Abstract
Hyperglycemia and renal fibrosis play critical roles in the occurrence and development of diabetic complications such as diabetic nephropathy (DN). Lupenone, a stable pentacyclic triterpenoid compound, has anti-hyperglycemic and anti-renal fibrosis activities. Previous research has confirmed that lupenone can improve renal fibrosis in type 2 diabetic nephropathy by regulating TGF-?/Smad/CTGF signaling pathway. However, the binding power of lupenone with its related targets has not been confirmed, and it is unclear whether it exerts anti-renal fibrosis effects as a prototype component. Therefore, the aim of this study was to identify the underlying mechanism of lupenone on anti-renal fibrosis based on the TGF-?/ Smad/CTGF signaling pathway and elucidate their binding ability using molecular docking and in vitro cell experiments. Molecular docking results suggested that lupenone combined well with fibronectin, TGF-?1, T?RI, T?RII, Smad2, Smad3, Smad4, Smad7 and Smurf2, respectively. And lupenone could significantly reduce high glucose-induced MCs cytotoxicity. Furthermore, lupenone significantly downregulated the mRNA and protein expression of collagen-I, collagen-IV, fibronectin, TGF-?1, p-T?RI/T?RI, T?RII, p-Smad2/ Smad2, p-Smad/Smad3, Smad4, Smurf2, and CTGF in high glucose-induced MCs, with the best effect observed in the high-dose lupenone group. These results concluded that lupenone could inhibit the generation of fibrosis factors collagen-I, collagen-IV, and fibronectin and delay the process of fibrosis by regulating the TGF-?/Smad/CTGF signaling pathway in MCs.
Keywords
Molecular docking, lupenone, TGF-?/Smad/CTGF signaling pathway, Renal fibrosis, Diabetic nephropathy
Introduction
According to the global epidemiological report on chronic kidney disease (CKD) published by The Lancet, CKD is recognized as a global public health problem with high morbidity and mortality [1]. Diabetic nephropathy (DN) is the most main microvascular complication of diabetes and is one of the leading causes of end-stage renal disease (ESRD) and CKD [2]. Renal fibrosis is the main pathological feature of DN, which manifests as glomerular hypertrophy, glomerular mesangial expansion, and thickening of the glomerular capillary basement membrane caused by hyperglycemia. With the development of DN, glomerular and tubulointerstitial fibrosis appear, eventually leading to renal failure [3,4]. Therefore, effectively alleviating kidney fibrosis to treat CKD is an important topic in contemporary medical research.
The mechanism of DN includes various factors, such as inflammation, oxidative stress, apoptosis, autophagy, and fibrosis, among which renal fibrosis is the final pathway of DN to ESRD. As a major signaling pathway, TGF-β/Smad/CTGF affects renal fibrosis in DN through various mechanisms [5-7]. TGF-β1 regulates cell proliferation, differentiation, apoptosis, autophagy, and extracellular matrix (ECM) production as a key regulator of fibrosis and is considered to be the induction and initiation factor of fibrosis development [8]. The TGF-β signaling pathway is mainly mediated by TGF-β receptor-regulated Smad and non-Smad signaling pathways [9]. When TGF-β is stimulated, regulatory Smads, such as Smad2 and Smad3, are recruited to TβRs, which are then activated and phosphorylated. Later, a trimeric complex is formed by phosphorylated Smad2 and Smad3 with Smad4, which transfers to the nucleus to induce ECM accumulation [10,11]. In contrast, Smad7 alleviate renal fibrosis by inhibiting the phosphorylation of Smad2 and Smad3 and promoting the degradation of TGF-β type I receptors [12]. Moreover, CTGF, as a downstream effector of TGF-β1, can stimulate the synthesis of extracellular matrix components such as fibronectin, collagen-I, and collagen-IV in glomerular mesangial cells and fibroblasts and promote the development of renal fibrosis [13-15]. Studies have found that the fibrotic process of glomerular mesangial cells is closely correlated to the development of DN [16], suggesting that glomerular mesangial cells are the target cells of DN. Renal fibrosis is accelerated in DN by promoting the production and accumulation of ECM. Therefore, a drug that can effectively alleviate the fibrosis process of glomerular mesangial cells is the key to treating DN.
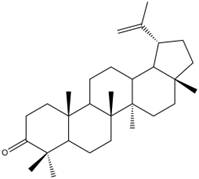
Lupenone (Lup, Figure 1) is a pentacyclic triterpenoid compound derived from the root of Musa basjoo Sied et Zucc. , the peel of Musa nana Lour. and Musa acuminata cv. Mas (AA) [17,18]. It can be found in Asteraceae, Iraceae, Bombacaceae, Leguminosae, and other plants [19]. Studies have shown that lupenone has anti-inflammatory, anti-type 2 diabetes, and anti-cancer activities [19-21]. Importantly, our previous studies have indicated that lupenone reduces fibrous tissue damage and inflammatory cell infiltration, which has protective and reparative effects on kidney injury [22]. In our preliminary research, in vivo research results show that lupenone can improve renal fibrosis in type 2 diabetic nephropathy by regulating TGF-β/Smad/CTGF signaling pathway [23]. However, the binding power of lupenone with its related targets has not been confirmed, and it is unclear whether it exerts anti-renal fibrosis effects as a prototype component. Moreover, molecular docking technology can predict the interaction and affinity between molecules and biological targets, and is widely used in the screening of active ingredients in traditional Chinese medicine and the determination of action targets [24-26].
Figure 1. Chemical structure of lupenone.
