Keywords
Influenza, COVID-19, Thoracic aortic aneurysms and dissection, Vaccination
Introduction
Acute type A aortic dissection (ATAAD) is the leading cause of morbidity and mortality for patients with thoracic aortic aneurysms, despite significant advances in the surgical treatment [1]. An aortic dissection is a life-threatening condition which occurs when blood enters through a tear in the wall of the aorta causing the aortic layers to separate or “dissect”. When the tear develops in the ascending aorta it is classified as type A aortic dissection and this represents a surgical emergency. While relatively rare, with an incidence of 4.4 per 100,000 person-years [2], aortic dissections carry a mortality rate of 18% with and 57% without surgery [3]. These numbers may be under reported, as one study showed that up to 50% patients with aortic dissection die before reaching the hospital [4]. While several modifiable (hypertension, smoking, BMI) and non-modifiable (age >65, male sex, heritable genetic variants in genes predisposing to thoracic aortic disease) risk factors are known to increase the risk for an acute aortic dissection [5], we recently reported that influenza may also increase the risk for acute aortic dissections by 30% [6].
As we are in the midst of a global health emergency [7] caused by the coronavirus disease of 2019 (COVID-19), it is an important time to review our knowledge of how viral syndromes may impact the incidence of acute aortic dissections in order to better predict and prepare for potential downstream impacts of this pandemic. While the most concerning aspect of this virus is the rapid deterioration into acute respiratory distress syndrome [8], infection with COVID-19 has serious implications for patients with cardiovascular risk factors, and could accelerate rates of major cardiovascular events [9].
Due largely to its predictable time course, high incidence and availability of a vaccination, the influenza virus has been wildly studied, and the relationship to cardiovascular effects has been well established [10]. Specifically, influenza infection has been linked with higher risk of myocardial infarction [11-15], with admission rates shown to follow seasonal patterns and peak during influenza pandemics [16]. Additionally, administration of influenza vaccination is associated with a significantly lower risk of major adverse cardiovascular events, with an effect observed most strongly in those with active coronary disease [17]. Through this research, the influenza vaccine has emerged as an important annual intervention to significantly reduce the risk of a cardiovascular event, and is a class IB recommendation for secondary prevention in individuals with coronary and other atherosclerotic vascular disease [18].
