Abstract
The incidence of allergic diseases has been increasing over the past few decades worldwide. Although allergic reactions are often characterized by an overzealous Th2 inflammatory response, the underlying mechanisms remain poorly defined. House dust mite (HDM) is one of the most common indoor allergens that cause allergic diseases such as asthma. Recent studies suggest that innate immunity activated by HDM allergens is critical for driving the development of a polarized type 2 response. HDM harbors not only allergic protein antigens, but also non-protein microbial products, e.g., LPS or dsRNA, which can trigger innate immune responses that either promote a type 2 inflammatory phenotype or suppress a severe type 2 immunopathologies in mice, respectively. In this review, we discuss how the innate sensing of immunomodulatory RNA in HDM allergens and viruses modulates type 2 inflammatory responses.
Keywords
Allergen immunotherapy, Dermatophagoides pteronyssinus, Dermatophagoides farina, Double-stranded RNA, Group 2 innate lymphoid cells, House dust mite, Toll-like receptor 3, Type 2 inflammation
Abbreviations
AIT: Allergen Immunotherapy; Dp: Dermatophagoides pteronyssinus; Df: Dermatophagoides farinae; dsRNA: double-stranded RNA; ILC2: group 2 Innate Lymphoid Cells; HDM: House Dust Mite; TLR3: Toll-Like Receptor 3
HDM-Induced Type 2 Inflammation
HDM is the main source of indoor aeroallergens that cause several allergic conditions including atopic dermatitis, conjunctivitis and asthma, in which patients commonly present type 2 immunopathologies characterized by the increased number of eosinophils and elevated levels of type 2 effector cytokines such as IL-4, IL-5, IL-9 and IL-13 [1,2]. Asthma, a common chronic airway disease, is estimated to affect more than 300 million people worldwide, among which most asthmatics are sensitized to HDM [3,4]. Both innate and adaptive immune responses contribute to the establishment of long-lasting type 2 inflammation caused by HDM (Figure 1). Therefore, there is a great need to better understand how HDM allergens and HDM-associated immunostimulatory components contribute to the development of type 2 immunopathologies [3,5].
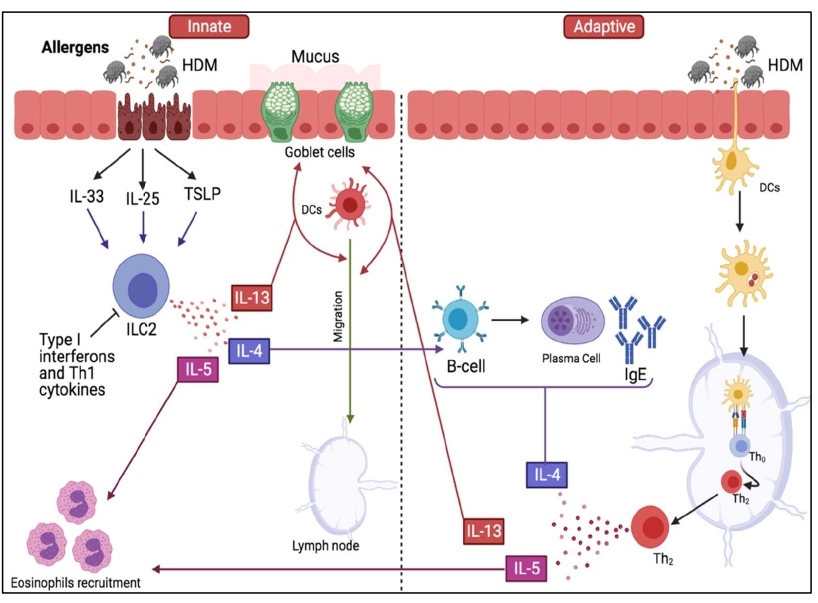
Figure 1: An illustration showing the roles of innate and adaptive immune systems in HDM-induced allergy. During initial exposure to HDM allergens, proteases damage epithelial cells resulting in the release of IL-33, IL-25 and TSLP. These cytokines stimulate ILC2s to produce Th2 cytokines: IL-4, IL-5 and IL-13 which promote IgE isotope switching, recruit eosinophils and promote DCs migration to the lymph nodes, respectively. IL-13 also induces mucus hypersecretion by goblet cells in addition to its role in DCs migration.
