Abstract
The diagnosis of azoospermia is a devasting one for men – and it represents the obliteration of so many unspoken hopes and expectations. Most causes of azoospermia are not amenable to treatment but those due to hypogonadotropic hypogonadism can respond to gonadotropin therapy. This can be a lengthy and expensive treatment, but it delivers an opportunity to men to restore, albeit temporarily, their production of sperm. Encouragingly, treatment is associated with good success rates, even outside of specialist clinics. In this commentary, we present an understanding of the underlying pathophysiology in hypogonadotropic hypogonadism, together with a simple outline from leading clinics on the treatments used to induce spermatogenesis. We review options to consider before embarking on fertility-altering treatment in medicine and in surgery.
Keywords
Gonadotrophins, Hypogonadotropic hypogonadism, Sperm count, Testicular volume (TV), Spermatogenesis, Children, Subfertility
Introduction
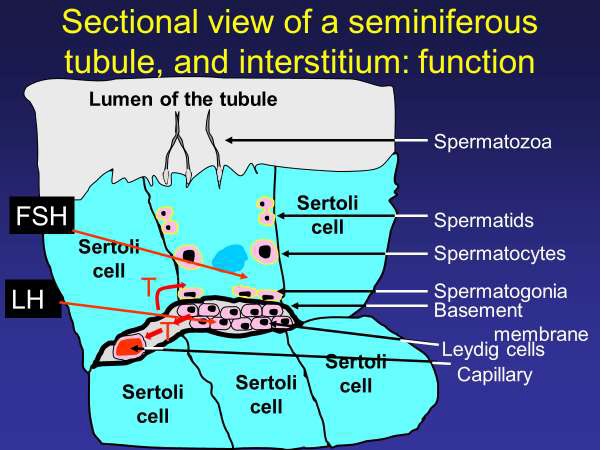
Male factor subfertility is present in as many as half of sub fertile couples and may be a sole contributor to subfertility in about 20% (1), with azoospermia in 6% [1]. The testes produce testosterone and spermatozoa. The Leydig cells produce testosterone (T) (Figure 1), the primary driver of virilisation and maintaining the male phenotype. Spermatogenesis occurs in the seminiferous tubules. Hypogonadism can refer to impairment of either or both functions [2].
A rare cause of male subfertility is hypothalamic–pituitary axis dysfunction. Inadequate pituitary gonadotroph functions leads to hypogonadotropic hypogonadism (HH), which can be congenital (CHH), or it can be acquired (AHH) – resulting from a range of intracerebral pathologies such as craniopharyngiomas, active pituitary adenomas, iron overload secondary to thalassaemia, pituitary surgery, head trauma or cranial/pituitary radiation [10]. The clinical presentation of HH depends on the age of onset of the condition and the severity (whether complete or partial deficiency) [3]. HH is a secondary hypogonadism characterised by low testosterone concentrations, reduced spermatogenesis and low or inappropriately normal concentrations of gonadotropins (Table 1) [4]. CHH can be further divided into those with anosmia i.e., Kallmann syndrome (KS) or those without [5]. KS is the commonest cause (60%) of an isolated GnRH deficiency [6] and will lead to an absent or blunted expression of puberty. In the general population, azoospermia is estimated to occur in 6% of couples [1, 7, 8] and in about 0.2%, hypogonadotropism will be the cause [1]. AHH may also result in absent puberty or puberty arrest depending on the timing of the insult to the hypothalamic-pituitary-gonadal axis. When pituitary tumours (functional or silent (60% of cases)) [9] lead to AHH, more than 50% of the tumours are benign in themselves [9], but their treatment leads to spermatogenetic failure.