In 2005, the investigators for the International Registry of Acute Aortic Dissection (IRAD) demonstrated higher incidence of acute aortic dissection during winter months that was independent of climate, suggesting other environmental factors may be involved [19]. Our research group recently reported on higher admission rates and in-hospital mortality for ATAAD during influenza season at our institution [6]. In this commentary article, we will describe the association between influenza infection and an increased incidence of ATAAD, with the hope that a better understanding of the vascular consequences of a viral syndrome could provide important insight into the potential cardiovascular impact of COVID-19.
Methods and Results
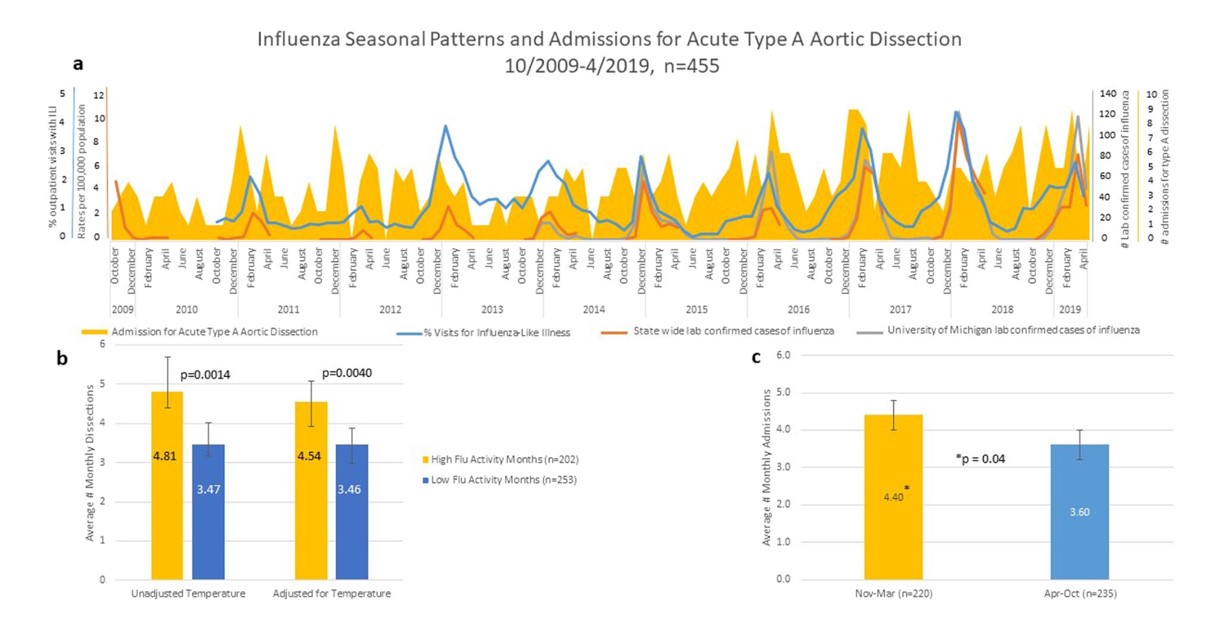
In our recent publication, we obtained Society of Thoracic Surgeons (STS) data elements from the University of Michigan Cardiac Surgery Data Warehouse for all patients who presented to with an ATAAD between October 2009 and April 2019 (n=455). We compared admission rates against Michigan specific influenza activity, as determined by metrics reported on the Center for Disease and Prevention (CDC) website as well as lab confirmed cases at the University of Michigan. We also gathered monthly ambient air temperatures reported by the National Oceanic and Atmospheric Administration (NOAA) to control for seasonal weather changes. Overall, we found a significantly higher admission rate for ATAAD during winter months (November-March) compared to the rest of the year (4.4 v. 3.6 admissions per month, p=0.04). We also found significantly higher admissions during months with high flu activity compared to months with low flu activity, both unadjusted (4.81 v. 3.47, p=0.0014) and adjusted (4.34 v 3.46, p=0.004) for weather as shown in Figure 1. The baseline characteristics of the patients with ATAAD was 59 years of age, 68% were male, 76% had hypertension, 18% coronary artery disease, 6% Diabetes and 3% Marfan syndrome; there was no difference in baseline characteristics among patient admitted during influenza season or non-influenza season.
Figure 1: A. Influenza patterns and average admissions per month for acute type A aortic dissection for all 455 patients admitted between 10/2009 and 4/2019. B. Data stratified by influenza monthly activity determined by monthly rates of influenza-like illness, state lab confirmed influenza hospitalization and University of Michigan lab confirmed cases of influenza. Data both unadjusted and adjusted for temperature. C. Data stratified by month between winter season (yellow, average temperature <35 F) and non-winter season (blue, average temperature >35 F). (Reproduced with permission from Ashur et. al. [6]).
Importantly, clinical outcomes for patients undergoing urgent surgical repair was different for patients during influenza season or non-influenza season. In-hospital mortality was 10.99% during influenza season and 5.79% otherwise (p=0.024) and 30-day mortality was 9.68% and 5.44%, p=0.048, respectively. However, there was no difference in length of admission or post op pneumonia in patients treated during months with high influenza activity compared to months with low influenza activity as shown as in Table 1.
