were passaged.
To ensure the line of C2C12 cells used within this experiment could undergo differentiation, a T80 flask was seeded at a density of 12,500 cells/cm2 and cultured for 96 hours until 100% confluent. Once confluency had been achieved the GM was replaced with differentiation media (DM) to promote myoblast fusion (2% Horse Serum, 1% P/S, and DMEM) (Sigma-Aldrich, Haverhill, UK) and left to culture for a further 48 hours. The cells were then fixed and stained for immunocytochemistry (Figure 3).
Figure 3: Murine C2C12’s in culture immunostained for desmin (red) with a DAPI nuclear counter stain (blue). Note the formation of myotubes indicating the line of C2C12’s used were able to undergo fusion into multinucleated myotubes, Scale bar = 20 µm.
Once a passage number of seven was attained the cells were split and seeded into eight, six well plates (NUNC A/S, Denmark) in 2 ml of Growth medium at a density of 1500 cells/cm2. The plate layout consisted of three wells for qPCR, one well for immunocytochemistry containing three 13 mm sterile glass coverslips, one well for cell counts and microscopy, and one well for checking the oxygen concentration within the microenvironment of the C2C12’s (Figure 4).
Figure 4: Representation of the six well plate layout.
Cells were sampled for RNA and immunostaining every 24 hours up to a 96 hour timepoint. During this time, the C2C12 cells were cultured in a humidified incubator (Heracell 240i CO2 Incubator, Thermo Fisher Scientific Inc, USA) at 37 °C under normoxic conditions (21% pO2). The hypoxic treatment involved an acute exposure of hypoxia during the final 24 hours of culture under what would be expected to be a physiological hypoxic condition (5% pO2). This was achieved by placing the cells within a SANYO CO2/O2 hypoxic incubator (MCO-5M, Panasonic, USA) at 37°C (5% O2, 5% CO2, 90% N2). Once the plates were removed from their incubators, they were sampled immediately. A schematic of the experimental design is shown in Figure 5. The GM was refreshed within the plates every 24 hours, prior to being placed in either the normoxic or hypoxic incubators. The experiment was repeated three times using three separate lines of C2C12’s.
Figure 5: Schematic diagram of the study design. N: Normoxic Exposure; H: Normoxic with 24 hour hypoxic exposure.
To ensure the microenvironment in which the cells were cultured was at a concentration of 5% O2 during the hypoxic exposure, a dip-type oxygen microelectrode (MI-730 O2 Microelectrode, Microelectrodes Inc, Bedford, UK) along with PowerLab 4/25T with LabChart Software (ADinstruments, New Zealand) was used. The probe was calibrated according to manufacture instructions and placed within the GM of one of the C2C12 inhabited wells through a drilled hole on the roof of the plate. Oxygen concentrations were checked at the end of each 24 hour time period for 3 minutes to ensure a constant 5% concentration of oxygen had been reached.
Cell counts and microscopy
The hypoxic and normoxic exposed cells were removed from their incubators and analysed using an LED microscope (Leica DM IL LED, Leica Microsystems, Wetzler, Germany) at 24 hour time points throughout the four day culture period and ten images were taken within the designated well for imagery. A cell count of viable cells was performed using a haemocytometer with Trypan blue exclusion. This was achieved by removing the GM from the monolayer of cells and adding 1 ml of Trypsin. Warm Phosphate Buffered Saline solution (PBS) was not placed on the cells so a count of the non-viable adhered cells could be carried out. Once the Trypsin was added, the plate was incubated at 37°C for five minutes with occasional disturbance to displace the cells from the wells. Using the LED microscope, the cells were checked for detachment followed by adding 2 ml of growth media (GM) to neutralise the effect of the Trypsin. If cells were still confluent, the serum was carefully triturated using a pipette or passed through a filter.