Figure 1: Where do FSH and LH have their effects within the testis? An illustration of the Leydig and Sertoli cells, with the immunological basement membrane barrier separating them. The Leydig cells producing testosterone under LH stimulation, that testosterone crossing the basement membrane into Sertoli cells and priming them to respond to FSH. This response is expressed as sperm cell production and extrusion, into the lumen of the seminferous tubule. FSH: Follicle Stimulating hormone; LH: Luteinizing hormone; T: Testosterone.
|
Clinical condition |
FSH |
LH |
Testosterone |
PRL |
|
Normal spermatogenesis |
Normal |
Normal |
Normal |
Normal |
|
Hypogonadotropic hypogonadism |
Low |
Low |
Low |
Normal |
|
Abnormal spermatogenesisi |
High/Normal |
Normal |
Normal |
Normal |
|
Complete testicular failure/hypergonadotropic hypogonadism |
High |
High |
Normal/Low |
Normal |
|
PRL-secreting pituitary tumour |
Normal/low |
Normal/low |
Low |
High |
|
imarkedly elevated isolated serum FSH is indicative of an abnormality in spermatogenesis. |
||||
Underlying pathophysiology
Endocrine control of spermatogenesis
Spermatogenesis occurs in the seminiferous tubules of the testis [10,11]. Spermatogenesis requires two gonadotrophins, follicle-stimulating hormone (FSH) and luteinizing hormone (LH) [12]. In women, these hormones are just as important as in men – with FSH being the main driver to folliculogenesis and LH acting on FSH-primed cells to induce ovulation. In men, endocrine function is reversed with LH being the main stimulator of testosterone production acting on the Leydig cells, and testosterone then primes the Sertoli cells lining the seminiferous tubules by inducing receptors to FSH (Figure 1). The two principle active cell groups in the testis (Sertoli and Leydig) are kept separate from each other by a basement membrane, whose tight junctions between cells acts as an immunological barrier between the Leydig cells and the seminiferous tubules (as the maturing haploid spermatozoa in the tubules are biologically and immunologically dissimilar to the remaining diploid cells in the rest of the body). LH stimulates the Leydig cells to produce testosterone (T), whilst FSH stimulates the Sertoli cells, which require testosterone for receptor induction, to maintain the process of spermatogenesis [12]. The concentration of circulating FSH correlates strongly with Sertoli cell number and testis size in adulthood [13] and in the absence of FSH, or its receptor, the Sertoli cell number is reduced by as much as 30–45% [12]. A reliable marker of Sertoli cell activity (cell growth and differentiation) is the production of Inhibin B [14-16].
Underlying causes for HH
These can occur at the upper two of the three levels (hypothalamus, pituitary) of the HPG axis. The lower of the three levels, testicular, is associated with a hypergonadotropic state. HH can also develop as some medications act to supress the HPG axis.
In the hypothalamus, GnRH deficiency is most often a congenital condition classified according to the presence or absence of the sense of smell. Congenital HH with anosmia is referred to as Kallmann syndrome and along with CHH with a normal sense of smell, both forms can be inherited through a variety of different genetic mutations [17].
Many forms of pituitary pathology, including mass lesions or iatrogenic causes, can interrupt or suppress gonadotropin secretion [18]. Masses, such as adenomas or craniopharyngiomas, within the sella turcica can affect gonadotroph cells due to a pressure effect. A prolactinoma (the most common pituitary adenoma) may result in hyperprolactinemia, which independently suppresses the hypothalamic-pituitary-gonadal axis and causes HH [19]. Both trans-sphenoidal surgery and cranial radiation can result in hypopituitarism [4,19].
Medications can exert an effect on the HPG axis, leading to infertility. Long term opioid therapy results in interruption of hypothalamic GnRH secretion leading to hypogonadism, present in up to 63% of male opioid users [20]. Glucocorticoids inhibit GnRH synthesis which results in low serum androgens. For example, prednisone can suppress serum T levels within three days in daily doses of 15 mg or higher. The suppressive effect is dose dependent [21,22].
Treatment regimens
Successful treatment depends on appropriate diagnosis, and the instigation of appropriate therapy. HH is the only cause of male infertility that can be successfully treated using gonadotrophin replacement. Spermatogenesis protocols using gonadotrophins replicate the natural endocrine control of spermatogenesis [23]. Men with HH are typically azoospermic [24], and response to therapy falls into three broad groups based upon their baseline characteristics. The mildest phenotype, with the most favourable outcomes, are men with adult?onset HH and secondary subfertility [25,26]. The next group, approximately one?third of CHH patients, experience some degree of spontaneous puberty rather than a complete failure of puberty. Typically their puberty arrests early, meaning these men often have a testicular volume (TV) > 4 mL and no history of cryptorchidism with a reasonable prognosis for spermatogenesis [23].The worst treatment prognosis is associated with the third group, CHH and a history of bilateral cryptorchidism and small testes (TV ≤ 4 mL) [25]. The seminiferous tubules (the site of spermatogenesis) account for approximately 90% of TV; hence, the size of the testes is a critical indicator of fertility potential [27].
