Introductory Remarks
Aging is associated with a progressive increase in the incidence of neurodegenerative diseases, both in laboratory animals and humans. In rats, aging is accompanied by degenerative and/or atrophic processes in the cholinergic system of the forebrain, as well as morphological changes which parallel a reduction in spatial learning capacity [1]. Likewise, there is solid evidence that, in this species, the decline in learning capacity and spatial reference memory that occurs with age is preceded by a multiplicity of structural, cellular, and molecular alterations at the hippocampal level many of which are comparable to those that occur during the aging of the human brain [2]. In 2013 our group started a systematic study initially aimed at characterizing the cognitive impairment and morphological changes in the dorsal hippocampus of very old female rats [3]. Our long-term goal was to explore therapeutic strategies able to partially reverse the age-related decline in learning performance and spatial memory in old female rats. Ultimately, we wanted to rejuvenate the hippocampus of old rats.
Reproductive Aging
In middle-aged (MA) female rats, we have demonstrated that intrahypothalamic gene therapy for insulin-like growth factor-I (IGF-I) extends the regular cyclicity of the animals beyond 10 months (the age at which MA rats stop ovulating). In MA female rats, both the reproductive capacity and frequency of regular estrous cycles decrease compared to their young adult counterparts [4]. Very recently we implemented long-term OSKM gene therapy in the hypothalamus of young female rats. The main goal was to extend fertility in the treated animals (see below).
Aging and Epigenetics
The discovery of animal cloning [5,6] and the subsequent development of cell reprogramming technology by means of the four reprogramming factors, Oct4, Sox2, Klf4, c-Myc, also known as the Yamanaka genes [7], ushered in a technological and conceptual revolution that led to the achievement of cell rejuvenation by full reprogramming and to the emerging view of aging as a reversible epigenetic process where cumulative DNA damage does not appear to play a central role as was long thought [8].
Regenerative Effects of Yamanaka Gene Therapy