|
|
High influenza activity months (n=191) |
Low influenza activity months (n=191) |
p-value |
|
Surgery delay (days) |
1.32 |
0.93 |
0.13 |
|
Length of Admission (days) |
13.51 |
13.95 |
0.33 |
|
Post-Op Pneumonia |
16.23% |
15.70% |
0.44 |
|
Emergent Status |
83.68% |
84.30% |
0.43 |
|
In hospital Mortality |
10.99% |
5.79% |
0.024 |
|
30-day Mortality |
9.68% |
5.44% |
0.048 |
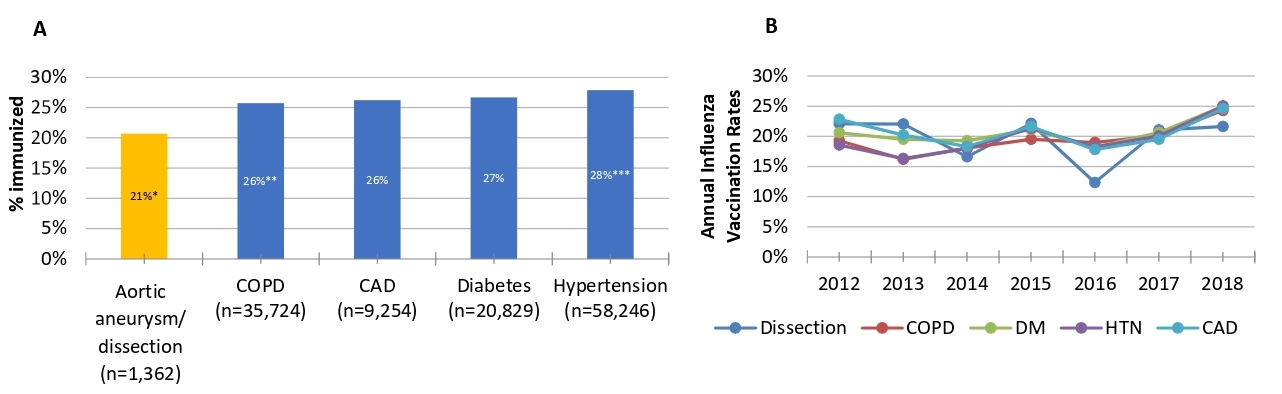
Finally, we conducted a search of the electronic medical record using the online tool DataDirect to search over four million unique patients in the University of Michigan healthcare system. We compared influenza vaccination rates among different patient populations and searched DataDirect from 1/1/2012-5/20/2019. We included only patients with a primary care physician in the University of Michigan Health system to prevent a referral bias and to exclude patients transferred in from other health care systems who are less likely to have a documented influenza vaccination in the University of Michigan database. Of the 292,221 patients identified in this search, those with a ICD 9/10 diagnosis code for thoracic aortic aneurysms or dissection had an influenza vaccination rate of 21%, which was significantly lower than patients with ICD9/10 diagnosis code for chronic pulmonary disease (26%, p<0.0001), coronary artery disease (26%, p<0.0001), diabetes mellitus (27%, p<0.0001) and hypertension (28%, p<0.0001), as shown in Figure 2A. As shown in Figure 2B, there was no significant change in vaccination prevalence by one-year intervals from 2012-2018, and the rate remains flat around 20%.
Figure 2: A. Cumulative influenza vaccination rate (2012-2018) among patients with ICD9/10 codes for different chronic diseases; *p<0.0001 compared to all, **p=0.01 compared to DM and ***p<0.0007 compared to all. B. Annual influenza vaccination rates.
Discussion
Mechanism of cardiac and vascular injury from a viral syndrome
The mechanisms by which influenza increases the risk for ATAAD is not clear, however the pathogenesis for cardiac complications of influenza such as myocardial infarction, myocardial injury and heart failure is thought to be due to inflammatory and immune mediated cardiac tissue injury, rather than direct influenza viral invasion of myocardial or vascular tissue [15,17]. While there have not yet been studies testing for influenza RNA in human aortic tissue with type A aortic dissection, mouse studies demonstrated localization of influenza virus mRNA in aortic tissue following intranasal inoculation with H3N2 influenza. Those mice also demonstrated increased mRNA levels for MCP-1, IP-10, RANTES, IL-6, IL-1 and others genes consistent with a picture of influenza virus-mediated aortitis [20]. However, influenza viremia rarely occurs in humans and therefore it is not clear whether those findings from murine studies can be directly translated to human aortic disease.
