Abstract
Angiopoietin-like 4 (ANGPTL4) belongs to the angiopoietin-like protein family and mediates the inhibition of lipoprotein lipase activity. Emerging evidence suggests that ANGPTL4 has pleiotropic functions with anti- and pro-inflammatory properties. Here, we have reviewed the research progress on ANGPTL4 and systematically discussed the dual role of ANGPTL4 in inflammation and inflammatory diseases. Understanding the potential mechanisms of ANGPTL4 in inflammation will aid in drug discovery and treatment development.
Keywords
ANGPTL4, Inflammation, Post-translational modification, Cleavage
Abbreviations
AMI: Acute Myocardial Infarction; ANGPTL4: Angiopoietin-like Protein 4; C5a: Component 5a; cANGPTL4: c-terminal ANGPTL4; LPL: Lipoprotein Lipase; nANGPTL4: n-terminal ANGPTL4
Commentary
Angiopoietin-like 4 (ANGPTL4) is a multifaceted secreted protein discovered by three different institutions in 2000 simultaneously [1]. It is expressed in adipose tissues, liver, muscle, heart, kidney, skin, and other tissues. The nutritional, metabolic, and inflammatory status of the organism regulate the expression of ANGPTL4 [2-4]. ANGPTL4 was initially found to inhibit lipoprotein lipase (LPL) activity thereby regulating the triglyceride levels [5-8]. Human genetic studies have shown that genetic inactivation of ANGPTL4 could reduce the risk of diabetes and coronary artery disease progression significantly [7-10]. Unfortunately, lacking ANGPTL4 in mice consumed dietary saturated fat induces a pro-inflammatory and ultimately lethal phenotype, including fibrinopurulent peritonitis, and ascites [11]. Antibodies against ANGPTL4 result in lymphadenopathy [9] and ascites in mice or monkeys [12]. These unexpected reports led us to focus on the role of ANGPTL4 in inflammatory processes [13]. While extensive research portray ANGPTL4 as an inflammatory mediator [14-18], there is a considerable body of evidence ascribing that ANGPTL4 protects against the severe pro-inflammatory effects of saturated fat and increases the number of anti-inflammatory macrophages in peritonitis and myocardial infarction (AMI) [11,19]. ANGPTL4’s role in inflammation appears to be bidirectional, and its exact mechanism is not fully understood.
In a recent issue, we systematically reviewed the functions, potential underlying mechanisms, and therapeutic value of ANGPTL4 in inflammation, including lung injury, cardiovascular disease, pancreatitis, gastrointestinal disease and metabolic disorders [20]. ANGPTL4 has been reported to be involved in inflammation processes of acute pulmonary diseases including influenza pneumonia and LPS-induced acute lung injury, as well as chronic pulmonary diseases such as chronic obstructive pulmonary disease. ANGPTL4 expression is directly upregulated by influenza infection through the IL6-STAT3 signaling cascade. Meanwhile, cleavage of full-length ANGPTL4 (fANGPTL4) to c-terminal ANGPTL4 (cANGPTL4) due to furin activation results in severe lung injury characterized by extensive pulmonary hemorrhage and immune cell infiltration [16]. In both lung tissue from an acute injury mouse model and LPS-treated human alveolar epithelial cells, ANGPTL4 expression significantly increases and is positively correlated with the inflammation in lung tissue (TNF-α, IL-6, and neutrophil infiltration) [14,21]. Patients suffering from chronic obstructive pulmonary disease often have upregulated levels of circulating ANGPTL4, which are associated with pulmonary function and systemic inflammation [22].
In addition to lung injury, ANGPTL4 also plays a crucial role in the development and progression of pancreatitis. Variations in the lipoprotein lipase pathway regulatory genes, including LPL, APOC3, APOA5, ANGPTL3, and ANGPTL4, that increase plasma triglyceride levels, are associated with a higher risk of acute pancreatitis [23]. In the microarray analysis, ANGPTL4 exhibits the most pronounced up-regulation among genes in pancreatic tissues from both mild and severe acute pancreatitis mice models. Macrophage activation and infiltration into the pancreas are enhanced by elevated ANGPTL4 levels, leading to increased complement component 5a (C5a) through the PI3K/AKT signaling pathway. C5a receptor activation results in hypercytokinemia, which advances pancreatitis by accelerating acinar cell damage [17]. In conclusion, targeting ANGPTL4 is considered a prospective therapeutic approach for pancreatitis.