HDM Allergen Composition
HDM are microscopic insects found in many places such as mattresses, pillows, carpets and blankets. They feed on human skin flakes and prefer humid environments in order to proliferate. There are 13 HDM species, however, the most common ones are Dermatophagoides pteronyssinus (Dp), Dermatophagoides farinae (Df) and Euroglyphus maynei (Em) [1]. Dp and Df are the main species that are known to cause respiratory allergic diseases [1,6]. Natural forms of HDM allergens are composed of both protein antigens and non-protein immunomodulatory elements. There are over 10 protein allergens in HDM including a cysteine protease (Der p1), chymotrypsin (Der p6), tropomyosin (Der p10), and chitinases (Der p15) [7-9]. Der p1 alone is highly allergenic and sufficient to initiate the production of pro-inflammatory and pro-Th2 cytokines such as IL-4, IL-5, IL-9, and IL-13 [10-12]. In addition to protein allergens, HDM also contains various microbial components such as LPS, ß-Glucan (a component of fungal spores) and various bacterial cell wall components [13-15]. Microbial products may trigger innate immune responses to orchestrate the initiation and amplification of type 2 inflammation. For example, HDM-associated LPS was previously shown to activate TLR4 in airway epithelial cells and induce the expression of thymic stromal lymphopoietin (TSLP), interleukin-25 (IL-25) and IL-33 cytokines [5,15] which are all known as primary activators of group 2 innate lymphoid cells (ILC2). HDM-LPS is also believed to directly activate TLR4 to aid the Th2 polarization [16]. Moreover, other molecules associated with HDM such as ß-Glucans and lipoproteins or lipopeptides can elicit pro-allergenic innate immune responses during HDM allergen exposures [14,15]. Activation of C type lectin receptors and TLR2 by glycan-derived molecules or lipoproteins has also been seen to lead to the infiltration of eosinophils and induction of Th2 cytokines [15-17]. Furthermore, Der p2 and chitin in HDM have also been shown to act as innate immune stimulants to induce the release of pro-inflammatory cytokines such as TNFa [15]. Taken together, HDM acts not only as an aeroallergen but also a carrier for numerous allergenic epitopes (present mainly in fecal pellets) and immunomodulatory molecules that exacerbate type 2 inflammation upon allergen exposure.
While HDM components have been known to promote type 2 inflammation and have been characterized extensively, it is somehow counterintuitive to realize that HDM allergens might contain factors that are able to suppress or reduce type 2 immune reactions as we demonstrated that HDM species (Dp) and (Df) contain immunomodulatory dsRNA > 40bp. At present, it remains unclear whether dsRNA species exist in other aeroallergens. It is known that dsRNA is immunologically active and can induce the production of type-I and type-III interferons [13], which may suppress type 2 inflammation, at least in part, through restricting ILC2 functions [18]. ILC2 cells are very important initiators and amplifiers for type 2 immune responses (more details about the role of ILC2 will be discussed later in the review).
In summary, HDM is a very complex allergen consisting of pro-allergenic proteins (e.g., Der p1) and microbial adjuvants (e.g., LPS) (Table 1). Paradoxically, new evidence indicates that HDM may also contain anti-allergenic factors such as dsRNA, which seems to be able to trigger innate immune responses to inhibit allergic inflammation.
|
HDM allergen components |
Corresponding receptor |
Effect on type 2 inflammation |
References |
|
β-glucans |
TLR2 |
Pro-allergenic, exacerbates airway allergic inflammation |
[17,70] |
|
Chitin |
TLR2 |
Pro-allergenic, exacerbates asthma and airway inflammation |
[71,72] |
|
LPS |
TLR4 |
Pro-allergenic, exacerbates asthma in mice |
[5,73] |
|
dsRNA |
TLR3 |
Anti-allergenic, Inhibits type 2 inflammation |
[13] |
RNA Sensing Immunity
Although RNA sensors have been extensively studied in the context of viral infections, their roles in allergic responses remain controversial. Multiple RNA sensors have been discovered and can be classified into two types, the endosomal RNA sensor and the cytosolic RNA sensor, based on their cellular locations. The former includes TLR3, TLR7/8 and TLR13 while the latter includes RIG-I and MDA5. TLR3 binds to relatively short strands of dsRNA (>40 bp) whereas MDA5 binds to longer dsRNA sequences (>1 kb). TLR7/8 recognizes the GC-rich ssRNA while TLR13 recognizes bacterial 23S rRNA with an exquisite sequence specificity [19,20]. Finally, RIG-I binds to 5’ppp-dsRNA. Upon engagement of the corresponding RNA ligand, these RNA sensors initiate different signaling cascades that culminate in the common downstream transcriptional pathways including NF-κB and IRF3, which ultimately result in the production of pro-inflammatory cytokines (e.g., IL-1ß, TNFα) or type I interferons (IFNα/β) [16]. TLR7/8 and TLR13 recruit the adaptor protein Myd88. Stimulation of TLR3 leads to the activation and recruitment of Trif adaptor protein [16,21,22]. The cytosolic RNA sensors RIG-I and MDA5 recruit the adaptor protein MAVS [23]. Notably, TLR3, 7, 9 ligands have been tested in clinical trials for treating allergic rhinitis or asthma [24].