While no association has been made specifically between COVID-19 and aortic dissection, several reports of myocarditis, ischemic heart disease and vascular injury (i.e stroke) have been observed [21]. Overall, the proposed mechanism of injury seems to be similar to the viral syndrome associated with influenza and involves a cytokine storm combined with a stress response inducing indirect vascular and cardiac inflammation. Like other coronaviruses, such as SARS-CoV [22] and MERS-CoV [21], COVID-19 induces a T-helper-1 mediated cell response through increased production of pro-inflammatory cytokines in the serum (IL1B, IFNγ, IP10 and MCP1). This cytokine storm is directly proportional to disease severity, with higher amounts of GCSF, IP10, MCP1, MIP1A, and TNFα in those requiring ICU admission. However, unlike SARS-Cov and MERS-CoV, COVID-19 also activates the anti-inflammatory T-helper-2 response via cytokines IL4 and IL10.23 The acute respiratory distress induced by COVID-19 may also trigger elevations in IL-1, IL-6, and IL-12 that last for up to 2 weeks after disease onset [22]. While SARS-CoV viral RNA was seen in 35% of autopsied hearts during the Toronto SARS outbreak [24], COVID-19 has not yet been identified directly in cardiac or vascular tissue.
Impact of a viral syndrome on the cardiovascular system
Based on findings from early reporting of this pandemic, COVID-19 and influenza appear to impose similar negative effects on the cardiovascular system. Influenza myocarditis is a phenomena felt to occur in up to 13% on patients, most typically those who are severely ill [25]. When looking back at previous influenza pandemics, myocarditis was associated with 39.4% of fatal cases with Asian flu (H2N2, 1957) and 48% of fatal cases with Spanish flu (H1N1, 1918), most likely related to multi-organ failure involved in the terminally-ill state of an influenza infection. However, in the Japan Influenza A Pandemic (H1N1, 2009), cases of myocarditis were reported in patients without preceding severe viral pneumonia, suggesting affinity for heart tissue in some susceptible individuals [26]. In reports coming out of Wuhan, China, myocardial injury was present in 7.2% of 138 patients hospitalized with COVID-19, and 22% of patients requiring ICU care [27]. While patients with cardiovascular disease (CVD) may be more susceptible to complications from COVID-19 [23], reports from the National Health Commission of China showed that of patients who died from COVID-19, 11.8% without pre-existing CVD had cardiac injury or experienced a cardiac arrest during hospitalization [28].
COVID-19 susceptibility in patients with cardiovascular risk factors
Patients with CVD risk factors may be predisposed to COVID-19, and those who are affected have increased risk of cardiovascular complications. In a large meta-analysis of 46,248 COVID-19 patients, the most common comorbidity of those affected was hypertension (17±7%, 95% CI 14-22%), followed by diabetes (8±6%, 95% CI 6-11%) [29]. Blood pressure levels in COVID-19 patients treated in the ICU were also significantly higher than those not treated in the ICU [23]. One proposed explanation is the relationship between COVID-19 and Angiotensin-converting enzyme 2 (ACE2). ACE2, a part of the renin-angiotensin-aldosterone system and a component in the development of hypertension, also functions as a receptor for coronaviruses [30] and could explain why symptoms are more severe in patients with CVD, who may have higher expression of ACE2 than the general population [31]. While patients with CVD, and specifically those with hypertension and known aortic aneurysms, are likely to be on ACE inhibitors or angiotensin receptor blockers which potentially may interfere with this proposed mechanism of infection, almost all major societies have recommended against adding or stopping RAAS antagonist [32].