Cho et al. [19] proved that in a co-culture environment with macrophages, mesenchymal stem cells actively expressed ANGPTL4, which in turn attenuated the macrophage polarization towards the pro-inflammatory phenotype. In animal models of peritonitis and myocardial infarction, injection of ANGPTL4 protein significantly enhanced the anti-inflammatory effects of macrophage infiltration. Post-acute myocardial infarction myocardial reperfusion injury encompasses a sequence of pathological responses, including hemorrhage, hematoma formation, and inflammatory responses. Hypoxia-induced ANGPTL4 expression may modulate vascular injury, infarct size, and the occurrence of no-reflow in AMI [24]. Lee et al. [25] invented a paintable and adhesive hydrogel patch that could encapsulate and effectively release the anti-inflammatory protein ANGPTL4 into the infarcted heart to alleviate the inflammatory response. Heart tissues receiving ANGPTL4-loaded hydrogel patches exhibited the reduced presence of inflammatory macrophages and cytokines (IL-1β, IL-6, and TNF-α), enhanced vascularization and structural cardiomyocyte maturation were observed in heart tissues received ANGPTL4-loaded hydrogel patches [26]. Furthermore, ANGPTL4 serum levels could be used to predict the occurrence of no-flow in ST-elevation myocardial infarction patients after successful percutaneous coronary intervention [24].
Numerous gastrointestinal diseases manifest a prolonged and intensified inflammation, which can result in substantial tissue damage following hypercykinaemia. Dextran sulfate sodium treated ANGPTL4-/- mice exhibit an exacerbated chronic inflammation, as well as the enrichment of genes associated with leukocyte migration and infiltration, which resembles the patterns observed in inflamed ulcerative colitis. In human colon epithelial cells, ANGPTL4 upregulated tristetraprolin expression to mediate the chronic inflammatory response [18]. In the intestinal mucosa of inflammatory bowel disease patients, endoscopic inflammation was found to modulate bile acid-inducible microbial genes in the microbiota, which in turn regulate the intestinal inflammatory responses via ANGPTL4 [27].
Atherosclerosis, diabetes, and obesity are regarded as chronic low-grade inflammatory diseases, which are persistent, non-specific inflammatory conditions characterized by increased concentrations of C-reactive protein, TNF-α, IL-1 [28], and activations of immune cells [29]. Tong et al. [30] demonstrated that the ANGPTL4 gene SNP1044250 independently contributes to the development of metabolism syndrome when body weight increases. The impact of ANGPTL4 on atherogenesis appears to employ both positive and negative effects depending on the context [9,31-34]. Cho et al. [35] discovered the positive effect of ANGPTL4 on vascular stability and inflammation in atherosclerosis. ANGPTL4 treatment suppressed the phenotypic transformation of smooth muscle cells into macrophage-like and foam-like cells through direct KLF4 downregulation or by diminishing the NOX1 activation of KLF4. The thickness of fibrous caps and the number of SM22α(+), SMA(+), and SM-MHC(+) cells in atherosclerotic lesions are significantly higher, whereas the number of Mac2(+) and CD68(+) cells are lower in the ANGPTL4 treated atherosclerotic mice. However, numerous studies indicate that ANGPTL4 increases vascular inflammation and vascular permeability, ultimately promoting the development of atherogenesis [36]. In mesenteric lymph nodes, ANGPTL4 overexpression inhibited postprandial lipid uptake in macrophages, thus preventing macrophage transformation into foam cells and expression of inflammatory genes (CXCL2, CCR1, PTGS2, and GDF15) [11]. Silencing of ANGPTL4 in mice liver by antisense oligonucleotides decreases diet-induced obesity, dyslipidemia, glucose intolerance, and liver damage without generating severe safety issues [37,38].