In our study, molecular docking is used to predict the potential targets of lupenone against renal fibrosis based on TGF-β/Smad/CTGF signaling pathway. Next, a high-glucose induced MCS model was used to evaluate the effect of lupenone on alleviating renal fibrosis based on TGF-β/Smad/CTGF signaling pathway. The experimental research flowchart is shown in Figure 2.
Figure 2. The flowchart of the molecular docking and experimental validation.
Materials and Methods
Molecular docking
The binding ability and binding location of ligands and proteins can be predicted by molecular docking, so it is the most widely used technology in drug design [27]. The lupenone structure was transformed from the SDF to the mol2 format by Chem 3D software. The 3D structure of Fibronectin, TGFβ1, TβRI, TβR?, Smad2, Smad3, Smad4, Smad7, and Smurf2 were all downloaded from the Protein Data Bank (PDB) (https://www.rcsb.org/). The solvent molecules and ligands were removed by Pymol software. In addition, we also used AutoDock Tools 1.5.6 software to add hydrogen, calculate charges, assign atomic types, and perform other operations, and saved the processed structure in pdbqt format. Then, Lamarck genetic algorithm (LGA) was chosen to dock lupenone with the core protein targets. Lastly, the molecular docking results are visualized by Pymol software.
Drugs and antibodies
Lupenone (C30H48O, MW: 424.7, purity: ≥ 98%) was obtained from Drug Analysis Laboratory of Guizhou University of Traditional Chinese Medicine. Its chemical structure (Figure 1) was identified by NMR spectroscopy and high-resolution mass spectrometry [28]. Irbesartan was purchased from Sanofi (Paris, France). The penicillin, streptomycin, DMEM, and 5% FBS were purchased at Hyclone (Logan, USA). Anti-GAPDH, anti-Collagen-I, anti-Collagen-IV, anti-Fibronectin, anti-TGF β1, anti-TGF beta Receptor I, anti-TGF beta Receptor II, anti-SMURF 2, MADH7/SMAD7 antibody, anti-Smad4, anti-Smad3, anti-Smad2, anti-Smad3 (phospho S423 + S425), anti-Smad2 (phospho S467), and anti-CTGF were purchased from Abcam (Cambridge, MA, USA). TGF beta Receptor I (Phospho-Ser165) antibody was purchased from Biorbyt Company (Shanghai, China).
Cell culture
MCs, purchased from Procell Life Science&Technology Co., Ltd. (Wuhan, China), were cultured in 71.25% DMEM and 23.75% Ham's F-12 medium including 5% FBS, 100 U/mL of penicillin, and 100 μg/mL of streptomycin, at 37°C with 5% CO2.
Cell viability assay
The MTT method was used to detect the effect of lupenone on MCs viability. Briefly, cells were seeded in a 96-well plate (1 × 103 cells/well), cells were exposed to different concentrations of lupenone (0.001, 0.01, 0.1, 1, 10, and 100 μg/mL) for 24 h. After incubation, 10 μL MTT reagent was added to each well, followed by further incubation for 3 h. Subsequently, the culture medium was removed and 150 μL DMSO was added into each well and shaken evenly. Finally, the optical density (OD) values of each well were measured at 570 nm using a microplate reader (Thermo Fisher, Multiskan 51119000).
Cell proliferation assay
The inhibitory effect of lupenone on high glucose-induced MCs proliferation was also examined using the MTT assay. MCs were seeded into 96-wekk plate (1 × 103 cells/well). Cells were randomly divided into eight groups: (1) model group: high-glucose (30 mmol/L), (2) control group: without glucose, (3) HG + 0.001 μg/mL lupenone group, (4) HG + 0.01 μg/mL lupenone group, (5) HG + 0.1 μg/mL lupenone group, (6) HG + 1 μg/mL lupenone group, (7) HG + 10 μg/mL lupenone group, and (8) HG + 100 μg/mL lupenone group. After the cells adhere to the wall, 30 mM glucose and lupenone treated with DMSO were added into cells according to the groups for 24 h. An equal volume of DMSO was added to the control group. And 10 μL MTT reagent was added to each well and incubated for 3 h. Finally, the OD value of each well at 570 nm was measured using a microplate reader (Thermo Fisher, Multiskan 51119000).
Cell treatment
Cells were inoculated into 6-well plates and randomly divided into six groups: (1) control group: no glucose or other drugs, (2) model group: glucose (30 mM), (3) irbesartan group: glucose (30 mM) + irbesartan (4.3 μg/mL), (4) high-dose lupenone group (Lup-L): glucose (30 mM) + lupenone (0.1 μg/mL), (5) medium-dose lupenone group (Lup-M): glucose (30 mM) + lupenone (1 μg/mL), and (6) low-dose lupenone group (Lup-H): glucose (30 mM) + lupenone (10 μg/mL).
Real-time polymerase chain reaction (RT-PCR)
The treated cells were harvested, and the total RNA was isolated according to the instructions of the TRIzol kit (Takara Bio Inc., San Jose, USA). RNA concentration was determined, and subsequent experiments were performed using the PrimescriPt™ RT reagent kit with gDNA Eraser kit (Takara Bio Inc.). Relative quantification of mRNA levels was performed using the 2−??CT method and normalized to GAPDH expression. These sequences are listed in Table 1.
