Abstract
Given that viral infections may trigger either the development of inflammatory renal disease or the worsening of pre-existing renal disease, activated signaling through Toll-like receptor 3 (TLR3) reportedly plays a role in the pathogenesis of glomerular diseases (GN). Concerning TLR3 in intrinsic glomerular cells, it has been reported that the activation of TLR3 and downstream immune responses can be induced by both infectious organisms and endogenous ligands and leading to the development of “pseudo” antiviral immunity-related inflammations in the kidney. Therefore, this theory is probably involved in the pathophysiology of GN, especially in lupus nephritis (LN). Glomerular endothelial cells (GECs) are directly exposed to circulating viral particles in the glomerulus. Thus, the specific molecular mechanisms underlying the initiation of glomerular inflammation thorough the activation of endothelial TLR3 signaling need to be determined. We found that endothelial TLR3 activation leads to inflammatory chemokine and adhesion molecule expression in human GECs. We believe that elucidating the specific mechanisms of human endothelial TLR3 signaling in GECs will assist in the development of possible curative strategies for GN, particularly in LN in the future.
Keywords
Chloroquine, Glomerular endothelial cells, Lupus nephritis, Pseudoviral immunity, Toll-like receptor 3
Toll-Like Receptor 3 (TLR3) and Intrinsic Glomerular Cells
Since glomerular endothelial cells (GECs), the glomerular basement membrane, and podocytes are believed to contribute to the glomerular filtration barrier, damages to any of these intrinsic cell types reportedly lead to the development of glomerulonephritis (GN) [1]. In clinical practice, it is well known that viral infections can trigger the development of inflammatory GN or the worsening of pre-existing GN [2]. Recognition of the molecular pattern of viral pathogens by Toll-like receptor 3 (TLR3) found in intracellular endosomes and subsequent immunoreactions is important in host antiviral defenses [3,4]. TLR3 signaling cascades activate the TIR-domain-containing adapter-inducing interferon-β (TRIF)-dependent pathways via transcriptional factors, i.e., nuclear factor κB (NF-κB) and interferon regulatory factor 3 (IRF3), and the subsequent release of inflammatory chemokines, cytokines, adhesion molecules, and finally type I interferons (IFN) [3]. Notably, the activation of TLR3 and their downstream immune responses can be induced not only by infectious pathogens but also non-infectious stimulation such as endogenous ligands [3]. It has also been reported that the expression of TLR3 in residual glomerular cells is confirmed in biopsy specimens from patients with GN [2,5]. Thus, regional viral and “pseudo” viral immunoreactions via the activation of TLR3 signaling in the intrinsic glomerular cells have been postulated to be involved in the pathogenesis of some GN [2,6]. Interestingly, sustained activation of type I IFN is thought to be involved in the pathogenesis of lupus nephritis (LN) [6,7]. Therefore, the involvement of the TLR3 signaling system in the intrinsic glomerular cells is postulated at least in part in the pathogenesis of LN [6,8-10].
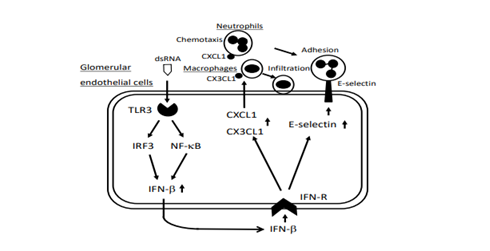
It has been reported that GECs express various adhesion molecules in response to proinflammatory cytokines and play a role in the pathogenesis of hemodynamic disturbances [11]. Furthermore, GECs are directly exposed to circulating viral particles in the glomerulus; therefore, endothelial antiviral and “pseudo” viral immunoreactions through TLR3 signaling are thought to play a role in the initiation of glomerular inflammation [12,13]. Thus far, considering the implications of TLR3 signaling in the pathogenesis of LN, we treated human GECs with polyinosinic-polycytidylic acid (poly IC), an authentic viral double-stranded RNA, and then examined the expressions of representative neutrophil chemoattractant C-X-C motif chemokine 1 (CXCL1)/GROα, the adhesion molecule E-selectin, and macrophage chemoattractant CX3CL1/fractalkine [13,14]. We found that the activation of TLR3 induced the endothelial expression of CXCL1, E-selectin, and CX3CL1, and that these involved TLR3 signaling cascades of