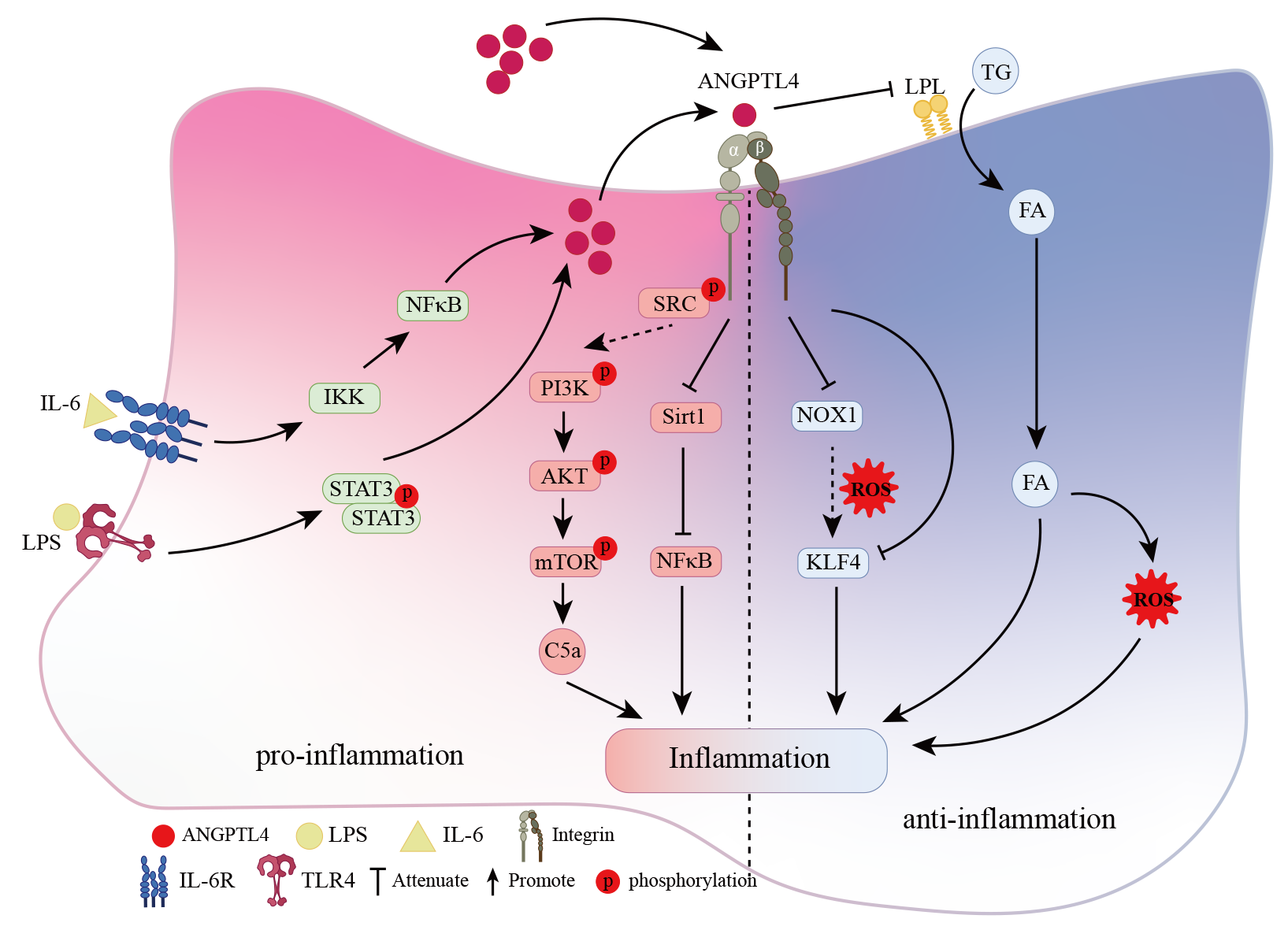
As discussed above, the role and potential mechanisms of ANGPTL4 in inflammation appear to be elusive (Figure 1). Based on the literature, we conclude that the following aspects may partly explain the current conflicting function. Post-translational modifications play vital roles in ANGPTL4 regulation, such as glycosylation, phosphorylation, and myristoylation. Treatment with N-glycosidase resulted in a reduction in the molecular weight of fANGPTL4 demonstrating that ANGPTL4 is an N-glycosylated protein [39]. N-glycosidase also led to a significant decrease in the molecular mass of cANGPTL4, implying that cANGPTL4 contains complex oligosaccharide structures [40]. Sialylation also affects the pro- or anti-inflammatory effects of ANGPTL4. The distinct sialylation form of ANGPTL4 produced in podocytes could affect albuminuria and proteinuria. Supplementation with the sialic acid precursor confers renoprotection in diabetic nephropathy [41].