|
Gene |
Forward Primer (5' -3') |
Reverse Primer (5' -3') |
|
GAPDH |
AACAGCAACTCCCACTCTTC |
CCTGTTGCTGTAGCCGTATT |
|
Collagen-? |
CAAGGTTCACCAGGGCTTAT |
GGGTCTCCTTTGTCACCTTT |
|
Collagen-? |
CAAGGTTCACCAGGGCTTAT |
GGGTCTCCTTTGTCACCTTT |
|
Fibronectin |
TCCTGTCTACCTCACAGACTAC |
GTCTACTCCACCGAACAACAA |
|
TGF-β1 |
CTGAACCAAGGAGACGGAATAC |
GGGCTGATCCCGTTGATTT |
|
TβRI |
GTTCCGAGAGGCAGAGATTTAT |
CGTCCATGTCCCATTGTCTT |
|
TβR? |
CGTCCATGTCCCATTGTCTT |
CCGACCTGTTGTTGGTCATTA |
|
Smad2 |
TGGACACGGTAGCAGTAGAA |
CGGACCTTCACAGTCATCAT |
|
Smad4 |
CTCTGGTAGTGGTACGGATTTAC |
GCAGAGTAATGCTCCAAGTATGT |
|
Smad7 |
CGAATGTCCTTCAGTGGGTAAG |
AGGCTGTGTTGCTGTGAA |
|
Smad3 |
AGAGAGTAGAGACGCCAGTT |
GGGAATGGAATGGCTGTAGT |
|
Smurf2 |
GTCTGACAGTGCCAAGATACA |
TCTCTTCCCTAGACACCTCAATA |
|
CTGF |
ACTATGATGCGAGCCAACTG |
CTCCAGTCTGCAGAAGGTATTG |
Protein detection via western blotting
Total protein was extracted from MCs using a RIPA lysis buffer, and the cells were centrifuged at 4°C for 10 min at 12,000 rpm. After centrifugation, a BCA protein assay was used to determine the total protein concentration. Then, a total of 30 μg protein sample was placed in a running gel phase with 10% isolate gel and 4% concentrate gel, and then the protein was transferred to a PVDF membrane. The membrane was incubated with primary antibodies against GAPDH, collagen-I, collagen-IV, fibronectin, TGF-β1, TGF-β receptor I, TGF-β receptor II, Smurf2, MADH7/Smad7, Smad4, Smad3, Smad2, phosphorylated Smad3, phosphorylated Smad2, and CTGF at 4°C overnight. Next, it was washed thrice with TBST at 25°C for 10 min each time. After incubation with the relevant secondary antibodies for 2 h at room temperature, relative protein expression was quantified using a Tanon 5200 chemiluminescence imager.
Statistical analysis
All statistical analyses were performed using one-way analysis of variance (ANOVA) in SPSS statistical software (version 26.0). Values are shown as the mean ± SD, and differences with P<0.05 were considered statistically significant.
Results
Molecular docking
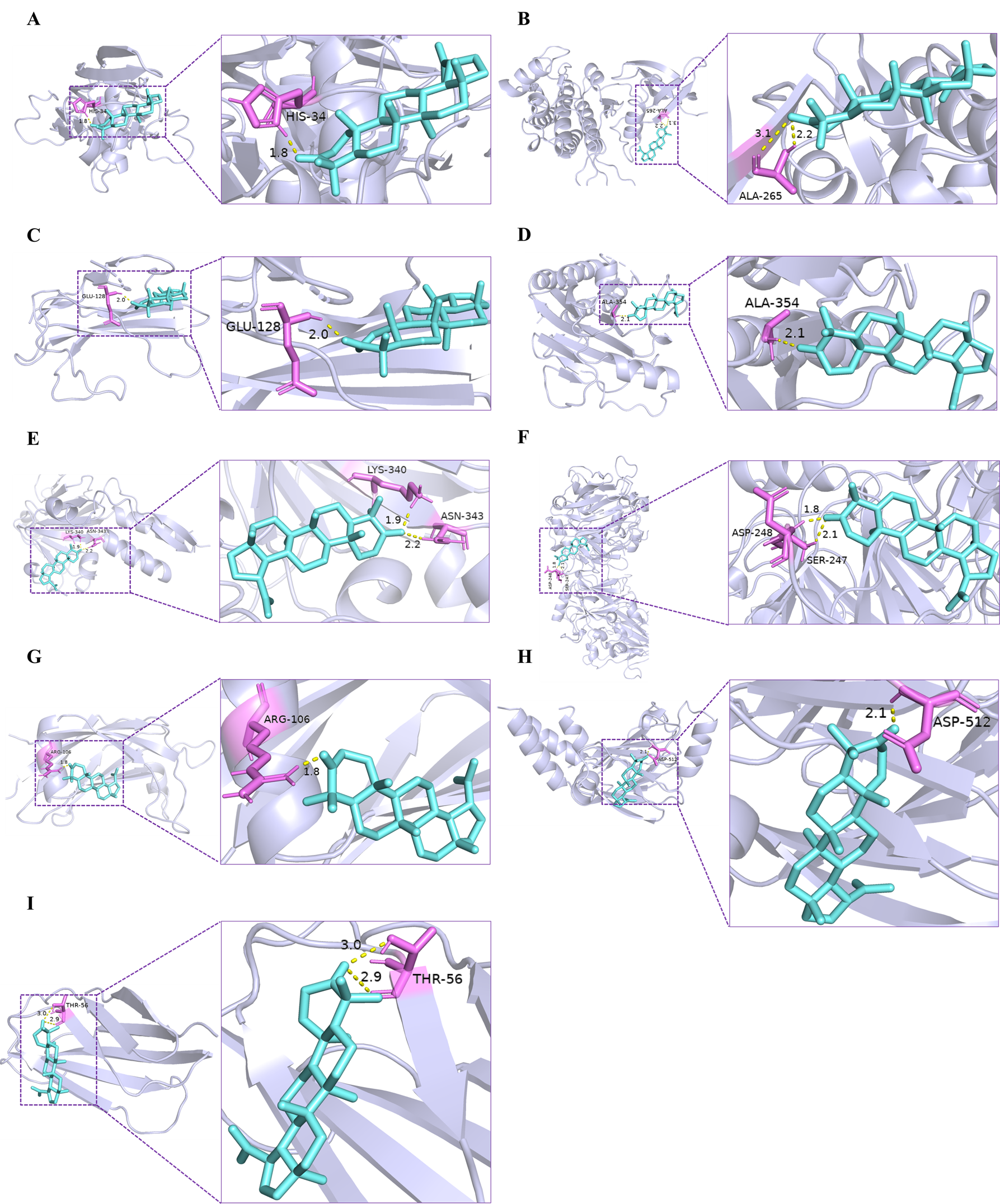
In order to explore the interactions between the lupenone and related proteins in the TGF-β/Smad/CTGF pathway, further molecular docking simulations were carried out. Based on the results of literature research, TGF-β/Smad/CTGF pathway plays a decisive role in renal fibrosis, and the core targets of this pathway were selected to dock with the lupenone acting on them, respectively. We identified the binding affinity between the lupenone and the core proteins (TGF-β1, TβRI, Smurf2, TβRII, Smad2, Smad3, Fibronectin, Smad4, Smad7) in TGF-β/Smad/CTGF pathway by molecular docking.