Immunocytochemistry
Within one of the wells of the six well plates, C2C12 cells were cultured on three 13 mm sterile glass coverslips (Sigma-Aldrich, Haverhill, UK). At the end of the designated exposure time in either the normoxic or hypoxic chamber, cells were fixed using ice-cold fixative solution consisting of one part methanol, to one part acetone (1 ml). The monolayer of cells were permeabilized using a solution of 1 x TRIS buffered saline solution (TBS) along with 5% normal goat serum (GS) and 0.2% Triton X-100 for 30 minutes. Following a thorough wash with TBS, a rabbit monoclonal anti-mouse desmin primary antibody (Sigma-Aldrich, Haverhill, UK) diluted 1 in 200 in TBS solution with 2% GS and 0.2% Triton X-100 was added to the cells and left at room temperature for two hours. A thorough TBS wash ensued before being treated with a goat anti-rabbit TRITC secondary antibody (Sigma-Aldrich, Haverhill, UK) diluted 1 in 200 in TBS with 2% GS and 0.2% Triton X-100 and left for one hour in room temperature with no light exposure. Finally, following a TBS wash, a fluorescent minor-groove DNA binding probe DAPI (4,6-diamidino-2-phenylindole; 1.0ng/ml; Sigma Aldrich, Haverhill, UK) was added to act as a nuclear stain and left for 10 minutes. Once completed the coverslips were thoroughly washed using TBS, dried, before being mounted on glass microscope slides using MOWIOL mounting medium. Examination was carried out by immunofluorescent microscopy using a Leica DM2500 microscope and Leica software LAC V3.7 (Leica Microsystems, Wetzler, Germany).
RNA extraction and quantitative PCR
Levels of proliferation and the onset of differentiation of the 2D culture was determined by quantitative PCR (qPCR) analysis of MyoD, Myf5, and Myogenin expression. Total RNA was extracted from the cells using the TRIzol® reagent protocol according to manufacturer instructions (Invitrogen). qPCR was run using the Stratagene Mx3005P PCR System (Applied Biosystems, Warrington UK). The PCR reaction mixture (10 µl SYBR Green, 0.2 µl RT mix, 0.15 µl forward primer, 0.15 µl reverse primer) was created for each gene to be analysed in duplicate over a 96 well plate (Applied Biosystems). Of this reaction mix, 10.5 µl of solution was added to each well along with 9.5 µl of solution containing 70 ng of RNA dissolved in RNAse-free water. The plate was incubated at 50°C for 10 minutes and 95°C for 5 minutes before undergoing 40 cycles of 95°C for 10 seconds and 60°C for 30 seconds, a thermal profile specifically designed by Applied Biosystems for use with assays designed according to Applied Biosystems assay design guidelines. The primer sequences used are presented in Table. 1.
|
Gene |
Primer Sequence |
|
MyoG |
F: CCAACTGAGATTGTCTGTC |
|
(GenBank NM_153798.2) |
R: GGTGTTAGCCTTATGTGAAT |
|
MyoD |
F: CATTCCAACCCACAGAAC |
|
(GenBank NM_010866) |
R: GGCGATAGAAGCTCCATA |
|
Myf5 |
F: CGTATTATGAACTCTCTC |
|
(GenBank NM_008656) |
R: CAAGACAGTATTTACAAC |
|
RPII-B (Housekeeper Gene) |
F: GGTCAGAAGGGAACTTGTGGTAT |
|
(GenBank NM_031189.2) |
R: GCATCATTAAATGGAGTAGCGTC |
A cycle threshold (CT) was defined as the fractional cycle number at which the fluorescence generated by cleavage of the probe exceeded a fixed threshold above the baseline. A Comparative CT method outlined by Applied Biosystems, was used to quantitate the amount of each target gene present. The mean CT values of the duplicate samples from each sample was determined and normalised to RPII, which acted as the endogenous housekeeping gene. Relative quantitation of mRNA expression was calculated using the ddCT method, using the 2-??Ct formula [34].
Statistical analysis
A mixed measures ANOVA was performed to assess differences across treatments (Hypoxia and Normoxia) and across time (24, 48, 72, and 96 hours). Paired t-tests were used to compare between Normoxia and Hypoxia treatment differences at individual time points. Significance was accepted when P<0.05. Statistical tests were conducted using SPSS version 19.0 (SPSS, Inc, Chicago, Illinois, USA). Data are reported as mean ± SD.