Our traditional view is that respiratory viral infections usually lead to the exacerbations of allergic diseases, however, emerging data from animal models suggests otherwise [25,26]. A certain number of viruses such as gammaherpesvirus or influenza virus have been shown to activate protective innate immune responses against the development of type 2 immunopathology [25,26]. Additionally, in the gastrointestinal system, enteric viruses were shown to activate innate RNA sensing immunity which is required for sustaining intestinal homeostasis via educating intestinal intraepithelial lymphocytes (IELs) [18,27,28]. These observations collectively suggest that RNA sensing immunity seems to play a beneficial role in host defense against both viral infections and allergic inflammation [29-32].
Role of RNA Sensing in Regulating Type 2 Inflammation
Emerging evidence suggest that RNA sensing immunity may play a significant role in modulating HDM-induced allergy. We have recently shown that HDM-associated dsRNA induced a protective responsive against type 2 inflammation and airway hyperresponsiveness [13]. Mechanistically, our data suggests that lung exposure to HDM-dsRNA induces the production of type I interferons through TLR3- and MAVS-mediated signaling pathways. Mice lacking both TLR3 and MAVS failed to activate interferon responses, and therefore developed much worse type 2 immunopathology upon exposure to HDM allergen [13]. Interferon responses induced by TLR3 were stronger compared to interferon responses induced by the RNA sensors upstream of MAVS, MDA5 and RIG-I [13]. This could be due to the ability of TLR3 to recognize nucleic acids sequence over 40 bp.
Human genetic studies have suggested that polymorphisms of TLR3 and TLR7/8 may be associated with asthma-related phenotypes in patients [33,34]. In prophylactic studies, dsRNA (TLR3 agonist) and ssRNA (TLR7/8 agonist) were used as immune adjuvants to prevent the onset of type 2 allergic inflammation [16]. Our data suggested the presence of dsRNA structures with >40 bp length in common HDM species (Df and Dp) [13,35,36]. Mechanistically, natural dsRNAs derived from either HDM or viruses, or a synthetic form, poly (I:C), mainly engage the TLR3-TRIF-IRF3-IFN pathway [13,36]. Unlike other HDM components that are known to be pro-allergenic, HDM dsRNA induces an anti-allergic interferon response which can efficiently suppress type 2 inflammation and airway hyperresponsiveness [13,16,37,38]. More importantly, we have shown that total RNAs derived from both DNA- and RNA- virus-infected cells were also able to suppress type 2 inflammation and airway hyperresponsiveness [39]. Thus, these findings collectively support the hygiene hypothesis, which states that early childhood microbial exposure confers protection against the development of allergic diseases [36,39].
Although we have clearly demonstrated in acute models of allergy that the dsRNA-activated interferon pathway is required for restraining the development of type 2 inflammation, it remains to be determined whether additional mechanisms are involved in negatively regulating the development of type 2 inflammation. Future studies would provide useful information for the clinical development of dsRNA as a vaccine adjuvant [40].
Modulation of ILC2-Mediated Type 2 Inflammation by Immune Sensing of HDM-Associated Immunomodulatory RNA
ILC2 are the innate counterpart of the T helper 2 lymphocytes (Th2) [41-44]. In response to alarmin cytokines such as IL-33, IL-25 and TSLP, ILC2s produce large amounts of the type 2 effector cytokines IL-5 and IL-13 [45-48], which promote the development of type 2 inflammatory responses characterized by eosinophilia, airway remodeling, mucus hypersecretion, and airway hyperresponsiveness (AHR) [45-53].
Damage of airway epithelial layers by proteases allergens often results in the release of cytokines like IL-25, IL-33 and TSLP, which potently activate ILC2 cells [54]. The function of ILC2 must be tightly regulated in order to prevent the onset of overzealous type 2 inflammation. Several mechanisms have been proposed for which ILC2 is regulated in allergic diseases [55]. Targeting ILC2 has shown some promising therapeutic effects in treating asthma and allergic rhinitis [55]. One of these strategies is to directly suppress ILC2 numbers and functions by interferons induced by activating TLRs [56]. Our lab has recently shown that a synthetic dsRNA analogue and natural dsRNA derived from DNA and RNA viruses can attenuate IL-33- and a fungal allergen-induced type 2 inflammation by blocking the activation of ILC2 via the TLR3-IFN signaling axis [36]. Our data suggest that dsRNA species induce the release of type I interferons in the lungs; this is sufficient to reduce airway inflammation as mice lacking interferon receptor had exacerbated lung inflammation [39]. Phenotypically, dsRNA-induced suppression of ILC2 function resulted in the decreased number of eosinophils by ~6 folds and reduced lung resistance in mice [36]. In addition to interferon-dependent suppression of ILC2, a number of studies demonstrated several other mechanisms to target these cells, for instance, prostaglandin E2, IL-27, regulatory T cells or their associated cytokines were all shown to suppress ILC2 activation, proliferation and functions, respectively [57-59].