The affinity degree of ingredients with protein targets was generally assessed by the binding energy. As shown in Table 2 and Figure 3, the docking result suggested that the binding energies of lupenone and their targets were all smaller than -5 kJ/mol, which indicates that the docking results were good. Together, lupenone could bind well with nine core targets, suggesting which lupenone might play key roles in anti-renal fibrosis through TGF-β/Smad/CTGF signaling pathway.
|
Protein |
PDB ID |
Affinity (kJ/mol) |
Protein |
PDB ID |
Affinity (kJ/mol) |
|
Fibronectin |
1FNA |
-6.80 |
Smad3 |
1MK2 |
-5.34 |
|
TGF-β1 |
1KLC |
-7.92 |
Smad4 |
1YGS |
-6.60 |
|
TβRI |
5E8S |
-6.54 |
Smad7 |
2DJY |
-5.81 |
|
TβR? |
1MGZ |
-6.30 |
Smurf2 |
1ZVD |
-5.79 |
|
Smad2 |
5XOD |
-5.51 |
|
|
|
Figure 3. Molecular models of lupenone binding to its predicted protein targets. (A) TGF-β1, (B) TβRI, (C) TβR?, (D) Smad2, (E) Smad3, (F) Smad7 (G) Smurf2, (H) Smad4, and (I) Fibronectin are shown interacting with lupenone molecule, represented by a purple stick model. The yellow dashed lines represent hydrogen bonds and the length was increased around the lines.
Lupenone protects MCs from high glucose-induced damage
First, we evaluated the cytotoxicity of lupenone on MCs. Lupenone treatment within the concentration range of 0.001-100 μg/mL had no significant effect on the viability of MCs (Figure 4A). Compared with the control group, the high-glucose group significantly promoted MCs proliferation, but lupenone (0.001-100 μg/mL) dose-dependently reduced high-glucose induced MCs proliferation (Figure 4B).
Figure 4. Molecular models of lupenone binding to its predicted protein targets. (A) TGF-β1, (B) TβRI, (C) TβR?, (D) Smad2, (E) Smad3, (F) Smad7, (G) Smurf2, (H) Smad4, and (I) Fibronectin are shown interacting with lupenone molecule, represented by a purple stick model. The yellow dashed lines represent hydrogen bonds and the length was increased around the lines.
Effect of lupenone on ECM-related gene expression in MCs
In the model group, the mRNA expression levels of collagen-I, collagen-IV, and fibronectin were significantly higher in MCs compared with the normal group. Notably, RT-PCR showed that the irbesartan group and lupenone treatment group reduced the mRNA levels of collagen-I, collagen-IV, and fibronectin in MCs (Figures 5A-5C).
Figure 5. Effect of lupenone on ECM-related gene expression in MCs. (A-C) mRNA expression of collagen-I, collagen-IV, and fibronectin in MCs determined via RT-PCR. Values are presented as the mean ± SD. ## P < 0.01 vs. the control group, ** P < 0.01 vs. the model group.
Effect of lupenone on ECM-related protein expression in MCs
The model group showed significantly elevated intracellular collagen-I, collagen-IV, and fibronectin protein levels (Figures 6A-6D) compared with the control group, indicating the successful establishment of a fibrotic model. However, irbesartan and high-dose and medium-dose lupenone significantly attenuated the levels of collagen-I, collagen-IV, and fibronectin in MCs. Furthermore, a low dose of lupenone markedly reduced the protein levels of collagen-IV and fibronectin, whereas the protein level of collagen-I displayed no significant discrepancy compared with the model group.
Figure 6. Effect of lupenone on ECM-related protein expression in MCs. (A-C) Protein expression of collagen-I, collagen-IV, and fibronectin as determined via western blotting in MCs; (D) Representative protein bands of collagen-I, collagen-IV, and fibronectin. Values are presented as the mean ± SD. ## P < 0.01 vs. the control group, ** P < 0.01 vs. the model group.