Figure 1. Proposed Mechanism of ANGPTL4 in Inflammation. The influenza infection and LPS activate ANGPTL4 through IKK/NFκB pathway or STAT3 phosphorylation. Through an autocrine/paracrine mechanism, ANGPTL4 interacts with integrins and activates SRC, which further triggers the PI3K/AKT and Sirt1/NFκB signaling pathways, contributing to inflammation and facilitating tissue destruction. Conversely, the anti-inflammatory effects of ANGPTL4 may be attributed to its targeted suppression of the NOX1 pathways. In light of existing evidence, the NFκB signaling pathway demonstrates bidirectional regulation in inflammation. ANGPTL4 assumes a role in inhibiting LPL activity, regulating FA uptake, and overseeing the regulation of circulating TG-rich lipoproteins. The increased expression of ANGPTL4 results in a diminished uptake of plasma TG-derived FA, subsequently mitigating FA-induced oxidative stress, lipid peroxidation, and inflammation.
Abbreviations: FA: Fatty Acid; LPL: Lipoprotein Lipase; TG: Triglyceride.
Cleavage and oligomerization also exert crucial effects. ANGPTL4 undergoes post-translational cleavage by pro-protein convertases (PCs) at its RRKR-consensus cleavage site upon secretion, releasing n-terminal ANGPTL4 (nANGPTL4) and cANGPTL4. nANGPTL4 forms disulphide-linked dimers and tetramers. Oligomerization could increase the stability of nANGPTL4 and the ability to inhibit LPL [42]. Whether ANGPTL4 oligomerization impacts systemic inflammatory paradigms remains unclear. In addition, the correlation between truncated forms of ANGPTL4 and PC expression or activity remains unknown. During influenza pneumonia, the concomitant increase in furin activity cleaves fANGPTL4 to generate cANGPTL4, resulting in extensive lung injury characterized by host immune cell infiltration. cANGPTL4 immunoneutralization accelerates lung recovery significantly [16]. Recent findings indicated that nANGPTL4 inhibited metastasis while cANGPTL4 facilitated tumor metastasis revealed opposing functions of ANGPTL4/cANGPTL4 compared with nANGPTL4 in angiogenesis [43]. The changes of fANGPTL4/cANGPTL4 in inflammation may have different effects.
The function of ANGPTL4 is also regulated by its subcellular localization in specific cells and tissues. Data from the Human Protein Atlas suggests that ANGPTL4 is localized to the nucleoplasm and vesicles. Recent findings indicated that ANGPTL4 is enriched in exosomes [44]. More attention should be paid to the ANGPTL4’s secretory function and its role in nucleoplasm and cytoplasm. Several other factors are also probably involved. The effect of ANGPTL4 in the early and late stages of stomatitis could lead to different outcomes [45]. Current reports rarely find ANGPTL4 receptor presence in inflammatory diseases, the detailed mechanism of ANGPTL4 in inflammation needs to be explored in further studies. Moreover, it is reported that ANGPTL4 is silenced by aberrant DNA methylation of CpG islands during the development of human gastric cancers and carcinomas [46,47]. DNA methylation-mediated downregulation of ANGPTL4 promotes colorectal cancer metastasis by activating the ERK pathway [48]. Whether ANGPTL4 methylation plays a key role in inflammatory diseases requires further studies.
The present study has several limitations. First, it is unclear whether ANGPTL4 directly regulates inflammation or indirectly regulates this process by affecting lipid metabolism. Second, most of the literature focus on ANGPTL4 only and ignore other ANGPTLs with similar functions, such as ANGPTL3 and ANGPTL8. Third, the pathogenesis of ANGPTL4 has not yet been fully elucidated, but both intrinsic pathway and extrinsic pathway are assumed to play a role.
In summary, there is strong evidence that ANGPTL4 plays a dual role in the inflammatory response. Further studies are needed to elucidate the regulatory mechanisms, including the conditions that elevate ANGPTL4 expression in specific diseases, the extracellular or intracellular signaling pathways that mediate ANGPTL4 signaling, or how the outcome of this regulation affects only the local cellular microenvironment or leads to systemic inflammation.
References
2. Guo L, Li SY, Ji FY, Zhao YF, Zhong Y, Lv XJ, et al. Role of Angptl4 in vascular permeability and inflammation. Inflammation Research. 2014 Jan;63:13-22.