Results
Cell counts and viability
The Haemocytometer assay with Trypan blue exclusion showed no significant difference in proliferation rates between cells cultured under normoxia or acute hypoxia at any of the 24 hour time points (Figure 6). However, collectively over time, there was a notable increase in Total Cell Count with significant differences between time points. There was no significant difference in cell counts between 24 hours and 48 hours, although following 72 hours of culture, cell counts were significantly higher than those taken after 48 hours (P<0.026). Total cell counts after 96 hours of culturing were significantly higher than those taken at 24 hours (P<0.003) and 48 hours (P<0.002). Also there was a notable rise in cell counts between 72 hours and 96 hours of culturing but this was found to be non-significant ( P<0.126).
Figure 6: Total cell count of adhered cells, taken every 24 hours over a 96 hour period in vitro, as determined by a haemocytometer. Values expressed as mean ± standard deviation. No significant differences were observed between Normoxic and Hypoxic exposure at any time point with total cell counts (P>0.05).
The percentage of viable cells within the total cell count did not differ significantly at any of the 24 hour time points between cells cultured under normoxia or acute hypoxia. Although there is a possible trend towards a reduction in cell viability after 48 hours before recovering at 72 and 96 hours. Over time, cell viability did not differ between any of the 24 hour time points over the 96 hour culture period (Figure 7).
Figure 7: Percentage of viable cells adhered to coverslips taken every 24 hours over a 96 hour period in vitro, as determined by haemocytometer and Trypan blue exclusion. Hypoxic treatment involved acute hypoxic exposure of 5% O2 during the final 24 hours of culturing. Values expressed as mean ± standard deviation.
Quantitative PCR analysis
Quantative PCR analysis found no significant difference in relative Myf5, MyoD and Myogenin mRNA expression levels between the two exposure conditions of normoxia or acute hypoxia following 24, 48, 72, and 96 hours of culturing (P>0.05) (Figure 8). Concerning changes in mRNA expression levels over time, no differences were found between any of the time points when analysing Myf5 and MyoD, however there does appear to be a trend toward Myf5 expression increasing over time in both conditions. Myogenin mRNA expression levels following 24 hours of culturing were significantly higher than those expressed following 48 hours (P<0.06), 72 hours (P<0.011) and 96 hours (P<0.014) of culturing with both treatments.
Figure 8: Relative Myf5, MyoD and Myogenin mRNA expression levels from 2D C2C12 cultures, taken every 24 hours over a 96 hour period in vitro, as determined by qPCR. CT values were normalised to an internal housekeeping gene (RPI) and expressed relative to levels recorded following 24 hours of culture in normoxic conditions n=6. Significant difference between timepoints at *P<0.05. Values expressed as mean ± standard deviation. No significant differences were observed between Normoxic and Hypoxic exposure at any time point with all three genes. (P>0.05).
Figure 9: Murine C2C12’s in vitro. C2C12 cells were cultured in GM for a total of four days. Cells were fixed every 24 hours. (A) Cells incubated under normoxic conditions (20% O2) (B) Cells incubated under normoxic conditions (20% O2) and exposed to acute hypoxic exposure (5% O2) during the final 24 hours of culturing. Images selected randomly from a selection of ten. Scale bar=20 µm.
Discussion
The aim of this study was to establish whether acute low oxygen exposure influences the proliferation rate, viability and MRF expression of C2C12 cells in vitro. This study has shown that C2C12 myoblasts are unaffected following acute exposure to a low oxygen environment during the proliferative phase of myogenesis. The hypoxic exposure which involved exposure of 5% O2 during the final 24 hours of culturing up to and including 96 hours of total culture time, elicited no significant change in proliferative rate, cell viability or mRNA expression of Myf5, MyoD and Myogenin compared to cells grown in atmospheric conditions. Further analysis of these images also indicated no myotube formation had occurred following both the normoxic and hypoxic treatments during this period.