Effect of lupenone on the mRNA expression of the TGF-β/Smad/CTGF pathway in MCs
Compared with the control group, the mRNA levels of TGF-β1, TβRI, Smurf2, TβRII, Smad2, CTGF, Smad3, and Smad4 were dramatically upregulated after high-glucose stimulation in MCs (Figures 7A-7H). In contrast, the level of Smad7 was significantly reduced in the model group (Figure 7I). However, lupenone treatment reduced the mRNA levels of TGF-β1, TβRI, TβRII, Smad2, Smad3, Smad4, Smurf2, and CTGF in high-glucose induced MCs, while significantly upregulated the mRNA expression of Smad7 (Figures 7A-7H).
Figure 7. Effect of lupenone on the mRNA expression of the TGF-β/Smads/CTGF signalling pathway in MCs. (A-I) mRNA expression of TGF-β1, TβRI, TβR?, Smad2, Smad3, Smad4, Smurf2, CTGF, and Smad7 in MCs as determined using RT-PCR. Values are presented as the mean ± SD. ## P < 0.01 vs. the control group, ** P < 0.01 vs. the model group.
Effect of lupenone on the protein expression of the TGF-β/Smad/CTGF signaling pathway in MCs
As illustrated in Figures 8A-8L, compared with the control group, the protein levels of TGF-β1, p-TβRI/TβRI, TβR?, p-Smad2/Smad2, p-Smad3/Smad3, Smad4, Smurf2, and CTGF were significantly increased in the model group. However, compared to the control group, the protein level of Smad7 was visibly reduced in the model group. Treatment with lupenone treatment downregulated intracellular TGF-β1, p-TβRI/TβRI, TβRII, p-Smad2/Smad2, p-Smad3/Smad3, Smad4, Smurf2, and CTGF protein levels. In contrast, the protein level of Smad7 was markedly elevated in these cells. And the results show a dose-effect relationship.
Figure 8. Effect of lupenone treatment on the protein expression of the TGF-β/Smad/CTGF signalling pathway in MCs. (A-F) Protein expression of TGF-β1, p-TβRI/TβRI, TβR?, p-Smad2/Smad2, p-Smad3/Smad3, and Smad4 in MCs as determined via western blotting; (G-I) Representative western blot protein bands of p-TβRI, TβRI, TGF-β1, TβR?, p-Smad2, Smad2, p-Smad3, Smad3, Smad4, Smurf2, CTGF, and Smad7 in each group; (J-L) Protein expression of Smurf2, CTGF, and Smad7 in MCs as determined via western blotting. Values are presented as the mean ± SD. ## P < 0.01 vs. the control group, ** P < 0.01 vs. the model group.
Discussion
DN is a serious glomerular complication associated with diabetes mellitus. It is characterized by an excessive deposition of ECM proteins, mesangial cell proliferation, and the thickening of the glomerular and tubular basement membranes, ultimately leading to glomerulosclerosis and tubulointerstitial fibrosis and the development of ESRD [29]. Glomerular mesangial cells are intrinsic cells of the kidney, which is mainly used for the production of ECM, and plays a crucial role in sustaining the tissue structure and physiologic function of the kidney [30]. Moreover, previous studies found that lupenone can reduce tissue tubular dilatation and inflammatory cell infiltration of kidney tissue in T2DN mice and reduce the degree of renal interstitial fibrosis in T2DN rats [23]. In the present study, the high glucose induced MCs model was used to investigate the mechanism of lupenone in preventing DN.
The proliferation of mesangial cells is promoted by high glucose levels, a significant increase in the proliferation capacity of mesangial cells is vital for exacerbating DN progression [31,32]. Our studies suggested that high glucose could promote the proliferation of MCs. Meanwhile, treatment with lupenone at the tested concentrations dose-dependently inhibited the high glucose-induced proliferation of MCs, indicating that lupenone could alleviate fibrosis in DN by inhibiting the proliferation of glomerular mesangial cells. In addition, previous research indicated that lupenone can improve renal fibrosis in type 2 diabetic nephropathy by regulating TGF-β/Smad/CTGF signaling pathway [23]. However, the binding power of lupenone with its related targets has not been confirmed, and it is unclear whether it exerts anti-renal fibrosis effects as a prototype component. Therefore, 9 proteins in the TGF-β/Smad/CTGF signaling pathway were selected for molecular docking in our study. The results showed that the lupenone had high affinity for fibronectin, TGF-β1, TβRI, TβRII, Smad2, Smad3, Smad4, Smad7 and Smurf2 targets.
The pathogenesis of renal fibrosis is complicated and includes many processes, such as oxidative stress, inflammation, apoptosis and fibrosis, then renal fibrosis are the final steps leading to the development of DN to ESRD [7]. The study found that TGF-β/Smad is an important signaling pathway that results in renal fibrosis in DN. It can activate renal interstitial fibroblasts, promote the phenotypic transformation of renal tubular epithelial cells to myofibroblasts, enhance ECM synthesis and inhibit its degradation, which then leads to excessive ECM accumulation and renal fibrosis in the diabetic kidney [33]. The important components of ECM, the massive deposition of fibronectin, collagen I and collagen IV are vital indictors of diabetic renal fibrosis and DN [34]. In our study, lupenone treatment downregulated the expression of mRNA and protein of fibronectin, collagen I, and collagen IV. The results suggest that lupenone reduces the expression of fibrosis factors, downgrades the accumulation of extracellular matrix, thereby alleviating the development of renal fibrosis.