3. Kersten S, Mandard S, Tan NS, Escher P, Metzger D, Chambon P, et al. Characterization of the fasting-induced adipose factor FIAF, a novel peroxisome proliferator-activated receptor target gene. Journal of Biological Chemistry. 2000 Sep 15;275(37):28488-93.
4. Hato T, Tabata M, Oike Y. The role of angiopoietin-like proteins in angiogenesis and metabolism. Trends in Cardiovascular Medicine. 2008 Jan 1;18(1):6-14.
5. Liu DJ, Peloso GM, Yu H, Butterworth AS, Wang X, Mahajan A, et al. Exome-wide association study of plasma lipids in> 300,000 individuals. Nature Genetics. 2017 Dec;49(12):1758-66.
6. Klarin D, Damrauer SM, Cho K, Sun YV, Teslovich TM, Honerlaw J, et al. Genetics of blood lipids among~ 300,000 multi-ethnic participants of the Million Veteran Program. Nature Genetics. 2018 Nov;50(11):1514-23.
7. Gusarova V, O’Dushlaine C, Teslovich TM, Benotti PN, Mirshahi T, Gottesman O, et al. Genetic inactivation of ANGPTL4 improves glucose homeostasis and is associated with reduced risk of diabetes. Nature Communications. 2018 Jun 13;9(1):2252.
8. Lotta LA, Stewart ID, Sharp SJ, Day FR, Burgess S, Luan JA, et al. Genetically-enhanced LPL mediated lipolysis, LDL cholesterol lowering alleles and risk of coronary disease and type 2 diabetes. JAMA Cardiology. 2018 Oct 10;3(10):957-66.
9. Dewey FE, Gusarova V, O’Dushlaine C, Gottesman O, Trejos J, Hunt C, et al. Inactivating variants in ANGPTL4 and risk of coronary artery disease. New England Journal of Medicine. 2016 Mar 24;374(12):1123-33.
10. Stitziel NO, Stirrups KE, Masca NG, Erdmann J, Ferrario PG, Koenig IR, et al. Coding Variation in ANGPTL4, LPL, and SVEP1 and the Risk of Coronary Disease. New England Journal of Medicine. 2016;374:1134-44.
11. Lichtenstein L, Mattijssen F, de Wit NJ, Georgiadi A, Hooiveld GJ, van der Meer R, et al. Angptl4 protects against severe proinflammatory effects of saturated fat by inhibiting fatty acid uptake into mesenteric lymph node macrophages. Cell Metabolism. 2010 Dec 1;12(6):580-92.
12. Desai U, Lee EC, Chung K, Gao C, Gay J, Key B, et al. Lipid-lowering effects of anti-angiopoietin-like 4 antibody recapitulate the lipid phenotype found in angiopoietin-like 4 knockout mice. Proceedings of the National Academy of Sciences. 2007 Jul 10;104(28):11766-71.
13. Aryal B, Price NL, Suarez Y, Fernández-Hernando C. ANGPTL4 in metabolic and cardiovascular disease. Trends in Molecular Medicine. 2019 Aug 1;25(8):723-34.
14. Guo L, Li S, Zhao Y, Qian P, Ji F, Qian L, et al. Silencing angiopoietin‐like protein 4 (ANGPTL4) protects against lipopolysaccharide‐induced acute lung injury via regulating SIRT1/NF‐kB pathway. Journal of Cellular Physiology. 2015 Oct;230(10):2390-402.
15. Brown R, Imran SA, Wilkinson M. Lipopolysaccharide (LPS) stimulates adipokine and socs3 gene expression in mouse brain and pituitary gland in vivo, and in N-1 hypothalamic neurons in vitro. Journal of Neuroimmunology. 2009 Apr 30;209(1-2):96-103.
16. Li L, Chong HC, Ng SY, Kwok KW, Teo Z, Tan EH, et al. Angiopoietin-like 4 increases pulmonary tissue leakiness and damage during influenza pneumonia. Cell Reports. 2015 Feb 10;10(5):654-63.
17. Jung KH, Son MK, Yan HH, Fang Z, Kim J, Kim SJ, et al. ANGPTL 4 exacerbates pancreatitis by augmenting acinar cell injury through upregulation of C5a. EMBO Molecular Medicine. 2020 Aug 7;12(8):e11222.