It is generally accepted that most stem cells that are cultivated in lower, physiologic oxygen environments, proliferate more than in 21% O2 environments [1,35,36]. This improvement in proliferative capacity in low oxygen has been found in a variety of stem cells including, murine CNS derived multipotent stem cells [37], marrow-derived mesenchymal stem cells [38], and adult murine skeletal muscle satellite cells [2,25]. Regarding the cultivation of skeletal muscle satellite cells, the increase in proliferative capacity has been found when satellite cells were cultured between 2% and 10% pO2. The enhanced proliferation of stem cells in lower oxygen may in part reflect the typical relatively low oxygen microenvironment that the satellite cell are found in vivo which is usually around 2-10% pO2 [39,40]. Although the C2C12 cells used in this study are a mouse myoblast cell line typically cultivated in atmospheric conditions. Therefore 21% O2 could be considered normoxic whilst 5% O2 would be deemed extremely hypoxic for C2C12 cells. Generally, the increase in proliferation rate elicited is believed to be down to the effect the change in O2 has on the signalling pathways that regulate stem cell proliferation and MRF expression. Specifically chronic low oxygen exposure (2 - 10% pO2) has been found to result in a significant up-regulation of MyoD, Myf5 and Myogenin in a number of skeletal muscle cell lines [25,41,42].
This experiment found no change in proliferative capacity or MRF expression when cells were cultured in a low oxygen environment, although this is the first experiment of its kind to look at the effects of acute hypoxic exposure (24 hours) at different time points during the proliferative phase of myogenesis, rather than chronic exposure. The design study aimed to establish whether acute exposure is effective at eliciting a change in proliferative capacity or MRF expression and if so then at which stage of the proliferative phase of myogenesis should it be used. Sato et al. looked at the effect of culturing C2C12’s continuously for 122 hours at 2%, 5% and 10% oxygen levels. Interestingly they found that growth rate of the cells in the hypoxic environments was the same as cells under normal conditions for up to 2 days after plating before growth rate increased [27]. Lui et al. [22] found that the initial 48 hours of culturing primary murine myoblasts in 1% pO2 affected myoblast fate decision by promoting self-renewal and inhibiting differentiation, but overall cell proliferation was not affected. However, earlier research by Csete et al. [25] did still find a significantly improved rate of proliferation of satellite cells found on cultured single muscle fibres following as little as 24 hours of hypoxic exposure at 6% O2. Nevertheless, the results of this study with that of recent literature, do tend to indicate 24 hours of exposure to a low, physiological oxygen environment may not be an adequate exposure time to induce an effect on cell proliferation rate. It is still unclear whether the hypoxic condition used in the present study reflects the drop in intramyocellular oxygen pressure induced by exercise, muscular damage or hypoxic exposure [27,43], however when using manipulation of O2 concentrations in improving muscle self renewal for damage repair or treating regenerative disease, it may be possible that exposure times must be longer than 24 hours to be effective.
Concerning the effects of acute low oxygen exposure has on MRF expression, this study found no difference over time with regards to Myf5, MyoD and Myogenin expression. As well as chronic low oxygen exposure being known to increase expression of all these transcription factors, short term exposure has been shown to upregulate the myogenic regulatory factors Myf5 and MyoD when at 6% O2 [25]. When culturing cells in more extreme hypoxic conditions (2 - 0.1% pO2), there appears to be a down regulation of Myf5 and MyoD expression [22,27,44]. As no research has yet looked at the effects of acute exposure at 5% O2 has on these specific MRF’s, it may be possible that 5% O2 is the cut-off point between which hypoxia either starts having an up-regulatory or down-regulatory effect on Myf5 and MyoD and expression. Concerning myogenin, its expression has been shown not to differ significantly by cells under normoxic or hypoxic environment for up to 96 hours after induction [27] supporting our findings and indicating as to why we did not see a significant change in myogenin after such a short hypoxic exposure. However myogenin, a transcription factor that stimulates myoblasts to undergo terminal differentiation and fusion into myofibers, typically does not undergo up-regulation until both Myf5 and MyoD are coexpressed, usually around 96 hours after plating of C2C12’s [15,27,45,]. The findings of this study found myogenin expression was significantly higher following 24 hours of culturing in both normoxic and hypoxic conditions, before a rapid down-regulation and no change between 48 – 96 hours of culturing (Figure 8). Explanations for this occurrence are challenging.