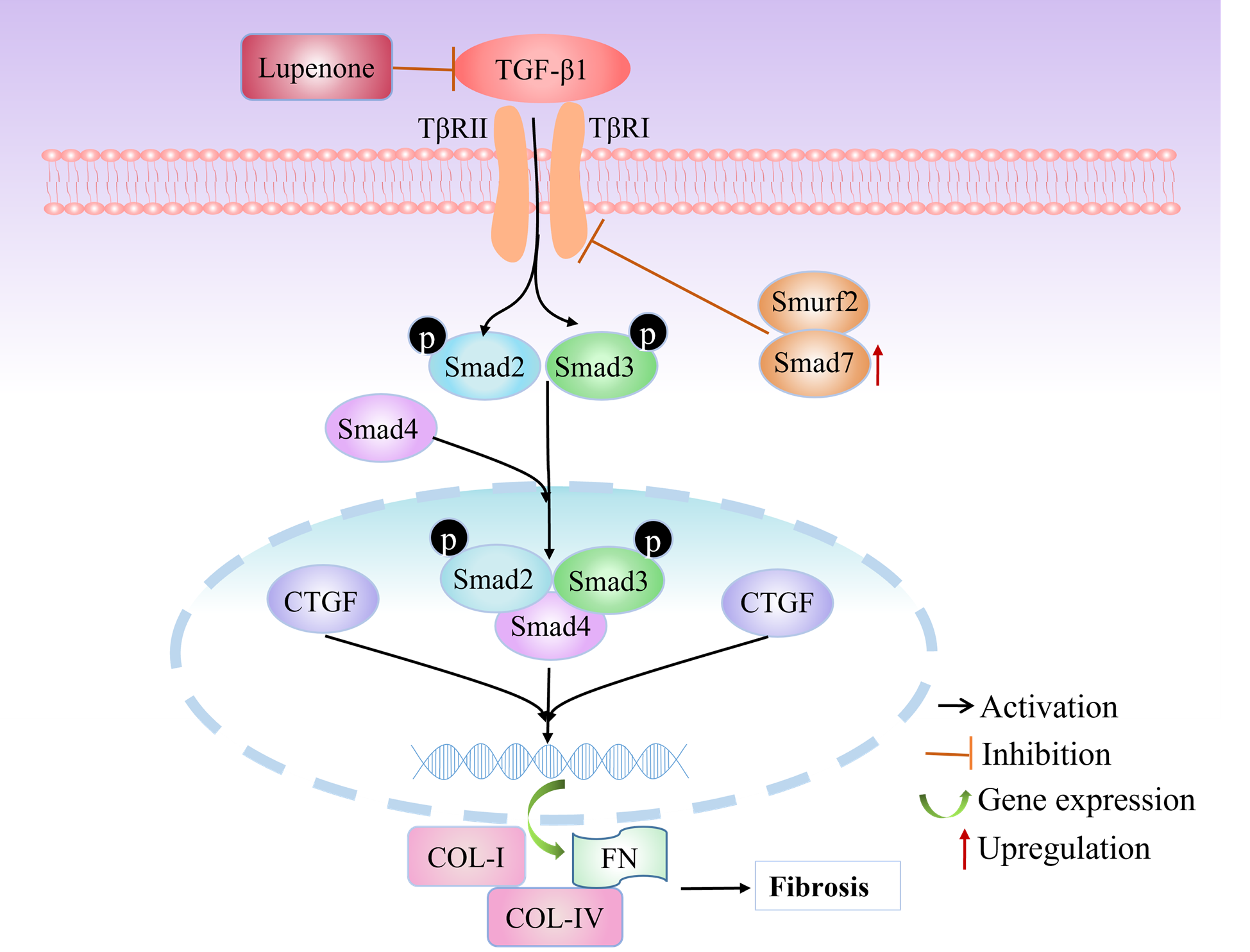
TGF-β1 is recognized as a major regulator of ECM accumulation and a vital driver of renal fibrosis [11]. The binding of TGF-β1 to TβRII can quickly activate TβRI-kinase, leading to the phosphorylation of Smad2 and Smad3. Later, they form the Smad complex with Smad4 that transfers into the nucleus to regulate the transcription of its target genes [10,11]. It has been reported that Smurf2 is a significant E3 ligase which takes part in regulating TGF-β-mediated signaling by targeting TβRI and Smad7 [35]. Smad7, an adaptor protein, can cause the degradation of TGF-β receptor-1 by helping Smurf2 conjugate to TGF-β receptor-1, resulting in the downregulation of TGF-β signaling [36-38]. CTGF, a molecule downstream of TGF-β, plays a critical role in connective tissue homeostasis and fibroblast proliferation, migration, adhesion, and ECM accumulation, controlling fibronectin and collagen gene expression [39]. We found that lupenone treatment downregulated the mRNA and protein expression of TGF-β1, TβRI, and TβRII, which inhibited Smad2 and Smad3 phosphorylation and CTGF expression. Then, we proved that treatment with lupenone lead to the significant inhibition of Smurf2 expression, blocking Smurf2-mediated ubiquitin-induced Smad7 degradation (Figure 9). These results suggested that lupenone could reduce fibrosis in glomerular mesangial cells by regulating the TGF-β/Smad/CTGF signaling pathway and preventing renal fibrosis in DN.
Figure 9. Schematic illustration summarizing the regulatory effects of lupenone on the TGF-β/Smad/CTGF signaling pathway. TGF-β1 transduces a transmembrane signal by binding to TβR-II and TβR-I, resulting in the phosphorylation of downstream Smad2/3. They form a complex with Smad4 and translocate into the nucleus to regulate the transcription of target genes. CTGF is a molecule downstream of TGF-β that stimulates ECM production. Moreover, an inhibitory Smad, Smad7, causes the degradation of TβR-I by helping Smurf2 conjugate to TβR-I. However, lupenone maybe suppresses the activity of the activated TGF-β1 and increases the expression of Smad7 in MCs, inhibiting the production and accumulation of ECM.