Overwhelmed healthcare system may not be fit to manage aortic dissection
The direct and indirect cardiovascular complications associated with COVID-19 [9] and the suggestion of a viral trigger for cardiovascular events in previous coronavirus and influenza pandemics [33] suggest that an increased incidence of aortic dissections and other cardiac events may be seen during the COVID-19 pandemic, at a time when the healthcare system is already overwhelmed. Notably, the majority of prior influenza pandemics have seen more patients die from cardiovascular events than all other causes [34]. With health care systems exceeding capacity, protocols will need to be in place to ensure that those presenting with aortic dissection receive the emergent care they need. Some hospitals have already put such emergency protocols into place for patients presenting with ST-segment elevation myocardial infarction (STEMI) using fibrinolytic therapy if delays to PCI are anticipated [35]. In order to preserve cardiovascular ICU accommodations and support staff for patients presenting with ATAAD at a time when many cardiac ICUs will be repurposed to care for patients with COVID-19, cardiac surgeries such as coronary artery bypass or valve replacement should instead be deferred for medical management or less invasive approaches such as percutaneous coronary intervention (PCI) or transcatheter valve replacement [9]. Prolonged mechanical ventilation following surgical repair for ATAAD is not uncommon [36], underscoring the need for a strategy to utilize ventilators at a time when supplies could be in shortage as a result of the COVID-19 pandemic [37].
Utility of the STS database
To date, there have been no reports demonstrating an association between COVID-19 and ATAAD. Future use of the STS database to identify patients presenting with an ATAAD during the months of the COVID-19 pandemic will be instrumental in retrospectively understanding this association. An increased incidence of ATAAD during the COVID-19 pandemic relative to baseline would provide further support for the role of viral mediation in ATAAD. In general however, identification of risk factors associated with ATAAD has been challenging due to the low incidence of disease and rapid fatality that is often attributed to other causes [38], with up to 50% of patients dying before reaching the hospital [4]. This may prove to be particularly true of data obtained during the time of the COVID-19 pandemic, where a limited availability of emergency departments and first responders may increase the incidence of ATAAD fatalities occurring before reaching the hospital, thus artificially lowering the true disease incidence.
Under vaccination in high risk population
Despite evidence that the influenza vaccine has been shown to reduce cardiovascular morbidity and mortality, vaccination rates remain low, with utilization significantly lower in patients with thoracic aortic aneurysms/dissection compared to other cardiovascular diseases. There are currently multiple candidate vaccines against COVID-19 under investigation [39], and it will be imperative that medical providers convey the necessity of vaccination to patients, especially those at high risk of complications. If trends for the COVID-19 vaccination are similar to those of influenza, quality improvement projects aimed at generating higher vaccination rates may be needed, particularly for those with aortic aneurysms and other cardiovascular risk factors. To explore methods aimed at increasing the rate of influenza vaccination in an at-risk population, we undertook a quality improvement project at the University of Michigan Cardiovascular Center, in which trained medical students approached patients in the clinic and discussed reasons for influenza vaccination and provided an educational flyer (Figure 3). Of patients who had not already received or planned to receive the influenza vaccination, 39% felt that intervention convinced them to get a flu shot during their visit. Widespread dissemination of similar educational flyers conveying the cardiovascular risks associated with COVID-19 may increase vaccine uptake and reduce the future burden of cardiovascular morbidity and mortality associated with COVID-19.
Figure 3: Informational flyer on influenza and cardiovascular disease disseminated to patients in the waiting room of a cardiology clinic.
References
2. DeMartino RR, Sen I, Huang Y, Bower TC, Oderich GS, Pochettino A, et al. Population-Based Assessment of the Incidence of Aortic Dissection, Intramural Hematoma, and Penetrating Ulcer, and Its Associated Mortality From 1995 to 2015. Circulation: Cardiovascular Quality and Outcomes. 2018 Aug;11(8):e004689.
3. Evangelista A, Isselbacher EM, Bossone E, Gleason TG, Eusanio MD, Sechtem U, et al. Insights from the International Registry of Acute Aortic Dissection: a 20-year experience of collaborative clinical research. Circulation. 2018 Apr 24;137(17):1846-60.
4. Howard DP, Banerjee A, Fairhead JF, Perkins J, Silver LE, Rothwell PM. Population-based study of incidence and outcome of acute aortic dissection and premorbid risk factor control: 10-year results from the Oxford Vascular Study. Circulation. 2013 May 21;127(20):2031-7.