Regarding cell viability, this study found that 24 hours of acute physiologically hypoxic exposure did not influence cell viability during the proliferative phase of C2C12’s culturing in vitro. This is in contrast to the research looking at the effects of chronic physiological hypoxic exposure that has found various stem cells undergo significantly less apoptosis of cultures in low oxygen than in the traditional 21% O2 conditions [25,46]. Furthermore. the usual reduction in apoptosis has been found to lead to the increased yield of cultured stem cells during the proliferative phase of cells cultured within low oxygen conditions [37]. Specifically looking at murine muscle satellite stem cells, C2C12’s undergo significantly less apoptosis in 6% O2 than in 20% O2 following 3 days of exposure [25]. The possible mechanisms suggested for the anti-apoptotic effect of culturing in low oxygen conditions, is that fewer reactive oxygen species are generated in cells cultured in low oxygen conditions, compared to higher oxygen conditions. Specifically, primary murine myoblasts cultured in 6% pO2 generated around 1/3rd fewer reactive oxygen species compared to cells cultured in 20% oxygen [36]. On the contrary Ramirez et al. [35] found following three days of culturing in 5% pO2 there is a significant rise in oxidative stress leading to increased apoptosis. It must be reiterated that these studies looked at the effect of chronic hypoxic exposure, with at least 48 hours of exposure. This study used similar oxygen concentrations during the hypoxic exposure, but the exposure time during this study was only 24 hours and deemed acute. Therefore, the reason that no difference in cell viability was found, leads to the possible suggestion that acute exposure does not allow for an adequate change in reactive oxygen species concentrations and therefore an effect on cell viability. Research that has looked at the apoptosis of neural stem cells in hypoxic conditions, did not find a significant difference until 72 hours of exposure in 1%, 2.5% and 5% pO2 [47].
It should be noted that the physiologic hypoxic environment in which the cells were cultured (5% pO2) was regulated using the Sanyo O2/CO2 Incubator. Although oxygen sensors embedded within the incubator would ensure the atmospheric environment inside were kept at a constant, it is also important to ensure the oxygen tension seen at the level of the tissue culture monolayer is also expressive of a physiologic hypoxic environment. The oxygen tension seen at a cellular level can vary considerably to the level of oxygen that the incubators deliver, and a number of factors can influence this variation, including the depth of medium, the density of cells, and cellular respiration [36,48]. Within this study to monitor the oxygen tension seen by the cells, a hole was drilled into the top of a single well of the plates and a dip-type oxygen microelectrode was placed within the growth medium at the cellular level. This approach to incorporate multiple oxygen sensors embedded at various levels to monitor oxygen tension is a novel approach that is yet to be seen within studies that culture C2C12 in hypoxic environments. The results indicated that the oxygen tension seen at the cell level was also 5% pO2 at the end of the 24 hour exposure time in the hypoxic incubator and it can be confirmed that the cells were exposed to a physiologically hypoxic environment.
Discussing study insufficiency, a more accurate account of cell viability and proliferation rates could have been achieved by centrifuging the removed medium and re-suspending these cells prior to total cell counts being performed to account for non-adhered cells. Independent of this observation, when analysing the cell viability of adhered cells, it can be suggested that the acute hypoxic exposure did not influence the percentage of viable cells as well as the proliferation rate. A further limitation is that although the incubator allowed control of oxygen levels during cultivation, it is impossible to prevent reperfusion of atmospheric conditions when the cells are removed from the incubators before being fixed [36]. In order to ensure the cells are exposed to relatively constant levels of oxygen, it would be preferential for cells to be cultured and manipulated in a closed workstation that allows for oxygen and CO2 control.