It is of significant interest that Yamanaka gene therapy in the retina of an experimental-glaucoma mouse model and in middle-aged mice ameliorated their visual acuity [9]. In progeric mice, transgenic for OSKM factors, cyclic partial reprogramming attenuated several signs of aging in visceral tissues and extended by 50% the survival of experimental versus control counterparts [10]. Furthermore, in a very recent report [11] it was shown that intravenous gene therapy with a regulatable AAV9 vector system that expresses the OSK genes, implemented in senile mice (29.2 months), prolonged the survival of the animals by two months compared to control counterparts.
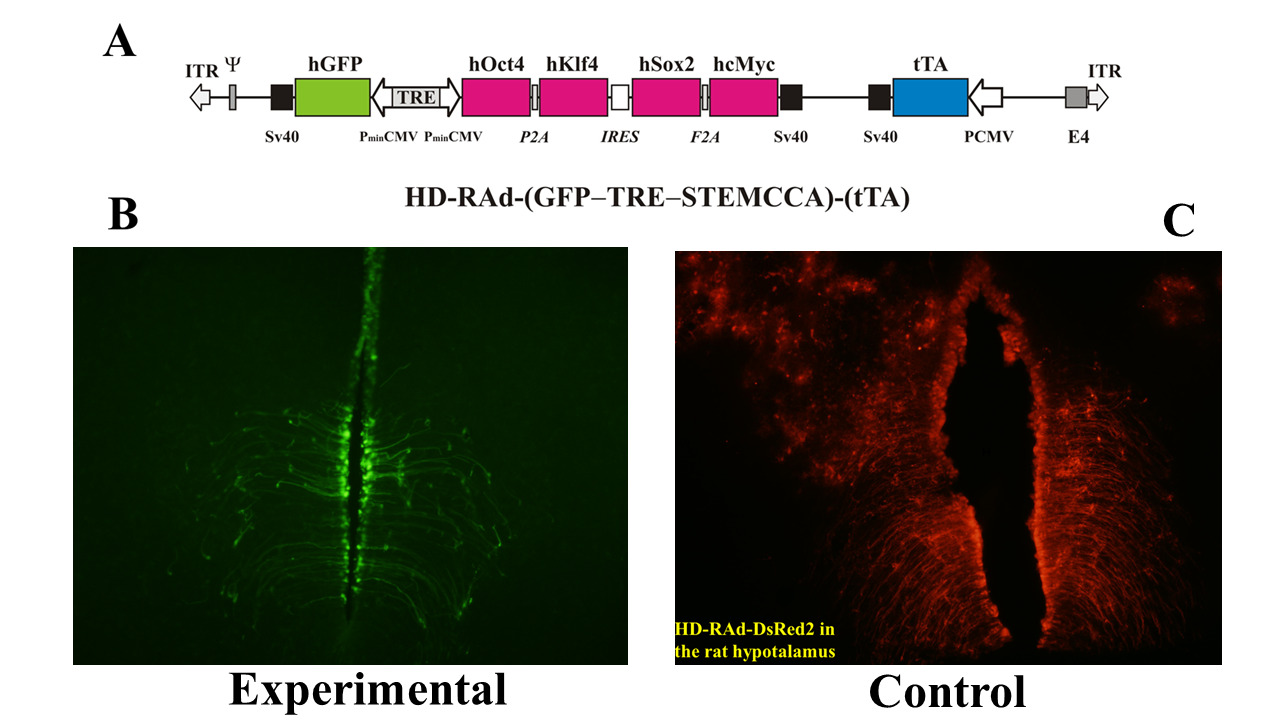
This line of evidence prompted us to implement OSKM gene therapy in the hippocampus of old female rats using an adenovector constructed by us that expresses a polycistronic cassette (the STEMCCA system) harboring the OSKM genes as well as the GFP gene, all under the control of a bidirectional regulatable promoter [12] (Figure 2 panel A).
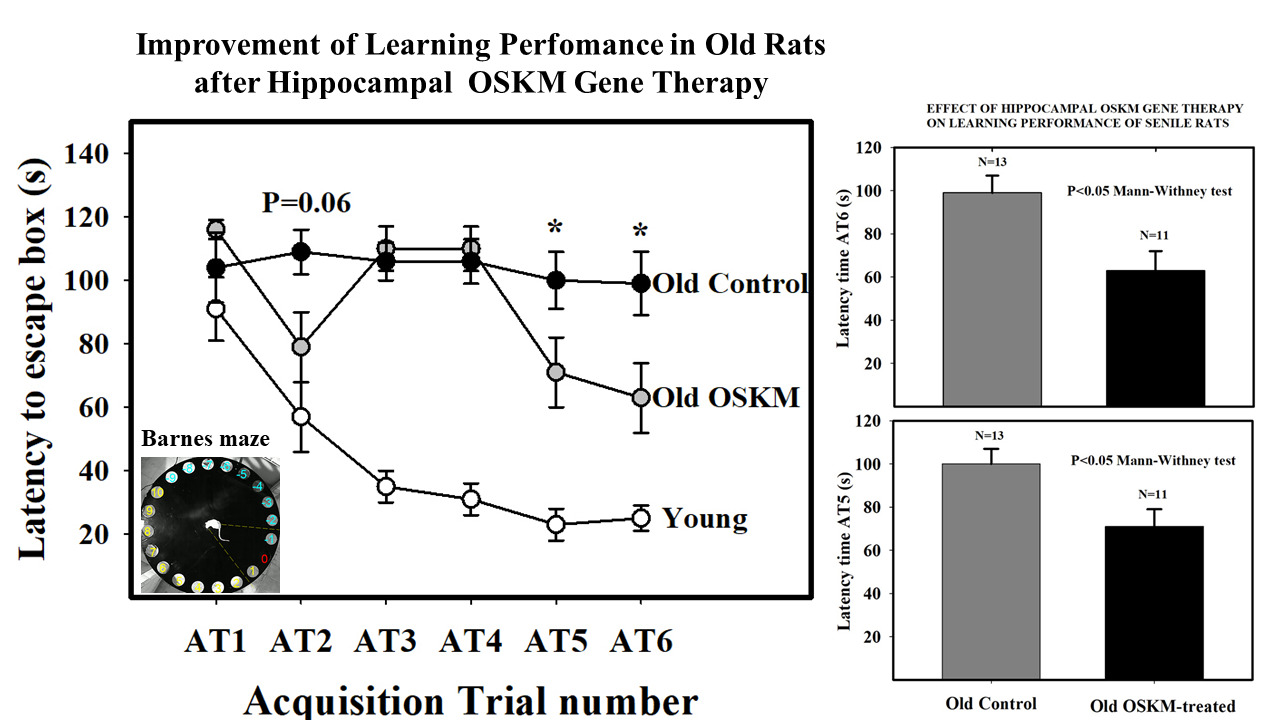
We assessed the cognitive performance of young (3.5 mo.) untreated rats and old (25.3 mo.) treated and control rats. Treatment was carried out by intrahippocampal injection of OSKM adenovector. Learning and spatial memory performance were assessed by means of the Barnes maze test. The learning performance of the OSKM-treated old rats was significantly improved compared to that of the control old counterparts (Figure 1). A marginal (P=0.06) improvement in the spatial memory was recorded in the treated versus control old rats. OSKM gene expression induced no pathological changes in the brain. The morphology and number of hippocampal cell populations like astrocytes and mature neurons did not show any changes with the treatment in the old rats as compared with the control old counterparts. The rat pan tissue DNAm age marker (Horvath epigenetic clock) revealed that old OSKM gene-treated rats show a trend towards a decrease in epigenetic age [13].
Figure 1: Effect of OSKM genes on learning performance in old rats. Main plot shows escape hole latency throughout training in young intact, old control and old OSKM treated rats. The training consists of six training sessions (acquisition trials, AT) in which the animals are given 120 seconds per AT to learn the location of the escape box, placed underneath hole 0. The latency time (the time, in s, that takes the animal to find the escape box since it is released from the starting box). Latency time falls rapidly in the young rats while in control old rats latency time remains as a plateau line during the whole training period. In contrast, old treated rats show little improvement from AT1 through AT4 but at AT5 and AT6 show a significant fall in latency time as compared with old controls [13]. Bar plots on the right show the differences between old controls and old treated rats at AT5 and AT6.