5. Gawinecka J, Schönrath F, von Eckardstein A. Acute aortic dissection: pathogenesis, risk factors and diagnosis. Swiss medical weekly. 2017 Sep 5;147:w14489.
6. Ashur C, Norton E, Farhat L, Conlon A, Willer C, Froehlich JB, et al. Higher admission rates and in-hospital mortality for acute type A aortic dissection during Influenza season: a single center experience. Scientific reports. 2020 Mar 13;10(1):1-6.
7. Organization. WH. Coronavirus disease 2019 (COVID-19), Situation report 64; March 24, 2020
8. Goh KJ, Choong MC, Cheong EH, Kalimuddin S, Wen SD, Phua GC, et al. Rapid Progression to Acute Respiratory Distress Syndrome: Review of Current Understanding of Critical Illness from COVID-19 Infection. Annals of the Academy of Medicine, Singapore. 2020 Jan 1;49(1):1.
9. Driggin E, Madhavan MV, Bikdeli B, Chuich T, Laracy J, Bondi-Zoccai G, et al. Cardiovascular considerations for patients, health care workers, and health systems during the coronavirus disease 2019 (COVID-19) pandemic. Journal of the American College of Cardiology. 2020 Mar 19.
10. Public Health Weekly Reports for NOVEMBER 11, 1932. Public Health Reports. 1932;47(46):2159-2189.
11. Guan XR, Li X, Xin XM, Jiang LX, Cui LY, Wang LF, Li HY. Influenza virus infection and risk of acute myocardial infarction. Inflammation. 2008 Aug 1;31(4):266-72.
12. Madjid M, Aboshady I, Awan I, Litovsky S, Casscells SW. Influenza and cardiovascular disease: is there a causal relationship?. Texas Heart Institute Journal. 2004;31(1):4.
13. Bainton D, Jones GR, Hole D. Influenza and ischaemic heart disease-a possible trigger for acute myocardial infarction?. International Journal of Epidemiology. 1978 Sep 1;7(3):231-9.
14. Smeeth L, Thomas SL, Hall AJ, Hubbard R, Farrington P, Vallance P. Risk of myocardial infarction and stroke after acute infection or vaccination. New England Journal of Medicine. 2004 Dec 16;351(25):2611-8.
15. Kwong JC, Schwartz KL, Campitelli MA, Chung H, Crowcroft NS, Karnauchow T, et al. Acute myocardial infarction after laboratory-confirmed influenza infection. New England Journal of Medicine. 2018 Jan 25;378(4):345-53.
16. Foster ED, Cavanaugh JE, Haynes WG, Yang M, Gerke AK, Tang F, et al. Acute myocardial infarctions, strokes and influenza: seasonal and pandemic effects. Epidemiology & Infection. 2013 Apr;141(4):735-44.
17. Udell JA, Zawi R, Bhatt DL, Keshtkar-Jahromi M, Gaughran F, Phrommintikul A, et al. Association between influenza vaccination and cardiovascular outcomes in high-risk patients: a meta-analysis. JAMA. 2013 Oct 23;310(16):1711-20.
18. Smith SC, Allen J, Blair SN, Bonow RO, Brass LM, Fonarow GC, et al. AHA/ACC guidelines for secondary prevention for patients with coronary and other atherosclerotic vascular disease: 2006 update: endorsed by the National Heart, Lung, and Blood Institute. Journal of the American College of Cardiology. 2006 May 16;47(10):2130-9.
19. Mehta RH, Manfredini R, Bossone E, Fattori R, Evagelista A, Boari B, et al. The winter peak in the occurrence of acute aortic dissection is independent of climate. Chronobiology International. 2005 Jan 1;22(4):723-9.
20. Haidari M, Wyde PR, Litovsky S, Vela D, Ali M, Casscells SW, et al. Influenza virus directly infects, inflames, and resides in the arteries of atherosclerotic and normal mice. Atherosclerosis. 2010 Jan 1;208(1):90-6.