The effects exercise and altitude have on intramyocellular oxygen pressure is well documented [43,49,50]. It is also known that a drop in intramyocellular oxygen pressure can be induced by exercise, altitude or pathological damage [27,43]. Further to these, in vitro studies have found that culturing cells in a moderately hypoxic environment (6-15% pO2) promotes muscle differentiation and induces skeletal muscle hypertrophy [25,33]. Further to this, in vivo research has shown that a moderately hypoxic muscle environment may partially contribute to muscle hypertrophic responses during blood flow restriction training and resistance training under hypoxic conditions [30-32]. Although these studies are supportive of one another, a major difference between the in vivo and in vitro studies exists in the duration of the hypoxic exposure. In vitro studies to date expose the culturing cells to chronic hypoxia, while the in vivo hypoxic exposure through training is acute lasting no more than a few hours. Although moderate hypoxia may contribute to muscle hypertrophic responses during resistance training, the current study shows that acute hypoxic exposure alone is not sufficient to elicit a hypertrophic response without a mechanical stress.
It can be concluded that the effect of acute moderate hypoxia lasting 24 hours appears to have no effect on the proliferation rate, viability or transcription factor expression of C2C12’s during the proliferative phase of myogenesis. When manipulating intramyocellular oxygen pressures, it is therefore suggested 24 hours of exposure at 5% O2 is insufficient in duration to promote muscle cell proliferation.
References
2. Chakravarthy MV, Spangenburg EE, Booth FW. Culture in low levels of oxygen enhances in vitro proliferation potential of satellite cells from old skeletal muscles. Cellular and Molecular Life Sciences CMLS. 2001 Jul 1;58(8):1150-8.
3. Stamati K, Mudera V, Cheema U. Evolution of oxygen utilization in multicellular organisms and implications for cell signalling in tissue engineering. Journal of Tissue Engineering. 2011 Jan;2(1):2041731411432365.
4. Weidemann A, Johnson RS. Biology of HIF-1 α. Cell Death & Differentiation. 2008 Apr;15(4):621-7.
5. Lin Q, Kim Y, Alarcon RM, Yun Z. Oxygen and cell fate decisions. Gene Regulation and Systems Biology. 2008 Jan;2:GRSB-S434.
6. Mauro A. Satellite cell of skeletal muscle fibers. The Journal of biophysical and biochemical cytology. 1961 Feb 1;9(2):493.
7. Kuang S, Gillespie MA, Rudnicki MA. Niche regulation of muscle satellite cell self-renewal and differentiation. Cell Stem Cell. 2008 Jan 10;2(1):22-31.
8. Miller JB, Schaefer L, Dominov JA. 6 Seeking Muscle Stem Cells. InCurrent Topics in Developmental Biology 1998 Jan 1;43: 191-219.
9. Bischoff R, Heintz C. Enhancement of skeletal muscle regeneration. Developmental Dynamics. 1994 Sep;201(1):41-54.
10. Le Grand F, Rudnicki MA. Skeletal muscle satellite cells and adult myogenesis. Current Opinion in Cell Biology. 2007 Dec 1;19(6):628-33.
11. Kuang S, Rudnicki MA. The emerging biology of satellite cells and their therapeutic potential. Trends in Molecular Medicine. 2008 Feb 1;14(2):82-91.
12. Majmundar AJ, Skuli N, Mesquita RC, Kim MN, Yodh AG, Nguyen-McCarty M, et al. O2 regulates skeletal muscle progenitor differentiation through phosphatidylinositol 3-kinase/AKT signaling. Molecular and Cellular Biology. 2012 Jan 1;32(1):36-49.
13. Cornelison DD, Olwin BB, Rudnicki MA, Wold BJ. MyoD−/− satellite cells in single-fiber culture are differentiation defective and MRF4 deficient. Developmental Biology. 2000 Aug 15;224(2):122-37.
14. Seale P, Ishibashi J, Holterman C, Rudnicki MA. Muscle satellite cell-specific genes identified by genetic profiling of MyoD-deficient myogenic cell. Developmental Biology. 2004 Nov 15;275(2):287-300.
15. Yablonka-Reuveni Z, Rivera AJ. Temporal expression of regulatory and structural muscle proteins during myogenesis of satellite cells on isolated adult rat fibers. Developmental Biology. 1994 Aug 1;164(2):588-603.