In a recent study in young (2.6 mo) and old (26.6 mo) rats we found that 1,090 CpGs methylation sites exhibited increased methylation in the hippocampal DNA of the old rats. Additionally, an enrichment pathway analysis revealed that neuron fate commitment, brain development, and central nervous system development were processes whose underlying genes were enriched in hypermethylated CpGs in the old animals [14]. In the old rat hippocampi of the cited study, the methylation levels of CpGs proximal to transcription factors associated with genes Pax5, Lbx1, Nr2f2, Hnf1b, Zic1, Zic4, Hoxd9; Hoxd10, Gli3, Gsx1 and Lmx1b, and Nipbl showed a significant inverse regression with spatial memory performance. Furthermore, regression analysis of different memory performance indices with hippocampal DNAm age was significant. Those results suggested that age-related hypermethylation of transcription factors related to certain gene families, like Zic and Gli, may play a causal role in the decline in spatial memory with age [14].
Since in a similar hippocampal DNA methylation analysis in the above described study [13], we found a subset of 174 hypomethylated CpGs in the hippocampal DNA from old OSKM-treated rats and young controls, both compared with old control hippocampi, it means that in the hippocampal DNA there is a common set of CpGs which are hypermethylated during aging and are demethylated by the OSKM genes. This observation can be interpreted by stating that the hypermethylation induced by aging (174 CpGs were hypermethylated in old versus young animals) is reversed by the demethylation effect of the OSKM genes on the same 174 CpGs. The observation is consistent with the well-established rejuvenation effects of OSKM genes. This hypomethylation effect of OSKM genes is likely to derepress a number of hippocampal genes some of which may be involved in the improvement of the learning performance and spatial memory documented by the results of the Barnes test. The results are also consistent with a report indicating that in the hippocampus of female rats, 210 genes are differentially expressed in senile as compared with young counterparts, most of them being downregulated [15].