21. Sellers SA, Hagan RS, Hayden FG, Fischer WA. The hidden burden of influenza: a review of the extra‐pulmonary complications of influenza infection. Influenza and Other Respiratory Viruses. 2017 Sep;11(5):372-93.
22. Wong CK, Lam CW, Wu AK, Ip WK, Lee NL, Chan IH, et al. Plasma inflammatory cytokines and chemokines in severe acute respiratory syndrome. Clinical & Experimental Immunology. 2004 Apr;136(1):95-103.
23. Huang C, Wang Y, Li X, Ren L, Zhao J, Hu Y, et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. The Lancet. 2020 Feb 15;395(10223):497-506.
24. Booth CM, Matukas LM, Tomlinson GA, Rachlis AR, Rose DB, Dwosh HA, et al. Clinical features and short-term outcomes of 144 patients with SARS in the greater Toronto area. Jama. 2003 Jun 4;289(21):2801-9.
25. Kodama M. Influenza myocarditis. Circulation Journal. 2010;74(10):2060-1.
26. Ukimura A, Izumi T, Matsumori A. A national survey on myocarditis associated with the 2009 influenza A (H1N1) pandemic in Japan. Circulation Journal. 2010:1008040838-.
27. Wang D, Hu B, Hu C, Zhu F, Liu X, Zhang J, et al. Clinical characteristics of 138 hospitalized patients with 2019 novel coronavirus–infected pneumonia in Wuhan, China. JAMA. 2020 Mar 17;323(11):1061-9.
28. Zheng YY, Ma YT, Zhang JY, Xie X. COVID-19 and the cardiovascular system. Nature Reviews Cardiology. 2020 Mar 5:1-2.
29. Yang J, Zheng Y, Gou X, Pu K, Chen Z, Guo Q, et al. Prevalence of comorbidities in the novel Wuhan coronavirus (COVID-19) infection: a systematic review and meta-analysis. International Journal of Infectious Diseases. 2020 Mar 12.
30. Turner AJ, Hiscox JA, Hooper NM. ACE2: from vasopeptidase to SARS virus receptor. Trends in Pharmacological Sciences. 2004 Jun 1;25(6):291-4.
31. Zheng YY, Ma YT, Zhang JY, Xie X. COVID-19 and the cardiovascular system. Nature Reviews Cardiology. 2020 Mar 5:1-2.
32. Clerkin KJ, Fried JA, Raikhelkar J, Sayer G, Griffin JM, Masoumi A, et al. Coronavirus disease 2019 (COVID-19) and cardiovascular disease. Circulation. 2020 Mar 21.
33. Madjid M, Safavi-Naeini P, Solomon SD, Vardeny O. Potential effects of coronaviruses on the cardiovascular system: a review. JAMA Cardiology. 2020 Mar 27.
34. Madjid M, Casscells SW. Of birds and men: cardiologists' role in influenza pandemics. Lancet. 2004 Oct 9;364(9442):1309.
35. Zeng J, Huang J, Pan L. How to balance acute myocardial infarction and COVID-19: the protocols from Sichuan Provincial People’s Hospital. Intensive Care Medicine. 2020 Mar 11:1-3.
36. Jin M, Ma WG, Liu S, Zhu J, Sun L, Lu J, et al. Predictors of prolonged mechanical ventilation in adults after acute type-A aortic dissection repair. Journal of Cardiothoracic and Vascular Anesthesia. 2017 Oct 1;31(5):1580-7.
37. Truog RD, Mitchell C, Daley GQ. The toughest triage—allocating ventilators in a pandemic. New England Journal of Medicine. 2020 Mar 23.
38. Auer J, Berent R, Eber B. Aortic dissection: incidence, natural history and impact of surgery. Journal of Clinical and Basic Cardiology. 2000 Jan 1;3(3):151-4.
39. Chen WH, Strych U, Hotez PJ, Bottazzi ME. The SARS-CoV-2 vaccine pipeline: an overview. Current Tropical Medicine Reports. 2020 Mar 3:1-4.