Pulsatile GnRH
Induction of spermatogenesis in men with HH may be achieved by replacing GnRH in a pulsatile manner via an infusion pump, thus recreating normal physiology [23]. Normal T levels can be achieved in over 90% of patients by 6 months [14]. With pulsatile GnRH therapy, men that have undergone partial or complete puberty can achieve normal TV and normal median sperm concentration when compared to men with absent puberty (median sperm concentration of 21 (partial), 36 (complete) and 3 (absent) x 106/ml; P = 0.05) (14). In that study, time to spermatogenesis varies from 2 to 24 months, all men with partial or complete puberty achieved spermatogenesis within 6 months compared to only 50% of men with absent puberty [14]. However, GnRH agonist pump therapy is not widely available. They are currently only used in research settings due to the limited availability of the drug, a suitable infusion device, and the clinical expertise required to monitor the treatment [23]. This treatment modality is not appropriate for patients with a non-functioning pituitary gland, whether this stems from a disease process or an iatrogenic consequence of treatment. When comparing fertility outcomes for pulsatile GnRH and gonadotrophin regimens, no advantage to GnRH agonist pump has been shown [25].
HCG monotherapy
Men with AHH following complete pubertal development have an excellent chance of achieving spermatogenesis following gonadotrophin treatment. Human chorionic gonadotrophin (hCG) contains a common alpha subunit with LH, FSH and TSH. The beta subunit is what confers a structural differentiation; however, all forms of hCG and LH bind to a common receptor. An important distinction between the two hormones is the difference in half-life. LH has a half-life of approximately 25 to 30 minutes, while hCG has a much longer half-life at 37 hours [28]. This allows hCG to serve as a long?acting LH?analogue that can be self?administered by subcutaneous injection 2?3 times per week [23 29]. hCG monotherapy is successful in inducing testicular development and spermatogenesis and fertility is typically achieved within 3?24 months [29,30]. Monotherapy with hCG is best used to stimulate spermatogenesis in those CHH men with partial or near complete testicular development (TV > 4ml) [30]. Finkel et al. demonstrated that 1/15 of men with absent puberty achieved spermatogenesis with hCG-only treatment in comparison to 6/6 men who had completed puberty (P ≤ 0.002)(31). Following the addition of human menopausal gonadotrophin (hMG), 5/7 men without cryptorchidism achieved spermatogenesis [31]. Long term (that is, up to 18 months) hCG monotherapy achieved spermatogenesis in only 34/84 men with absent puberty [32]. Therefore, if following hCG monotherapy, spermatogenesis is not achieved within 6 months, or the count remains suboptimal (without conception), FSH should be added to the regimen (i.e., 75?300 IU, 3 times per week) [23,30]. Semen analysis parameters will not be within World Health Organisation criteria [33]. However, this does not preclude natural fertility and may enable successful treatment with intracytoplasmic sperm injection (ICSI) [29].