18. Phua T, Sng MK, Tan EH, Chee DS, Li Y, Wee JW, et al. Angiopoietin-like 4 mediates colonic inflammation by regulating chemokine transcript stability via tristetraprolin. Scientific Reports. 2017 Mar 13;7(1):44351.
19. Im Cho D, Kang HJ, Jeon JH, Eom GH, Cho HH, Kim MR, et al. Antiinflammatory activity of ANGPTL4 facilitates macrophage polarization to induce cardiac repair. JCI Insight. 2019 Aug 8;4(16):e125437.
20. Zuo Y, He Z, Chen Y, Dai L. Dual role of ANGPTL4 in inflammation. Inflammation Research. 2023 Jun 10;72:1303-13.
21. Wang Y, Chen H, Li H, Zhang J, Gao Y. Effect of angiopoietin-like protein 4 on rat pulmonary microvascular endothelial cells exposed to LPS. International Journal of Molecular Medicine. 2013 Sep 1;32(3):568-76.
22. Wu YQ, Shen YC, Wang H, Zhang JL, Li DD, Zhang X, et al. Serum angiopoietin-like 4 is over-expressed in COPD patients: association with pulmonary function and inflammation. European Review for Medical & Pharmacological Sciences. 2016 Jan 1;20(1):44-53.
23. Hansen SE, Madsen CM, Varbo A, Tybjærg-Hansen A, Nordestgaard BG. Genetic variants associated with increased plasma levels of triglycerides, via effects on the lipoprotein lipase pathway, increase risk of acute pancreatitis. Clinical Gastroenterology and Hepatology. 2021 Aug 1;19(8):1652-60.
24. Bouleti C, Mathivet T, Serfaty JM, Vignolles N, Berland E, Monnot C, et al. Angiopoietin-like 4 serum levels on admission for acute myocardial infarction are associated with no-reflow. International Journal of Cardiology. 2015 May 6;187:511-6.
25. Lee M, Kim YS, Park J, Choe G, Lee S, Kang BG, et al. A paintable and adhesive hydrogel cardiac patch with sustained release of ANGPTL4 for infarcted heart repair. Bioactive Materials. 2024 Jan 1;31:395-407.
26. Galaup A, Gomez E, Souktani R, Durand M, Cazes A, Monnot C, et al. Protection against myocardial infarction and no-reflow through preservation of vascular integrity by angiopoietin-like 4. Circulation. 2012 Jan 3;125(1):140-9.
27. Hernández-Rocha C, Borowski K, Turpin W, Filice M, Nayeri S, Raygoza Garay JA, et al. Integrative analysis of colonic biopsies from inflammatory bowel disease patients identifies an interaction between microbial bile acid-inducible gene abundance and human angiopoietin-like 4 gene expression. Journal of Crohn's and Colitis. 2021 Dec 1;15(12):2078-87.
28. Musso G, Gambino R, Cassader M. Interactions between gut microbiota and host metabolism predisposing to obesity and diabetes. Annual Review of Medicine. 2011 Feb 18;62:361-80.
29. Niccoli G, Montone RA, Sabato V, Crea F. Role of allergic inflammatory cells in coronary artery disease. Circulation. 2018 Oct 16;138(16):1736-48.
30. Tong Z, Peng J, Lan H, Sai W, Li Y, Xie J, et al. Cross-talk between ANGPTL4 gene SNP Rs1044250 and weight management is a risk factor of metabolic syndrome. Journal of Translational Medicine. 2021 Dec;19:72.
31. Do R, Willer CJ, Schmidt EM, Sengupta S, Gao C, Peloso GM, et al. Common variants associated with plasma triglycerides and risk for coronary artery disease. Nature Genetics. 2013 Nov;45(11):1345-52.
32. Adachi H, Fujiwara Y, Kondo T, Nishikawa T, Ogawa R, Matsumura T, et al. Angptl 4 deficiency improves lipid metabolism, suppresses foam cell formation and protects against atherosclerosis. Biochemical and Biophysical Research Communications. 2009 Feb 20;379(4):806-11.
33. Aryal B, Rotllan N, Araldi E, Ramírez CM, He S, Chousterman BG, et al. ANGPTL4 deficiency in haematopoietic cells promotes monocyte expansion and atherosclerosis progression. Nature Communications. 2016 Jul 27;7(1):12313.