16. Cornelison DD, Wold BJ. Single-cell analysis of regulatory gene expression in quiescent and activated mouse skeletal muscle satellite cells. Developmental Biology. 1997 Nov 15;191(2):270-83.
17. Warkentin KM. Hatching timing, oxygen availability, and external gill regression in the tree frog, Agalychnis callidryas. Physiological and Biochemical Zoology. 2002 Mar;75(2):155-64.
18. Das R, Jahr H, van Osch GJ, Farrell E. The role of hypoxia in bone marrow–derived mesenchymal stem cells: considerations for regenerative medicine approaches. Tissue Engineering Part B: Reviews. 2010 Apr 1;16(2):159-68.
19. Li X, Zhu L, Chen X, Fan M. Effects of hypoxia on proliferation and differentiation of myoblasts. Medical Hypotheses. 2007 Jan 1;69(3):629-36.
20. Zhou Z, Bornemann A. MRF4 protein expression in regenerating rat muscle. Journal of Muscle Research & Cell Motility. 2001 May 1;22(4):311-6.
21. Bentzinger CF, Wang YX, Rudnicki MA. Building muscle: molecular regulation of myogenesis. Cold Spring Harbor Perspectives in Biology. 2012 Feb 1;4(2):a008342.
22. Liu W, Wen Y, Bi P, Lai X, Liu XS, Liu X, et al. Hypoxia promotes satellite cell self-renewal and enhances the efficiency of myoblast transplantation. Development. 2012 Aug 15;139(16):2857-65.
23. Pittman RN. Oxygen gradients in the microcirculation. Acta Physiologica. 2011 Jul;202(3):311-22.
24. Poole DC, Copp SW, Hirai DM, Musch TI. Dynamics of muscle microcirculatory and blood–myocyte O2 flux during contractions. Acta Physiologica. 2011 Jul;202(3):293-310.
25. Csete M, Walikonis J, Slawny N, Wei Y, Korsnes S, Doyle JC, et al. Oxygen-mediated regulation of skeletal muscle satellite cell proliferation and adipogenesis in culture. Journal of Cellular Physiology. 2001 Nov;189(2):189-96.
26. Roy S, Khanna S, Bickerstaff AA, Subramanian SV, Atalay M, Bierl M, et al. Oxygen sensing by primary cardiac fibroblasts: a key role of p21Waf1/Cip1/Sdi1. Circulation Research. 2003 Feb 21;92(3):264-71.
27. Sato K, Saida K, Yanagawa T, Fukuda T, Shirakura K, Shinozaki H, et al. Differential responses of myogenic C2C12 cells to hypoxia between growth and muscle-induction phases: growth, differentiation and motility. Journal of Physical Therapy Science. 2011;23(1):161-9.
28. Di Carlo A, De Mori R, Martelli F, Pompilio G, Capogrossi MC, Germani A. Hypoxia inhibits myogenic differentiation through accelerated MyoD degradation. Journal of Biological Chemistry. 2004 Apr 16;279(16):16332-8.
29. Reecy JM, Miller SA, Webster M. Recent advances that impact skeletal muscle growth and development research. Journal of Animal Science. 2003 Jan 1;81(13_suppl_1):E1-8.
30. Abe T, Yasuda T, Midorikawa T, Sato Y, CF K, Inoue K, et al. Skeletal muscle size and circulating IGF-1 are increased after two weeks of twice daily “KAATSU” resistance training. International Journal of KAATSU Training Research. 2005;1(1):6-12.
31. Nishimura A, Sugita M, Kato K, Fukuda A, Sudo A, Uchida A. Hypoxia increases muscle hypertrophy induced by resistance training. International Journal of Sports Physiology and Performance. 2010 Dec 1;5(4):497-508.
32. Kurobe K, Huang Z, Nishiwaki M, Yamamoto M, Kanehisa H, Ogita F. Effects of resistance training under hypoxic conditions on muscle hypertrophy and strength. Clinical Physiology and Functional Imaging. 2015 May;35(3):197-202.
33. Sakushima K, Yoshikawa M, Osaki T, Miyamoto N, Hashimoto T. Moderate hypoxia promotes skeletal muscle cell growth and hypertrophy in C2C12 cells. Biochemical and Biophysical Research Communications. 2020 Mar 12;525(4):921-927.
34. Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2− ΔΔCT method. Methods. 2001 Dec 1;25(4):402-8.
35. Ramirez MA, Pericuesta E, Yáñez‐Mó M, Palasz A, Gutiérrez‐Adán A. Effect of long‐term culture of mouse embryonic stem cells under low oxygen concentration as well as on glycosaminoglycan hyaluronan on cell proliferation and differentiation. Cell Proliferation. 2011 Feb;44(1):75-85.
36. Csete M. Oxygen in the cultivation of stem cells. Annals of the New York Academy of Sciences. 2005 May;1049(1):1-8.
37. Studer L, Csete M, Lee SH, Kabbani N, Walikonis J, Wold B, et al. Enhanced proliferation, survival, and dopaminergic differentiation of CNS precursors in lowered oxygen. Journal of Neuroscience. 2000 Oct 1;20(19):7377-83.
38. Lennon DP, Edmison JM, Caplan AI. Cultivation of rat marrow-derived mesenchymal stem cells in reduced oxygen tension: effects on in vitro and in vivo osteochondrogenesis. Journal of Cellular Physiology. 2001 Jun;187(3):345-55.
39. Greenbaum AR, Etherington PJ, Manek S, O'hare D, Parker KH, Green CJ, et al. Measurements of oxygenation and perfusion in skeletal muscle using multiple microelectrodes. Journal of Muscle Research & Cell Motility. 1997 Mar 1;18(2):149-59.
40. Richardson RS. Oxygen transport: air to muscle cell. Medicine and science in sports and exercise. 1998 Jan 1;30(1):53-9.
41. Yun Z, Lin Q, Giaccia AJ. Adaptive myogenesis under hypoxia. Molecular and cellular biology. 2005 Apr 15;25(8):3040-55.
42. Kook SH, Son YO, Lee KY, Lee HJ, Chung WT, Choi KC, et al. Hypoxia affects positively the proliferation of bovine satellite cells and their myogenic differentiation through up-regulation of MyoD. Cell Biology International. 2008 Aug;32(8):871-8.
43. Howald H, Hoppeler H. Performing at extreme altitude: muscle cellular and subcellular adaptations. European Journal of Applied Physiology. 2003 Oct 1;90(3-4):360-4.
44. Koning M, Werker PM, van Luyn MJ, Harmsen MC. Hypoxia promotes proliferation of human myogenic satellite cells: a potential benefactor in tissue engineering of skeletal muscle. Tissue Engineering Part A. 2011 Jul 1;17(13-14):1747-58.
45. Rudnicki MA, Le Grand F, McKinnell I, Kuang S. The molecular regulation of muscle stem cell function. InCold Spring Harbor symposia on quantitative biology 2008 Jan 1;73: 323-331.
46. Erkkilä K, Pentikäinen V, Wikström M, Parvinen M, Dunkel L. Partial oxygen pressure and mitochondrial permeability transition affect germ cell apoptosis in the human testis. The Journal of Clinical Endocrinology & Metabolism. 1999 Nov 1;84(11):4253-9.
47. Santilli G, Lamorte G, Carlessi L, Ferrari D, Nodari LR, Binda E, Delia D, Vescovi AL, De Filippis L. Mild hypoxia enhances proliferation and multipotency of human neural stem cells. PloS one. 2010 Jan 5;5(1):e8575.
48. Muschler GF, Nakamoto C, Griffith LG. Engineering principles of clinical cell-based tissue engineering. Journal of Bone and Joint Surgery. 2004 Jul 1;86(7):1541-58.
49. Poole DC, Copp SW, Hirai DM, Much TI. Oxygen partial pressure (PO2) in heavy exercise. Mooren FC (Ed.). Encyclopedia of Exercise Medicine in Health and Disease. Berlin: Springer, 2012; p.683-688.
50. Hoppeler H, Klossner S, Vogt M. Training in hypoxia and its effects on skeletal muscle tissue. Scandinavian Journal of Medicine & Science in Sports. 2008 Aug;18:38-49.