Combination gonadotrophin treatment with hCG and FSH
Men with CHH, absent puberty, and prepubertal testes (TV < 4 mL) have the poorest outcomes for sperm induction treatment [40]. Follicle?stimulating hormone preparations, in the form of purified urinary gonadotrophins (hMG) or recombinant FSH (recFSH) are recognised to be effective in spermatogenesis [27]. The time to achieve spermatogenesis ranges from 3 to 19 months with a median treatment time of 9?12 months range with varying regimens [27,34,35]. In a long term (up to 10 years) gonadotrophin therapy, only 36% of the CHH men with prepubertal testes developed sperm compared to 71% with some testicular development [36]. Another study of a larger cohort of men with CHH, using a standardised combined gonadotrophin regime, found that nearly 90% of the men achieved sperm production. The median time to spermatogenesis was 7 months and, with 38/75 men becoming fathers, the median time to conception was 28 months [26]. Reinforcing the findings from this study, a combined analysis of several studies with a structured combined gonadotrophin protocol found that 84% of men with HH developed sperm and 69% achieved sperm concentrations ≥1.5 x106/mL [34]. Their analysis also found that small TV was a negative predictor of treatment outcome, also reported elsewhere [29]. CHH men with smaller testes (≤4 mL) are much more likely to have had cryptorchidism [36] and typically require longer treatment courses to achieve spermatogenesis [35,37]. Those men with smaller testes at outset of treatment also achieve lower sperm counts compared to those with a larger TV [14]. A commonly recognised combined gonadotrophin regime is hCG monotherapy with injections i.e., 1000 - 2000 Units two to three times per week. Treatment with FSH can be initiated immediately, but in most cases, hCG monotherapy is continued for 3 - 6 months. If no sperm are present in the ejaculate, then FSH is added (i.e. 75 - 150 IU every other day/three times per week) [10,27,29,38].
Sequential/combined therapy with FSH and hCG
Approximately 90% of testicular volume is determined by the seminiferous tubules – the site of spermatogenesis [27]. Stimulation of seminiferous tubule development, via the proliferation of Sertoli and germ cells, is an essential step for achieving spermatogenesis, especially in men with prepubertal testes [10]. Poor Sertoli cell proliferation means that monotherapy with hCG is ultimately less effective in the treatment of CHH with absent puberty. Therefore, it would seem reasonable to recreate the physiology of puberty by providing a course of unopposed FSH to cause Sertoli cell proliferation prior to maturation with hCG [38, 39]. Studies into the effect of pre-treatment with recombinant FSH in males with prepubertal testes have shown increase in TV, serum Inhibin B and AMH levels [15,16].
A randomized controlled trial (RCT) compared 4-months of recFSH treatment prior to gonadotrophin therapy versus a standard treatment protocol in CHH men with prepubertal testes [39]. In four months the recFSH treatment induced normal serum Inhibin B levels [14-16] and TV doubled. Histology samples revealed Sertoli cells and spermatogonia proliferation as well as cytoskeletal rearrangements. All men receiving recFSH pre-treatment developed sperm in their ejaculate (7/7) compared to 4/6 in the standard protocol. The recFSH pre-treatment group also trended towards higher maximal sperm counts [46]. These trends support the sequential treatment approach, including pre-treatment with FSH, in inducing testicular growth and fertility in CHH men with prepubertal testes. However, these studies were underpowered, and lack reliability [39]. When considering the effect of FSH on Sertoli cell, an in-vitro study on porcine Sertoli cells, found that differing preparations have slightly different molecular effects [40]. The authors postulated that this may allow for individualising FSH treatment to the specific phenotype of HH. However, no human studies have looked at this effect.
Monitoring and side effects of treatment
Some potential adverse effects of fertility?inducing treatment exist, though none are life-threatening. Local irritation at the site of injection is possible [41] and gynecomastia is the most common side effect of hCG treatment, resulting from hCG induced oestradiol secretion, and can affect as many as one third of patients [42]. Elevated haematocrit, likely as a response to rising T levels, is also a common adverse effect of hCG [23,27]. When monitoring the response to treatment it is important to consider the half?life of hCG as 36?hours and therefore trough testosterone concentrations are most useful [27].
Clinically, assessment of testicular volume gives an important insight into a man’s response to treatment [43]. However, men with limited testicular growth can still have sperm in the ejaculate [44]. It is prudent to offer sperm cryopreservation on successful achievement of spermatogenesis [23,27].
Fertility-Altering Treatment in Medicine and in Surgery
In an adult male, with a functioning HPG axis, who may be at risk of iatrogenic hypogonadism that will impair spermatogenesis, it is salutary to consider pre-emptively storing sperm in freeze storage. will avoid the expense and inconvenience of gonadotrophin therapy to induce spermatogenesis after the fact. Far too often in reproductive medicine practice, instances arise in which a little thought on the part of surgical, medical, or oncological colleagues would obviate the need for gonadotrophin therapy [45]. While 91% of oncologists agreed that sperm banking should be offered to all men at risk of infertility as a result of cancer treatment, 48% never bring up the topic or mention it to less than 25% of eligible men [45].