HDM allergen not only polarize to type 2 allergic responses by acting on epithelial cells but also contain proteases that could direct DCs, via ILC2-derived IL-13, to the lymph nodes where they present antigens to Th2 cells, hence, leading to establishment of adaptive Th2 responses [49]. Given the critical role of ILC2 in amplifying and exacerbating type 2 inflammation, it is necessary to explore new methods for targeting these cells.
Although we have clearly showed that dsRNA seizes the proliferation and expansion of ILC2 in acute models of allergic inflammation (Figure 2) [36], it has not been determined whether viral or HDM-dsRNA inhibition of ILC2 could extend to chronic models of allergic inflammation. Notably, DCs play very important role at early stages of sensitization by allergens. DCs take up allergen, then migrate to adjacent lymph nodes where they present allergen peptides to MHCII-restricted T cells, thus, leading to polarized Th2 responses. Given that dsRNA can be used to promote antigen-specific Th1-polarized responses, it would be interesting to evaluate whether DCs could be targeted by dsRNA to manipulate allergen sensitization process [60].
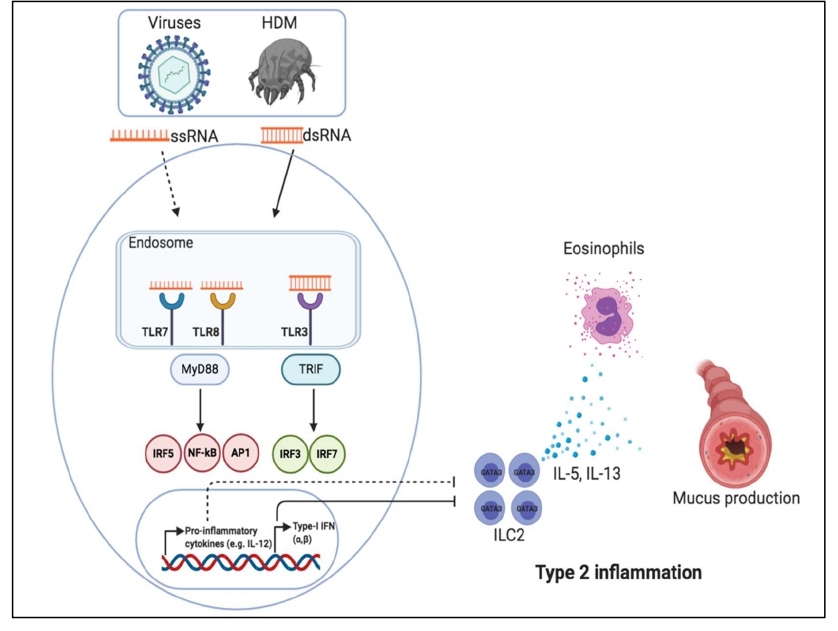
Figure 2: Immunogenic ssRNA or dsRNA derived from viruses or HDM suppress ILC2-mediated type 2 inflammation through triggering MyD88-IFN axis or TRIF-IFN axis. Cytokines such as IL-12 and interferons restrain ILC2 functions thus inhibiting the production of IL-5 and IL-13 which are responsible for eosinophils recruitment and mucus production, respectively.
Implication of RNA-Sensing in HDM Specific Immunotherapy
Allergen immunotherapy (AIT) is an effective approach to treat allergic diseases. Although not fully defined, AIT is thought to suppress Th2 responses [61]. Treating HDM-sensitized patients with both forms of immunotherapy, subcutaneous and sublingual (SCIT and SLIT), has been shown to effectively reduce the disease severity [62]. However, the process of immunotherapy is complicated and often takes many years to reach satisfying therapeutic effects. In order to shorten the treatment period, immune adjuvants that stimulate innate immune sensors such as TLR4 and TLR9 have been used clinically [63].
HDM extracts used in AIT contain not only protein allergens, but also other microbial components, of which some could be pro-allergenic while others like TLR3 agonist (dsRNA) are anti-allergic [13]. In fact, commercial HDM extracts from 10 different manufacturers induces variable IgE reactivity profiles in patients [64]. To minimize the element of variability in AIT, it would be important to clearly define the immune stimulatory nature of HDM-associated components.