Summing up the OSKM results, 39-day OSKM gene therapy in the dorsal hippocampus of old rats significantly reverses the typical learning deficits displayed by aged rats and demethylates part of the hippocampal CpGs methylated during aging With the above OSKM-GFP vector (Figure 2, Panel A), we also implemented long-term (5.8 months) regenerative gene therapy in the hypothalamus of young females with the goal to extend fertility in the treated animals. We hypothesized that the long-term expression of the OSKM genes in the hypothalamus of young female rats could slow down the rate of decline of fertility in the animals as they near the age of ovulatory cessation (10 months in our rat colony).
Figure 2: Panel A The figure illustrates the basic components of HD-RAd-STEMCCA-GFP-Tet-Off genome [16]. Panel B- Expression of GFP from the adenovector HD-RAd-STEMCCA-GFP in the third ventricle of the rat hypothalamus 6 days after vector injection in the lateral ventricles. Notice the processes of the ependymal tanycytes forming an extensive network in the hypothalamic parenchyma. Obj. 20X. Panel C- Expression of DsRed2 from the control adenovector HD-RAd-DsRed2 in the rat hypothalamus 30 days after vector injection. Notice the processes of the ependymal tanycytes forming an extensive network in the hypothalamic parenchyma. Obj. 10X. Abbreviations- GFP: Humanized Green Fluorescent Protein; TRE: Tetracycline Responsive Element; tTA: Chimeric Regulatory Protein; PminCMV: Cytomegalovirus Minimal Promoter; SV40pA: Polyadenylation Signal; ITR: Inverted Terminal Repeats; ψ: Packaging Signal.
At 4 months of age 12 female rats received an intrahypothalamic injection of our OSKM-GFP vector (treated rats); 12 control rats received a vector expressing the Ds-Red fluorescent protein (control rats). Both, the HD-RAd OSKM-GFP experimental vector and the HD-RAd Tet-Off-control vectors were stereotaxically injected in the medial basal hypothalamus (MBH) of young female rats (Figure 2, panel B). Six days after injection, hGFP expression of the OSKM-GFP vector was strong. The processes of the ependymal tanycytes formed an extensive network in the hypothalamic parenchyma (Figure 2, Panel B). When our control vector HD-RAd-DsRed2 vector was injected into the hypothalamic parenchyma of young rats, DsRed 2 expression remained strong for at least a month (Figure 2, panel C).
At 9.3 months of age, middle-aged (MA) control and MA treated rats were mated with young males. A group of 12 young intact female rats was also mated. The rate of pregnancy recorded was 83%, 10% and 30% for young, MA control and M-A OSKM-treated animals, respectively. The BW of the pups from the young females remained lower than that of the M-A counterparts, a fact that may be accounted for by the higher number of pups the young mothers had to feed as compared with the OSKM and control MA rats (8 vs. 3, respectively). The lower BW of Control versus OSKM pups at weaning suggests that milk production and /or quality declines with age, a change that may have been attenuated by hypothalamic OSKM gene therapy.
The total number of pups born from Y, MA-C and MA-OSKM was 83, 3 and 9, respectively. All pups survived and showed similarly normal behavior in the three groups.
The results revealed that hypothalamic OSKM gene therapy at a young age slowed down the age-related decline in fertility of female rats [16].
Concluding Remarks
The reviewed results constitute emerging evidence that viral vector-mediated delivery of the Yamanaka genes in the brain has strong regenerative actions without adverse side effects.
Since one of the evolutionary purposes of the Yamanaka genes is rejuvenation [8,17], experimental studies aimed at exploring their functional effects on the brain and other organs, in adult and old animals seem to be a promising avenue of research in regenerative medicine and perhaps, ultimately, a path towards whole animal rejuvenation. Indeed, the idea of harnessing the unique reprogramming power of rejuvenation genes in order to achieve organismal rejuvenation does not seem unrealistic.
In clinical terms, OSK /OSKM gene therapy offers the opportunity of effective treatment for neurodegenerative pathologies. Furthermore, there already are ongoing clinical trials using OSK gene therapy for the treatment of retinal degenerative pathologies.