Clinicians dealing with breast and blood malignancies seem, in practice, to be most aware of this need. Other colleagues would be advised to consider this in any man about to have radiotherapy or surgery which is likely to have an impact on the normal functioning of the HPG axis.
Discussion
Medical treatments can improve the chances of men with congenital or acquired HH to father a biological child. The strength of evidence for some of them is weak. Excluding those men whose clinical and biochemical findings are negative predictors of success, most men in research studies [8] and clinical practice [29] are likely to produce sufficient sperm to use in assisted reproduction, though probably not for natural conception. Negative predictive factors for the response of men to treatment include small testicular volume (<4ml); increased baseline gonadotrophins; absent puberty; and cryptorchidism [25,26,31]. Pubertal status, and the absence of puberty, reduces the chance of achieving spermatogenesis compared to a cohort of pre and post pubertal men by almost 20% [68% vs. 84% 95% CI, p = 0.011] [24]. Increased gonadotropin levels at baseline may indicate a degree of primary testicular failure contributing to the hypogonadism [24].
Findings from large reviews of data on treatment are not wholly optimistic. The most recent Cochrane review concluded that as numbers of trials and participants are small, evidence is insufficient to allow final conclusions [46]. A meta-analysis from the same time found hCG/hMG to be equivalent in effectiveness to the GnRH pump, with success rates of 75% and 75% in achieving spermatogenesis (though some studies considered that meant finding one sperm) with mean sperm concentrations obtained of 5.9 and 4.3 million/mL for gonadotropin and GnRH therapy [25]. Table 2 summaries descriptive papers published since then, examining the value of gonadotrophin therapy in spermatogenesis.
|
Subjects |
Drugs used |
Effectiveness |
Reference |
|
13 |
urinary hCG x 3/12 hMG/recFSH added thereafter, up to 20 months. Increase in dose up to 225 iu |
Spermatogenesis in all subjects within 3-19 months |
[8] |
|
36 |
hCG/hMG |
23 with testicular volume (TV) <4 ml, 13 with TV >4 ml. |
[36] |
|
117 |
hCG/hMG |
22% achieved spermatogenesis, in the range 5-10 x106 sperm/ml |
[50] |
|
16 |
hCG/hMG |
13 men produced sperm, 7 fathered a pregnancy, one of which was spontaneous, remainder with AR |
[29] |
|
21 |
hCG/hMG |
6 subjects with normal puberty achieved spermatogenesis with hCG only, 7 of the remainder did so with added hMG |
[31] |
|
6 |
GnRH pump x 24 months |
4 produced sperm, 1 conceived without AR, 1 had several unsuccessful AR attempts |
[39] |
| hCG: human Chorionic Gonadotrophin; hMG: human Menopausal Gonadotrophin; recFSH: recombinant Follicle Stimulating Hormone; iu: international units; AR: Assisted Reproduction; GnRH: Gonadotrophin Releasing Hormone. The annotation hCG/hMG reflects hCG treatment for 2 -3 months followed by hMG for 4 months. | |||
This paper focusses on the challenges for clinical care in men with hypogonadotrophic hypogonadism, using gonadotrophins. Our experience in 30 years of clinical practice has been that gonadotrophin use in men with normal gonadotrophins is fruitless, while others seem to suggest that this is a feasible option for these men [47]. We would not agree that this is a clinically or financially reasonable approach to promote, and do not consider that their data support that approach.
Conclusion, Challenges and Future Directions
Encountering a diagnosis of azoospermia associated with any form of hypogonadotrophic hypogonadism (HH) is the only form of azoospermia that is amenable to treatment. Best results are achieved in men who go through a normal puberty and then develop an acquired form of HH thereafter, along with other negative predictive factors described above. Therapies leading to iatrogenic hypogonadism should be preceded by adequate directive counselling on fertility options.