Future Directions
TLR agonists targeting TLR2, TLR3, TLR4, TLR7/8, TLR9 seem to be effective in reducing eosinophilic inflammation by inducing the production of interferons and Th1 cytokines such as IL-12 in animal models and in clinical trials [24,56,65,66]. The hygiene hypothesis states that microbial exposures early in life may confer a protective immunity against the development of allergy in healthy individuals. Accumulating evidence from experimental and epidemiological studies in recent years supports this notion [25,67]. Mechanistically, interferon responses induced by TLR agonists have been shown to play an important role in ameliorating type 2 inflammation in animal models of asthma and allergy [17]. Other protective pathways beyond interferons should be explored in the future studies. Besides dsRNA, ssRNA can be generated during microbial infections and it would be important to evaluate whether ssRNA species derived from viruses or bacteria could lead to the activation of TLR7/8, and therefore regulation of allergen-induced type 2 inflammation.
HDM allergens cause various allergic diseases including allergic rhinitis, eczema, conjunctivitis and asthma [68]. Over 50% of asthmatic patients are sensitized to HDM. Immunotherapy with HDM extract has already been utilized to desensitize allergic patients [64,69]. We recently identified that the medical-grade HDM extracts endogenously contain long dsRNA species that potently induce innate immune responses in mouse lungs [13], which might contribute to some immunological effects during the HDM immunotherapy in patients. Besides immunotherapy, other forms of treatments are impermanent and not suitable for all groups of patients, hence, identifying immunomodulatory RNA within HDM allergen could provide an alternative therapeutic approach to control the development of airway inflammation induced by HDM or other related allergens in clinical settings.
Acknowledgments
H.H.A. is supported by the Department of Clinical Laboratory Sciences, College of Applied Medical Sciences, Al Jouf University, Sakaka, Saudi Arabia. X.D.L. is supported by the UT Health San Antonio School of Medicine Startup Fund and the Max and Minnei Voelcker Fund
References
2. Emran H, Chieng CS, Taib S, Cunningham AC. House dust mite sensitisation and association with atopic dermatitis in Brunei. Clinical and Translational Allergy. 2019 Dec;9(1):1-4.
3. Gaffin JM, Phipatanakul W. The role of indoor allergens in the development of asthma. Current Opinion in Allergy and Clinical Immunology. 2009 Apr;9(2):128.
4. Nunes C, Pereira AM, Morais-Almeida M. Asthma costs and social impact. Asthma Research and Practice. 2017 Dec;3(1):1-1.
5. Hammad H, Chieppa M, Perros F, Willart MA, Germain RN, Lambrecht BN. House dust mite allergen induces asthma via Toll-like receptor 4 triggering of airway structural cells. Nature Medicine. 2009 Apr;15(4):410-6.
6. Thomas WR. House dust allergy and immunotherapy. Human Vaccines & Immunotherapeutics. 2012 Oct 16;8(10):1469-78.
7. Miller JD. The role of dust mites in allergy. Clinical Reviews in Allergy & Immunology. 2019 Dec;57(3):312-29.
8. Reithofer M, Jahn-Schmid B. Allergens with protease activity from house dust mites. International Journal of Molecular Sciences. 2017 Jul;18(7):1368.
9. Wong L, Huang CH, Lee BW. Shellfish and house dust mite allergies: is the link tropomyosin?. Allergy, Asthma & Immunology Research. 2016 Mar;8(2):101.
10. Dai X, Sayama K, Tohyama M, Shirakata Y, Hanakawa Y, Tokumaru S, et al. Mite allergen is a danger signal for the skin via activation of inflammasome in keratinocytes. Journal of Allergy and Clinical Immunology. 2011 Mar 1;127(3):806-14.
11. Asokananthan N, Graham PT, Stewart DJ, Bakker AJ, Eidne KA, Thompson PJ, et al. House dust mite allergens induce proinflammatory cytokines from respiratory epithelial cells: the cysteine protease allergen, Der p 1, activates protease-activated receptor (PAR)-2 and inactivates PAR-1. The Journal of Immunology. 2002 Oct 15;169(8):4572-8.
12. King C, Brennan S, Thompson PJ, Stewart GA. Dust mite proteolytic allergens induce cytokine release from cultured airway epithelium. The Journal of Immunology. 1998 Oct 1;161(7):3645-51.
13. She L, Alanazi HH, Yan L, Zou Y, Sun Y, Dube PH, et al. Immune sensing of aeroallergen-associated double-stranded RNA triggers an IFN response and modulates type 2 lung inflammation. The Journal of Immunology. 2019 Nov 1;203(9):2520-31.