References
2. Sugaya K, Chouinard M, Greene R, Robbins M, Personett D, Kent C, Gallagher M, McKinney M. Molecular indices of neuronal and glial plasticity in the hippocampal formation in a rodent model of age-induced spatial learning impairment. J Neurosci. 1996 May 15;16(10):3427-43.
3. Morel GR, Andersen T, Pardo J, Zuccolilli GO, Cambiaggi VL, Hereñú CB, Goya RG. Cognitive impairment and morphological changes in the dorsal hippocampus of very old female rats. Neuroscience. 2015 Sep 10; 303:189-99.
4. Zheng W, Jimenez-Linan M, Rubin BS, Halvorson LM. Anterior pituitary gene expression with reproductive aging in the female rat. Biol Reprod. 2007 Jun;76(6):1091-102.
5. Gurdon JB. The developmental capacity of nuclei taken from intestinal epithelium cells of feeding tadpoles. J Embryol Exp Morphol. 1962 Dec; 10:622-40.
6. Wilmut I, Schnieke AE, McWhir J, Kind AJ, Campbell KH. Viable offspring derived from fetal and adult mammalian cells. Nature. 1997 Feb 27;385(6619):810-3.
7. Takahashi K, Yamanaka S. Induction of pluripotent stem cells from mouse embryonic and adult fibroblast cultures by defined factors. Cell. 2006 Aug 25;126(4):663-76.
8. Goya RG, Lehmann M, Chiavellini P, Canatelli-Mallat M, Hereñú CB, Brown OA. Rejuvenation by cell reprogramming: a new horizon in gerontology. Stem Cell Res Ther. 2018 Dec 17;9(1):349.
9. Lu Y, Brommer B, Tian X, Krishnan A, Meer M, Wang C, et al. Reprogramming to recover youthful epigenetic information and restore vision. Nature. 2020 Dec;588(7836):124-9.
10. Ocampo A, Reddy P, Martinez-Redondo P, Platero-Luengo A, Hatanaka F, Hishida T, et al. In Vivo Amelioration of Age-Associated Hallmarks by Partial Reprogramming. Cell. 2016 Dec 15;167(7):1719-33.e12.
11. Macip CC, Hasan R, Hoznek V, Kim J, Lu YR, Metzger LE 4th, et al. Gene Therapy-Mediated Partial Reprogramming Extends Lifespan and Reverses Age-Related Changes in Aged Mice. Cell Reprogram. 2024 Feb;26(1):24-32.
12. Lehmann M, Canatelli-Mallat M, Chiavellini P, Morel GR, Reggiani PC, Hereñú CB, et al. Regulatable adenovector harboring the GFP and Yamanaka genes for implementing regenerative medicine in the brain. Gene Ther. 2019 Nov;26(10-11):432-40.
13. Horvath S, Lacunza E, Mallat MC, Portiansky EL, Gallardo MD, Brooke RT, et al. Cognitive rejuvenation in old rats by hippocampal OSKM gene therapy. 2024, in press. bioRxiv 2023.06.13.544719.
14. Chiavellini P, Lehmann M, Canatelli Mallat M, Zoller JA, Herenu CB, Morel GR, et al. Hippocampal DNA Methylation, Epigenetic Age, and Spatial Memory Performance in Young and Old Rats. J Gerontol A Biol Sci Med Sci. 2022 Dec 29;77(12):2387-94.
15. Pardo J, Abba MC, Lacunza E, Francelle L, Morel GR, Outeiro TF, et al. Identification of a conserved gene signature associated with an exacerbated inflammatory environment in the hippocampus of aging rats. Hippocampus. 2017 Apr;27(4):435-49.
16. Gallardo MD, Girard M, Bigres A, Lehmann M, Rodriguez SS, Goya RG. Regenerative Gene therapy in the hypothalamus prolongs fertility in female rats. bioRxiv. 2023 May 25:2023-05.
17. Kerepesi C, Zhang B, Lee SG, Trapp A, Gladyshev VN. Epigenetic clocks reveal a rejuvenation event during embryogenesis followed by aging. Sci Adv. 2021 Jun 25;7(26):eabg6082.