The biggest current challenge with this treatment is the prolonged nature of it, whether monotherapy with hCG or combined therapy with FSH is used. This is considerable strain on the individual to maintain compliance over months of thrice-weekly treatment. If oral gonadotrophins were available, this would certainly ease the burden but not the duration of treatment. Other therapeutic options such as clomiphene, aromatase inhibitors and nasal testosterone are proposed but without clinical data to support their use for spermatogenesis [48].
Conflicts of Interest
Neither Dr. Morris, nor Prof. Cahill have any conflicts of interest in writing this paper.
Funding
Neither Dr. Morris, nor Prof. Cahill received any funding in the writing of this paper.
Acknowledgments
This work is our own and we have received no external assistance in its preparation or writing.
References
2. Bhasin S, Cunningham GR, Hayes FJ, Matsumoto AM, Snyder PJ, Swerdloff RS, et al. Testosterone therapy in men with androgen deficiency syndromes: an Endocrine Society clinical practice guideline. J Clin Endocrinol Metab. 2010;95(6):2536-59.
3. Seminara SB, Hayes FJ, Crowley WF, Jr. Gonadotropin-releasing hormone deficiency in the human (idiopathic hypogonadotropic hypogonadism and Kallmann's syndrome): pathophysiological and genetic considerations. Endocr Rev. 1998;19(5):521-39.
4. Basaria S. Male hypogonadism. Lancet. 2014;383(9924):1250-63.
5. Rohayem J, Sinthofen N, Nieschlag E, Kliesch S, Zitzmann M. Causes of hypogonadotropic hypogonadism predict response to gonadotropin substitution in adults. Andrology. 2016;4(1):87-94.
6. Balasubramanian R, Crowley WF, Jr. Isolated GnRH deficiency: a disease model serving as a unique prism into the systems biology of the GnRH neuronal network. Mol Cell Endocrinol. 2011;346(1-2):4-12.
7. MacColl G, Quinton R, Bouloux PM. GnRH neuronal development: insights into hypogonadotrophic hypogonadism. Trends Endocrinol Metab. 2002;13(3):112-8.
8. Oldereid NB, Abyholm T, Tanbo TG. Spermatogenesis and fertility outcome in male hypogonadotrophic hypogonadism. Hum Fertil (Camb). 2010;13(2):83-9.
9. Torregrosa-Quesada ME, García-Martínez A, Sánchez-Barbie A, Silva-Ortega S, Cámara R, Fajardo C, et al. The silent variants of pituitary tumors: demographic, radiological and molecular characteristics. J Endocrinol Invest. 2021.
10. Boehm U, Bouloux P-M, Dattani MT, de Roux N, Dodé C, Dunkel L, et al. European Consensus Statement on congenital hypogonadotropic hypogonadism—pathogenesis, diagnosis and treatment. Nature Reviews Endocrinology. 2015;11(9):547-64.
11. Smith LB, Walker WH. The regulation of spermatogenesis by androgens. Semin Cell Dev Biol. 2014;30:2-13.
12. Oduwole OO, Peltoketo H, Huhtaniemi IT. Role of Follicle-Stimulating Hormone in Spermatogenesis. Front Endocrinol (Lausanne). 2018;9:763.
13. Allan CM, Garcia A, Spaliviero J, Zhang FP, Jimenez M, Huhtaniemi I, et al. Complete Sertoli cell proliferation induced by follicle-stimulating hormone (FSH) independently of luteinizing hormone activity: evidence from genetic models of isolated FSH action. Endocrinology. 2004;145(4):1587-93.
14. Pitteloud N, Hayes FJ, Dwyer A, Boepple PA, Lee H, Crowley WF, Jr. Predictors of outcome of long-term GnRH therapy in men with idiopathic hypogonadotropic hypogonadism. J Clin Endocrinol Metab. 2002;87(9):4128-36.
15. Raivio T, Wikstrom AM, Dunkel L. Treatment of gonadotropin-deficient boys with recombinant human FSH: long-term observation and outcome. Eur J Endocrinol. 2007;156(1):105-11.