14. Gregory LG, Lloyd CM. Orchestrating house dust mite-associated allergy in the lung. Trends in Immunology. 2011 Sep 1;32(9):402-11.
15. Jacquet A. The role of innate immunity activation in house dust mite allergy. Trends in Molecular Medicine. 2011 Oct 1;17(10):604-11.
16. Zakeri A, Russo M. Dual role of toll-like receptors in human and experimental asthma models. Frontiers in immunology. 2018 May 15;9:1027.
17. Hadebe S, Kirstein F, Fierens K, Redelinghuys P, Murray GI, Williams DL, et al. β-Glucan exacerbates allergic airway responses to house dust mite allergen. Respiratory Research. 2016 Dec;17(1):1-3.
18. Liu L, Gong T, Tao W, Lin B, Li C, Zheng X, et al. Commensal viruses maintain intestinal intraepithelial lymphocytes via noncanonical RIG-I signaling. Nature Immunology. 2019 Dec;20(12):1681-91.
19. Li XD, Chen ZJ. Sequence specific detection of bacterial 23S ribosomal RNA by TLR13. Elife. 2012 Oct 30;1:e00102.
20. Oldenburg M, Krüger A, Ferstl R, Kaufmann A, Nees G, Sigmund A, et al. TLR13 recognizes bacterial 23S rRNA devoid of erythromycin resistance–forming modification. Science. 2012 Aug 31;337(6098):1111-5
21. Maney SK, Xu HC, Huang J, Pandyra AA, Ehlting C, Aguilar-Valenzuela R, et al. RAIDD Mediates TLR3 and IRF7 Driven Type I Interferon Production. Cellular Physiology and Biochemistry. 2016;39(4):1271-80.
22. Ioannidis I, Ye F, McNally B, Willette M, Flaño E. Toll-like receptor expression and induction of type I and type III interferons in primary airway epithelial cells. Journal of virology. 2013 Mar 15;87(6):3261-70.
23. Seth RB, Sun L, Ea CK, Chen ZJ. Identification and characterization of MAVS, a mitochondrial antiviral signaling protein that activates NF-κB and IRF3. Cell. 2005 Sep 9;122(5):669-82.
24. Anwar MA, Shah M, Kim J, Choi S. Recent clinical trends in Toll‐like receptor targeting therapeutics. Medicinal Research Reviews. 2019 May;39(3):1053-90.
25. Machiels B, Dourcy M, Xiao X, Javaux J, Mesnil C, Sabatel C, et al. A gammaherpesvirus provides protection against allergic asthma by inducing the replacement of resident alveolar macrophages with regulatory monocytes. Nature Immunology. 2017 Dec;18(12):1310.
26. Duerr CU, McCarthy CD, Mindt BC, Rubio M, Meli AP, Pothlichet J, et al. Type I interferon restricts type 2 immunopathology through the regulation of group 2 innate lymphoid cells. Nature Immunology. 2016 Jan;17(1):65-75.
27. Yang JY, Kim MS, Kim E, Cheon JH, Lee YS, Kim Y, et al. Enteric viruses ameliorate gut inflammation via toll-like receptor 3 and toll-like receptor 7-mediated interferon-β production. Immunity. 2016 Apr 19;44(4):889-900.
28. Virgin HW. The virome in mammalian physiology and disease. Cell. 2014 Mar 27;157(1):142-50.
29. Weber F, Wagner V, Rasmussen SB, Hartmann R, Paludan SR. Double-stranded RNA is produced by positive-strand RNA viruses and DNA viruses but not in detectable amounts by negative-strand RNA viruses. Journal of Virology. 2006 May 15;80(10):5059-64.
30. Zhao Y, Ye X, Dunker W, Song Y, Karijolich J. RIG-I like receptor sensing of host RNAs facilitates the cell-intrinsic immune response to KSHV infection. Nature Communications. 2018 Nov 19;9(1):1-4.
31. Zhao Y, Karijolich J. Know thyself: RIG-I-like receptor sensing of DNA virus infection. Journal of virology. 2019 Dec 1;93(23).
32. Chiang JJ, Sparrer KM, van Gent M, Lässig C, Huang T, Osterrieder N, et al. Viral unmasking of cellular 5S rRNA pseudogene transcripts induces RIG-I-mediated immunity. Nature Immunology. 2018 Jan;19(1):53-62.
33. Zhang Q, Fu XL, Qian FH, Cao Q, Mao ZD, Bai JL, Du Q, Shi Y. Polymorphisms in Toll‐like receptor 3 are associated with asthma‐related phenotypes in the Chinese Han patients. International Journal of Immunogenetics. 2016 Dec;43(6):383-90.