34. Georgiadi A, Wang Y, Stienstra R, Tjeerdema N, Janssen A, Stalenhoef A, et al. Overexpression of angiopoietin-like protein 4 protects against atherosclerosis development. Arteriosclerosis, Thrombosis, and Vascular Biology. 2013 Jul;33(7):1529-37.
35. Cho DI, Ahn MJ, Cho HH, Cho M, Jun JH, Kang BG, et al. ANGPTL4 stabilizes atherosclerotic plaques and modulates the phenotypic transition of vascular smooth muscle cells through KLF4 downregulation. Experimental & Molecular Medicine. 2023 Feb;55(2):426-42.
36. Xu S, Ilyas I, Little PJ, Li H, Kamato D, Zheng X, et al. Endothelial dysfunction in atherosclerotic cardiovascular diseases and beyond: from mechanism to pharmacotherapies. Pharmacological Reviews. 2021 Jul 1;73(3):924-67.
37. Deng M, Kutrolli E, Sadewasser A, Michel S, Joibari MM, Jaschinski F, et al. ANGPTL4 silencing via antisense oligonucleotides reduces plasma triglycerides and glucose in mice without causing lymphadenopathy. Journal of Lipid Research. 2022 Jul 1;63(7):100237.
38. Singh AK, Chaube B, Zhang X, Sun J, Citrin KM, Canfrán-Duque A, et al. Hepatocyte-specific suppression of ANGPTL4 improves obesity-associated diabetes and mitigates atherosclerosis in mice. The Journal of Clinical Investigation. 2021 Sep 1;131(17):e140989.
39. Dijk W, Heine M, Vergnes L, Boon MR, Schaart G, Hesselink MK, et al. ANGPTL4 mediates shuttling of lipid fuel to brown adipose tissue during sustained cold exposure. elife. 2015 Oct 17;4:e08428.
40. Yang YH, Wang Y, Lam KS, Yau MH, Cheng KK, Zhang J,et al. Suppression of the Raf/MEK/ERK signaling cascade and inhibition of angiogenesis by the carboxyl terminus of angiopoietin-like protein 4. Arteriosclerosis, Thrombosis, and Vascular Biology. 2008 May 1;28(5):835-40.
41. Guo K, Pan P, Wu M, Ma Y, Lu J, Chen H. Hyposialylated angiopoietin-like-4 induces apoptosis of podocytes via β1 Integrin/FAK signaling in diabetic nephropathy. Molecular And Cellular Endocrinology. 2020 Apr 5;505:110730.
42. Ge H, Yang G, Yu X, Pourbahrami T, Li C. Oligomerization state-dependent hyperlipidemic effect of angiopoietin-like protein 4. Journal of Lipid Research. 2004 Nov 1;45(11):2071-9.
43. Hübers C, Abdul Pari AA, Grieshober D, Petkov M, Schmidt A, Messmer T, et al. Primary tumor–derived systemic nANGPTL4 inhibits metastasis. Journal of Experimental Medicine. 2022 Oct 21;220(1):e20202595.
44. Zhang Y, Liu X, Zeng L, Zhao X, Chen Q, Pan Y, et al. Exosomal protein angiopoietin-like 4 mediated radioresistance of lung cancer by inhibiting ferroptosis under hypoxic microenvironment. British Journal of Cancer. 2022 Nov 9;127(10):1760-72.
45. Tian MM, Wang YS, Xiao HB. Dual roles of ANGPTL4 in multiple inflammatory responses in stomatitis mice. Molecular Biology Reports. 2022 Oct;49(10):9195-204.
46. Kaneda A, Kaminishi M, Yanagihara K, Sugimura T, Ushijima T. Identification of silencing of nine genes in human gastric cancers. Cancer Research. 2002 Nov 15;62(22):6645-50.
47. Hattori N, Okochi‐Takada E, Kikuyama M, Wakabayashi M, Yamashita S, Ushijima T. Methylation silencing of angiopoietin‐like 4 in rat and human mammary carcinomas. Cancer Science. 2011 Jul;102(7):1337-43.
48. Zhang K, Zhai Z, Yu S, Tao Y. DNA methylation mediated down-regulation of ANGPTL4 promotes colorectal cancer metastasis by activating the ERK pathway. Journal of Cancer. 2021;12(18):5473-85.