16. Young J, Chanson P, Salenave S, Noël Ml, Brailly S, O’Flaherty Mn, et al. Testicular Anti-Müllerian Hormone Secretion Is Stimulated by Recombinant Human FSH in Patients with Congenital Hypogonadotropic Hypogonadism. The Journal of Clinical Endocrinology & Metabolism. 2005;90(2):724-8.
17. Cangiano B, Swee DS, Quinton R, Bonomi M. Genetics of congenital hypogonadotropic hypogonadism: peculiarities and phenotype of an oligogenic disease. Hum Genet. 2021;140(1):77-111.
18. Choy JT, Amory JK. Nonsurgical Management of Oligozoospermia. J Clin Endocrinol Metab. 2020;105(12):e4194-207.
19. Siemons LJ, Mahler CH. Hypogonadotropic hypogonadism in hemochromatosis: recovery of reproductive function after iron depletion. J Clin Endocrinol Metab. 1987;65(3):585-7.
20. Antony T, Alzaharani SY, El-Ghaiesh SH. Opioid-induced hypogonadism: Pathophysiology, clinical and therapeutics review. Clin Exp Pharmacol Physiol. 2020;47(5):741-50.
21. Calogero AE, Burrello N, Bosboom AM, Garofalo MR, Weber RF, D'Agata R. Glucocorticoids inhibit gonadotropin-releasing hormone by acting directly at the hypothalamic level. J Endocrinol Invest. 1999;22(9):666-70.
22. MacAdams MR, White RH, Chipps BE. Reduction of serum testosterone levels during chronic glucocorticoid therapy. Ann Intern Med. 1986;104(5):648-51.
23. Prior M, Stewart J, McEleny K, Dwyer AA, Quinton R. Fertility induction in hypogonadotropic hypogonadal men. Clin Endocrinol (Oxf). 2018;89(6):712-8.
24. Kumar R. Medical management of non-obstructive azoospermia. Clinics (Sao Paulo). 2013;68 Suppl 1(Suppl 1):75-9.
25. Rastrelli G, Corona G, Mannucci E, Maggi M. Factors affecting spermatogenesis upon gonadotropin-replacement therapy: a meta-analytic study. Andrology. 2014;2(6):794-808.
26. Liu PY, Baker HW, Jayadev V, Zacharin M, Conway AJ, Handelsman DJ. Induction of spermatogenesis and fertility during gonadotropin treatment of gonadotropin-deficient infertile men: predictors of fertility outcome. J Clin Endocrinol Metab. 2009;94(3):801-8.
27. Dwyer AA, Raivio T, Pitteloud N. Gonadotrophin replacement for induction of fertility in hypogonadal men. Best practice & research Clinical endocrinology & metabolism. 2015;29(1):91-103.
28. Cole LA. Biological functions of hCG and hCG-related molecules. Reprod Biol Endocrinol. 2010;8:102.
29. Morris GC, Lloyd-Evans E, Cahill DJ. Induction of spermatogenesis in men with hypogonadotropic hypogonadism. J Assist Reprod Genet. 2021.
30. Nieschlag E, Bouloux PG, Stegmann BJ, Shankar RR, Guan Y, Tzontcheva A, et al. An open-label clinical trial to investigate the efficacy and safety of corifollitropin alfa combined with hCG in adult men with hypogonadotropic hypogonadism. Reprod Biol Endocrinol. 2017;15(1):17.
31. Finkel DM, Phillips JL, Snyder PJ. Stimulation of spermatogenesis by gonadotropins in men with hypogonadotropic hypogonadism. N Engl J Med. 1985;313(11):651-5.
32. Yang L, Zhang SX, Dong Q, Xiong ZB, Li X. Application of hormonal treatment in hypogonadotropic hypogonadism: more than ten years experience. Int Urol Nephrol. 2012;44(2):393-9.
33. Cooper TG, Noonan E, von Eckardstein S, Auger J, Baker HW, Behre HM, et al. World Health Organization reference values for human semen characteristics. Hum Reprod Update. 2010;16(3):231-45.
34. Warne DW, Decosterd G, Okada H, Yano Y, Koide N, Howles CM. A combined analysis of data to identify predictive factors for spermatogenesis in men with hypogonadotropic hypogonadism treated with recombinant human follicle-stimulating hormone and human chorionic gonadotropin. Fertil Steril. 2009;92(2):594-604.