34. Møller-Larsen S, Nyegaard M, Haagerup A, Vestbo J, Kruse TA, Børglum AD. Association analysis identifies TLR7 and TLR8 as novel risk genes in asthma and related disorders. Thorax. 2008 Dec 1;63(12):1064-9.
35. Alanazi HH, She L, Li XD. Identification and Characterization of Immunogenic RNA Species in HDM Allergens that Modulate Eosinophilic Lung Inflammation. Journal of Visualized Experiments: Jove. 2020 May 30(159).
36. She L, Alanazi HH, Yan L, Brooks EG, Dube PH, Xiang Y, et al. Sensing and signaling of immunogenic extracellular RNAs restrain group 2 innate lymphoid cell-driven acute lung inflammation and airway hyperresponsiveness. Plos One. 2020 Jul 30;15(7):e0236744.
37. Mitchell C, Provost K, Niu N, Homer R, Cohn L. IFN-γ acts on the airway epithelium to inhibit local and systemic pathology in allergic airway disease. The Journal of Immunology. 2011 Oct 1;187(7):3815-20.
38. Koch S, Finotto S. Role of interferon-λ in allergic asthma. Journal of innate immunity. 2015;7(3):224-30.
39. Haspeslagh E, Heyndrickx I, Hammad H, Lambrecht BN. The hygiene hypothesis: immunological mechanisms of airway tolerance. Current Opinion in Immunology. 2018 Oct 1;54:102-8..
40. Bezemer GF, Sagar S, Van Bergenhenegouwen J, Georgiou NA, Garssen J, Kraneveld AD, et al. Dual role of Toll-like receptors in asthma and chronic obstructive pulmonary disease. Pharmacological Reviews. 2012 Apr 1;64(2):337-58.
41. Eberl G, Colonna M, Di Santo JP, McKenzie AN. Innate lymphoid cells. Innate lymphoid cells: a new paradigm in immunology. Science. 348: aaa6566.
42. Vivier E, Artis D, Colonna M, Diefenbach A, Di Santo JP, Eberl G, Koyasu S, Locksley RM, McKenzie AN, Mebius RE, Powrie F. Innate lymphoid cells: 10 years on. Cell. 2018 Aug 23;174(5):1054-66.
43. Artis D, Spits H. The biology of innate lymphoid cells. Nature. 2015 Jan;517(7534):293-301.
44. Barlow JL, McKenzie AN. Innate lymphoid cells of the lung. Annual Review of Physiology. 2019 Feb 10;81:429-52.
45. Liew FY, Girard JP, Turnquist HR. Interleukin-33 in health and disease. Nature Reviews Immunology. 2016 Nov;16(11):676.
46. Cayrol C, Girard JP. Interleukin‐33 (IL‐33): a nuclear cytokine from the IL‐1 family. Immunological Reviews. 2018 Jan;281(1):154-68.
47. Kubo M. Innate and adaptive type 2 immunity in lung allergic inflammation. Immunological Reviews. 2017 Jul;278(1):162-72.
48. Molofsky AB, Savage AK, Locksley RM. Interleukin-33 in tissue homeostasis, injury, and inflammation. Immunity. 2015 Jun 16;42(6):1005-19.
49. Halim TY, Steer CA, Mathä L, Gold MJ, Martinez-Gonzalez I, McNagny KM, et al. Group 2 innate lymphoid cells are critical for the initiation of adaptive T helper 2 cell-mediated allergic lung inflammation. Immunity. 2014 Mar 20;40(3):425-35.
50. Starkey MR, McKenzie AN, Belz GT, Hansbro PM. Pulmonary group 2 innate lymphoid cells: surprises and challenges. Mucosal Immunology. 2019 Mar;12(2):299-311.
51. Greenfeder S, Umland SP, Cuss FM, Chapman RW, Egan RW. Th2 cytokines and asthma the role of interleukin-5 in allergic eosinophilic disease. Respiratory Research. 2001 Apr;2(2):1-9.
52. Doran E, Cai F, Holweg CT, Wong K, Brumm J, Arron JR. Interleukin-13 in asthma and other eosinophilic disorders. Frontiers in Medicine. 2017 Sep 19;4:139.
53. McKenzie AN. Type-2 innate lymphoid cells in asthma and allergy. Annals of the American Thoracic Society. 2014 Dec;11(Supplement 5):S263-70.
54. Borger P, Oliver B, Heijink I, Hardavella G. Beyond the Immune System: The Role of Resident Cells in Asthma and COPD. Journal of Allergy. 2012;2012.
55. Doherty TA, Broide DH. Pathways to limit group 2 innate lymphoid cell activation. Journal of Allergy and Clinical Immunology. 2017 May 1;139(5):1465-7.