Our study uses molecular docking technology to explore the relationship between lupenone and the affinity of TGF-β/Smad/CTGF related fibrosis targets. The results suggest that lupenone may directly exert its anti-renal fibrosis effect as a prototype component. In addition, the high glucose induced MCs model was used to validate the mechanism of lupenone anti renal fibrosis, and this further confirmed the previous research results. This study provides a novel basis of the protective role of lupenone in ECM production and renal fibrosis. There are limitations in our research. In vivo and in vitro experiments have shown lupenone plays an anti-renal fibrosis role based on TGF-β/Smad/CTGF pathway, but it is not clear whether it’s target is exclusive. In future research, this could be explained through a combination of gene silencing and gene knockout studies.
Conclusions
In conclusion, our study demonstrated that lupenone significantly reduced the viability of high-glucose-induced MCs and decreased the mRNA and protein levels of collagen-I, collagen-IV, and fibronectin, which delayed the development of fibrosis induced by high glucose levels. Lupenone alleviated fibrosis in high glucose-induced MCs by regulating the protein levels of the TGF-β/Smad/CTGF signaling pathway and reducing the accumulation of ECM components. In summary, our study provides scientific evidence to support the therapeutic efficacy of lupenone on anti-renal fibrosis and expounds new interactive target proteins and mechanism against renal fibrosis.
Acknowledgments
The research was supported by grants from the National Natural Science Foundation of China (Grant No. 81860722).
References
References
2. Han W, Wang C, Yang Z, Mu L, Wu M, Chen N, et al. SRT1720 retards renal fibrosis via inhibition of HIF1A/GLUT1 in diabetic nephropathy. Journal of Endocrinology. 2019 Apr 1;241(1):85-98.
3. Liang G, Song L, Chen Z, Qian Y, Xie J, Zhao L, et al. Fibroblast growth factor 1 ameliorates diabetic nephropathy by an anti-inflammatory mechanism. Kidney International. 2018 Jan 1;93(1):95-109.
4. Xiang XJ, Zhang PP, Ye QQ, Xu YL, Chen HB. Study on effect Xiaoyu Xiezhuo decoction in preventing and treating renal fibrosis in diabetic nephropathy mice. Modern Applied Pharmacy in China. 2022;39:442-49.
5. Tzavlaki K, Moustakas A. TGF-β Signaling. Biomolecules. 2020 Mar 23;10(3):487.
6. Shan ML, Shi LJ. Advances in research on the pivotal signaling pathways in renal fibrosis. Life Sciences. 2021;33(9):1177-87.
7. Yang P, Liu TH, Wu LL, Qin LL, Zhang CF, Peng C, et al. Research progress on mechanism of saponin-like components of chinese medicine intervening in diabetic nephropathy: a review. Chinese Journal of Experimental Traditional Medical Formulae. 2023;29 :263-271.
8. Zhang HC, Han LH, Suo HL, Yuan B. Effect of Wenyang Yiqi decotion on regulating lung structural remodeling in rats with heart failure after myocardial infarction through regulating TGF-β1/Smad3 signaling pathway. Chinese Journal of Experimental Traditional Medical Formulae. 2018;24:114-120.
9. Geng XQ, Ma A, He JZ, Wang L, Jia YL, Shao GY, et al. Ganoderic acid hinders renal fibrosis via suppressing the TGF-β/Smad and MAPK signaling pathways. Acta Pharmacologica Sinica. 2020 May;41(5):670-7.
10. Hu HH, Chen DQ, Wang YN, Feng YL, Cao G, Vaziri ND, et al. New insights into TGF-β/Smad signaling in tissue fibrosis. Chemico-Biological Interactions. 2018 Aug 25;292:76-83.
11. Meng XM, Nikolic-Paterson DJ, Lan HY. TGF-β: the master regulator of fibrosis. Nature Reviews Nephrology. 2016 Jun;12(6):325-38.
12. Ma TT, Meng XM. TGF-β/Smad and renal fibrosis. Renal Fibrosis: Mechanisms and Therapies. 2019:347-64.
13. Ke B, Wu XF, Chen YX, Fang XD. Effect of erythropoietin on high glucose-induced fibrosis of human kidney mesangial cells and its possible mechanism. Chinese General Practice. 2015;18:3686-91.
14. Fu M, Peng D, Lan T, Wei Y, Wei X. Multifunctional regulatory protein connective tissue growth factor (CTGF): a potential therapeutic target for diverse diseases. Acta Pharmaceutica Sinica B. 2022 Apr 1;12(4):1740-60.
15. Ji J, He L. Effect of Kangxianling decoction on expression of TGF-β1/Smads and extracellular matrix deposition. Evidence-Based Complementary and Alternative Medicine. 2019 Jan 1;2019:5813549.
16. Ma DW, Wang QY, Chen FQ, Sun WB, Ma XY, Li J. High glucose induces the inflammatory response and fibrosis in human glomerular mesangial cells through the Rho/ROCK signaling pathway. Chinese Journal of Endocrinology and Metabolism. 2011;3:204-9.
17. Li XF, Wu HM, Wang XP. UPLC method to determine the Lupenone content in the skin and pulp of Musa nana Lour. and Musa acuminata cv. Mas (AA). Food Science. 2017;38:156-61.
18. Li XF, Wang YM, Wang XP, Wen XX, Wu HM. The HPLC method determined lupenone in the peel of Musa nana Lour., Musa balbisiana Colla. and Musa acuminata cv. Mas (AA) in five harvest periods. Chinese Traditional Patent Medicine. 2017;39:2630-2.