35. Barrio R, de Luis D, Alonso M, Lamas A, Moreno JC. Induction of puberty with human chorionic gonadotropin and follicle-stimulating hormone in adolescent males with hypogonadotropic hypogonadism. Fertil Steril. 1999;71(2):244-8.
36. Miyagawa Y, Tsujimura A, Matsumiya K, Takao T, Tohda A, Koga M, et al. OUTCOME OF GONADOTROPIN THERAPY FOR MALE HYPOGONADOTROPIC HYPOGONADISM AT UNIVERSITY AFFILIATED MALE INFERTILITY CENTERS: A 30-YEAR RETROSPECTIVE STUDY. The Journal of Urology. 2005;173(6):2072-5.
37. Bouloux P-MG, Nieschlag E, Burger HG, Skakkebaek NE, Wu FCW, Handelsman DJ, et al. Induction of Spermatogenesis by Recombinant Follicle-Stimulating Hormone (Puregon) in Hypogonadotropic Azoospermic Men Who Failed to Respond to Human Chorionic Gonadotropin Alone. J Androl. 2003;24(4):604-11.
38. Han TS, Bouloux PM. What is the optimal therapy for young males with hypogonadotropic hypogonadism? Clin Endocrinol (Oxf). 2010;72(6):731-7.
39. Dwyer AA, Sykiotis GP, Hayes FJ, Boepple PA, Lee H, Loughlin KR, et al. Trial of recombinant follicle-stimulating hormone pretreatment for GnRH-induced fertility in patients with congenital hypogonadotropic hypogonadism. J Clin Endocrinol Metab. 2013;98(11):E1790-5.
40. Arato I, Grande G, Barrachina F, Bellucci C, Lilli C, Jodar M, et al. “In vitro” Effect of Different Follicle—Stimulating Hormone Preparations on Sertoli Cells: Toward a Personalized Treatment for Male Infertility. Front Endocrinol (Lausanne). 2020;11(401).
41. Sohan K, Cahill D, Stegmann M. Local reaction to s.c. injections of a recombinant gonadotrophin preparation possibly related to the osmolality of the reconstituted solution. Hum Reprod. 1999;14(7):1921.
42. Maddock WO, NELSON WO. The effects of chorionic gonadotropin in adult men: increased estrogen and 17-ketosteroid excretion, gynecomastia. Leydig cell stimulation and seminiferous tubule damage*. The Journal of Clinical Endocrinology & Metabolism. 1952;12(8):985-1014.
43. Anawalt BD. Approach to Male Infertility and Induction of Spermatogenesis. The Journal of Clinical Endocrinology & Metabolism. 2013;98(9):3532-42.
44. Buchter D, Behre HM, Kliesch S, Nieschlag E. Pulsatile GnRH or human chorionic gonadotropin/human menopausal gonadotropin as effective treatment for men with hypogonadotropic hypogonadism: a review of 42 cases. European journal of endocrinology. 1998;139(3):298-303.
45. Schover LR, Brey K, Lichtin A, Lipshultz LI, Jeha S. Oncologists’ Attitudes and Practices Regarding Banking Sperm Before Cancer Treatment. Journal of Clinical Oncology. 2002;20(7):1890-7.
46. Attia AM, Abou‐Setta AM, Al‐Inany HG. Gonadotrophins for idiopathic male factor subfertility. Cochrane Database of Systematic Reviews. 2013(8).
47. Behre HM. Clinical Use of FSH in Male Infertility. Front Endocrinol (Lausanne). 2019;10(322).
48. Carrasquillo R, Chu K, Ramasamy R. Novel Therapy for Male Hypogonadism. Curr Urol Rep. 2018;19(8):63.
49. Diagnostic evaluation of the infertile male: a committee opinion. Fertil Steril. 2015;103(3):e18-25.
50. Lin J, Mao J, Wang X, Ma W, Hao M, Wu X. Optimal treatment for spermatogenesis in male patients with hypogonadotropic hypogonadism. Medicine (Baltimore). 2019;98(31):e16616.