56. Dong Z, Xiong L, Zhang W, Gibson PG, Wang T, Lu Y, et al. Holding the inflammatory system in check: TLRs and their targeted therapy in asthma. Mediators of Inflammation. 2016 Oct;2016.
57. Maric J, Ravindran A, Mazzurana L, Björklund ÅK, Van Acker A, Rao A, et al. Prostaglandin E2 suppresses human group 2 innate lymphoid cell function. Journal of Allergy and Clinical Immunology. 2018 May 1;141(5):1761-73.
58. Mchedlidze T, Kindermann M, Neves AT, Voehringer D, Neurath MF, Wirtz S. IL-27 suppresses type 2 immune responses in vivo via direct effects on group 2 innate lymphoid cells. Mucosal Immunology. 2016 Nov;9(6):1384-94.
59. Rigas D, Lewis G, Aron JL, Wang B, Banie H, Sankaranarayanan I, et al. Type 2 innate lymphoid cell suppression by regulatory T cells attenuates airway hyperreactivity and requires inducible T-cell costimulator–inducible T-cell costimulator ligand interaction. Journal of Allergy and Clinical Immunology. 2017 May 1;139(5):1468-77.
60. Jin B, Sun T, Yu XH, Liu CQ, Yang YX, Lu P, Fu SF, Qiu HB, Yeo AE. Immunomodulatory effects of dsRNA and its potential as vaccine adjuvant. Journal of Biomedicine and Biotechnology. 2010 Oct;2010:690438.
61. Matsuoka T, Shamji MH, Durham SR. Allergen immunotherapy and tolerance. Allergology International. 2013;62(4):403-13.
62. Tang RB. House dust mite-specific immunotherapy alters the natural course of atopic march. Journal of the Chinese Medical Association. 2020 Feb 1;83(2):109-12.
63. Gunawardana NC, Durham SR. New approaches to allergen immunotherapy. Annals of Allergy, Asthma & Immunology. 2018 Sep 1;121(3):293-305.
64. Yang L, Zhu R. Immunotherapy of house dust mite allergy. Human Vaccines & Immunotherapeutics. 2017 Oct 3;13(10):2390-6.
65. Aryan Z, Holgate ST, Radzioch D, Rezaei N. A new era of targeting the ancient gatekeepers of the immune system: toll-like agonists in the treatment of allergic rhinitis and asthma. International Archives of Allergy and Immunology. 2014;164(1):46-63.
66. Leaker BR, Singh D, Lindgren S, Almqvist G, Eriksson L, Young B, et al. Effects of the Toll-like receptor 7 (TLR7) agonist, AZD8848, on allergen-induced responses in patients with mild asthma: a double-blind, randomised, parallel-group study. Respiratory Research. 2019 Dec;20(1):1-1.
67. Strachan DP. Family size, infection and atopy: the first decade of the'hygiene hypothesis'. Thorax. 2000 Aug;55(Suppl 1):S2.
68. Li L, Qian J, Zhou Y, Cui Y. Domestic mite–induced allergy: Causes, diagnosis, and future prospects. 2018. 32: 2058738418804095.
69. Feng B, Xiang H, Jin H, Gao J, Huang S, Shi Y, Chen R, Chen B. Efficacy of sublingual immunotherapy for house dust mite-induced allergic rhinitis: a meta-analysis of randomized controlled trials. Allergy, Asthma & Immunology research. 2017 May;9(3):220..
70. Zhang Z, Myers JM, Brandt EB, Ryan PH, Lindsey M, Mintz-Cole RA, et al. β-Glucan exacerbates allergic asthma independent of fungal sensitization and promotes steroid-resistant TH2/TH17 responses. Journal of Allergy and Clinical Immunology. 2017 Jan 1;139(1):54-65.
71. Arae K, Morita H, Unno H, Motomura K, Toyama S, Okada N, et al. Chitin promotes antigen-specific Th2 cell-mediated murine asthma through induction of IL-33-mediated IL-1β production by DCs. Scientific Reports. 2018 Aug 6;8(1):1-5.
72. Choi JP, Lee SM, Choi HI, Kim MH, Jeon SG, Jang MH, et al. House dust mite-derived chitin enhances Th2 cell response to inhaled allergens, mainly via a TNF-α-dependent pathway. Allergy, Asthma & Immunology Research. 2016 Jul;8(4):362.
73. Lowe AP, Thomas RS, Nials AT, Kidd EJ, Broadley KJ, Ford WR. LPS exacerbates functional and inflammatory responses to ovalbumin and decreases sensitivity to inhaled fluticasone propionate in a guinea pig model of asthma. British Journal of Pharmacology. 2015 May;172(10):2588-603.