19. Xu F, Huang X, Wu H, Wang X. Beneficial health effects of lupenone triterpene: A review. Biomedicine & Pharmacotherapy. 2018 Jul 1;103:198-203.
20. Xu F, Zhang M, Wu H, Wang Y, Yang Y, Wang X. Study on the mechanism of lupenone for treating type 2 diabetes by integrating pharmacological evaluation and network pharmacology. Pharmaceutical Biology. 2022 Dec 31;60(1):997-1010.
21. Xu F, Yang L, Huang X, Liang Y, Wang X, Wu H. Lupenone is a good anti-inflammatory compound based on the network pharmacology. Molecular Diversity. 2020 Feb;24:21-30.
22. Wu HM, Wang XP, Yang LB, Zhang LL, Liang YQ, Li XF, et al. Application of lupenone in the preparation of drugs for the treatment of kidney damage. 2019;CN110448562A.
23. Wu H, Xu F, Huang X, Li X, Yu P, Zhang L, et al. Lupenone improves type 2 diabetic nephropathy by regulating NF-κB pathway-mediated inflammation and TGF-β1/Smad/CTGF-associated fibrosis. Phytomedicine. 2023 Sep 1;118:154959.
24. X.T. Wang, Y. Li, L. Zhang, M. Liu, C. Li, Q.S. Yang, et al, Screening of anti-fibrotic traditional Chinese medicine compounds based on machine learning, Journal of Beijing University of Chinese Medicine. 42 (2019) 30-36.
25. Hu N, Wang C, Dai X, Zhou M, Gong L, Yu L, et al. Phillygenin inhibits LPS-induced activation and inflammation of LX2 cells by TLR4/MyD88/NF-κB signaling pathway. Journal of Ethnopharmacology. 2020 Feb 10;248:112361.
26. An L, Lin Y, Li L, Kong M, Lou Y, Wu J, et al. Integrating network pharmacology and experimental validation to investigate the effects and mechanism of astragalus flavonoids against hepatic fibrosis. Frontiers in Pharmacology. 2021 Jan 22;11:618262.
27. Fu S, Zhou Y, Hu C, Xu Z, Hou J. Network pharmacology and molecular docking technology-based predictive study of the active ingredients and potential targets of rhubarb for the treatment of diabetic nephropathy. BMC Complementary Medicine and Therapies. 2022 Dec;22(1):210.
28. Wang XP, Hao JJ, Xu SN. The chemical constituents in ethyl acetate extraction from the rhizoma musae. Lishizhen Med. Mater. Med. Res. 2012;23:515-6.
29. Song LQ, Liu S, Song YX, Yun J, Zhang R, Luan ZQ. Effect of Chongcao Yishen Decotion on HMC proliferation and expression of CAT/MDA. Journal of Emergency in Traditional Chinese Medicine. 2016; 25:1833-36.
30. Gorin Y, Block K. Nox4 and diabetic nephropathy: with a friend like this, who needs enemies?. Free Radical Biology and Medicine. 2013 Aug 1;61:130-42.
31. Liu G, Ren G, Yang X. 1, 25 (OH) 2 D 3 regulates the proliferation, fibrosis, and autophagy of mesangial cells induced by high glucose via the VDR/mTOR pathway. Xi bao yu fen zi Mian yi xue za zhi= Chinese Journal of Cellular and Molecular Immunology. 2022 Mar 1;38(3):224-30.
32. Zoja C, Xinaris C, Macconi D. Diabetic nephropathy: novel molecular mechanisms and therapeutic targets. Frontiers in Pharmacology. 2020 Dec 21;11:586892.
33. Zhang NN, Ma Y, Deng K, Mao XM, Yao L. Anti-fibrosis effect and its related mechanism of coreopsis tinctoria flavonoids on high glucose and high fat-induced rat glomerular mesangial cells. Journal of Chinese Medicinal Materials. 2018;41:949-54.
34. Qiao S, Liu R, Lv C, Miao Y, Yue M, Tao Y, et al. Bergenin impedes the generation of extracellular matrix in glomerular mesangial cells and ameliorates diabetic nephropathy in mice by inhibiting oxidative stress via the mTOR/β-TrcP/Nrf2 pathway. Free Radical Biology and Medicine. 2019 Dec 1;145:118-35.
35. Yoon JJ, Park JH, Lee YJ, Kim HY, Han BH, Jin HG, et al. Protective effects of ethanolic extract from rhizome of Polygoni avicularis against renal fibrosis and inflammation in a diabetic nephropathy model. International Journal of Molecular Sciences. 2021 Jul 5;22(13):7230.
36. Zhao T, Sun S, Zhang H, Huang X, Yan M, Dong X, et al. Therapeutic effects of tangshen formula on diabetic nephropathy in rats. PloS one. 2016 Jan 25;11(1):e0147693.
37. Zhao TT, Zhang HJ, Lu XG, Huang XR, Zhang WK, Wang H, et al. Chaihuang-Yishen granule inhibits diabetic kidney disease in rats through blocking TGF-β/Smad3 signaling. PloS one. 2014 Mar 19;9(3):e90807.
38. Wang Q, Zhu TH, Zhang M, Chen D. Role of the E3 ubiquitin ligase Smurf family in bone morphogenetic proteins and transforming growth factor signal transduction. Progress in Biochemistry and Biophysics. 2006;5:423-30.
39. Twigg SM, Cooper ME. The time has come to target connective tissue growth factor in diabetic complications. Diabetologia. 2004 Jun;47:965-8.