NF-κB, IRF3, and IFN-β (Figure 1). Intense endothelial CXCL1 expression was observed in biopsy specimens from patients with crescentic GN [13]. Given that E-selectin, CXCL1 and CX3CL1 in GECs contribute to regional neutrophil and macrophage recruitment, which leads to the development of GN, TLR3 signaling in GECs may control initial inflammation in the glomerulus [13,14]. Further, we found that poly IC increased plasminogen activator inhibitor-1 (PAI-1) and interleukin-6 (IL-6) expressions in GECs [15,16]. Since PAI-1 inhibits fibrinolysis, an imbalance of activity between PAI-1 and tissue plasminogen activator contributes to glomerulosclerosis [17], endothelial TLR3 signaling is also involved in the chronic lesion of GN. Interestingly, cytosolic sensors of viral RNA, retinoic acid-inducible gene-I (RIG-I), and melanoma differentiation-associated gene 5 were found to be involved in IL-6 production via TLR3 signaling in GECs [16]. However, endothelial PAI-I expression did not depend on these cytosolic recognition sensors [15]. Thus, the expression of inflammatory molecules through TLR3 signaling in human GECs may be tightly regulated, although this theory is yet to be elucidated.
Figure 1: Postulated signaling pathways of TLR3, IRF3, NF-κB, and IFN-β in the expressions of neutrophil adhesion molecule, E-selectin, neutrophil chemoattractant, CXCL1, and macrophage chemoattactant, CX3CL1 induced by poly IC in GECs. TLR3: Toll-Like Receptor 3; IRF3: Interferon Regulatory Factor 3; GECs: Glomerular Endothelial Cells.
Despite some limitations, possible involvement of endothelial TLR3 signaling, which is associated with the continuous activation of type I IFN as well as a regional expression of various inflammatory molecules, is thought to be involved in the pathogenesis of GN, particularly in LN [6,7].
Chloroquine and Endothelial TLR3 Signaling
Although the European League against Rheumatism and the European Renal Association-European Dialysis and Transplant Association (EULAR/ERA-EDTA) recommended the use of an antimalarial agent, chloroquine for patients with LN [18], the detailed beneficial effects of chloroquine against glomerular inflammation is yet to be elucidated. Previously, we examined the effects of chloroquine on the representative TLR3/IFN-β-signaling axis, TLR3/IFN-β/RIG-I/chemokine (C-C motif) ligand 5 (CCL5) in human glomerular mesangial cells treated with poly IC and found that 10 μg/mL chloroquine attenuates mesangial TLR3 signaling in the early phase of NF-κB activation [19]. Subsequently, we examined the effect of chloroquine on the endothelial TLR3 signaling and found that pretreating cells with chloroquine but not dexasamethasone attenuated poly IC-induced CX3CL1 expression in GECs [14]. Further, we found that pretreating cells with chloroquine, and also hydroxychloroquine, but not dexamethasone, attenuated poly IC-induced PAI-1 expression in GECs [15]. Our results may further support regional renoprotective effects of the antimalarial agents, chloroquine in treating LN [14,15]. More detailed studies are required to confirm our preliminary findings.
In daily clinical practice, it has been reported that the minimum hydroxychloroquine target level to prevent flares in 171 lupus patients was, at least, more than 600 ng/mL [20]. Considering the blood concentration level, 10 μg/mL of chloroquine used in our experiments may be rather higher than postulated in this paper. However, the adequate blood level of the drug in patients with LN is yet to be determined. In addition, it is unclear whether adequate blood levels of the drug prevent activated TLR3 signaling in GECs. This issue should be clarified in future studies.
The activation of the TLR3 signaling cascade in residual renal cells and cross- talk of infiltrating monocytes, neutrophils, and lyomphocytes results in inducing type I IFNs release, which may be involved in the pathogenesis of LN [6,8,9]. Studies of drugs that have been useful in controlling glomerular inflammatory pathways mediated by the innate immunity are now underway.
Conflict of Interest
There are no conflicts of interest to declare.
Funding
This study was supported by Grants-in-Aid of the Japan Society for Promotion of Science (JSPS KAKENHI Grant number: 16K10055 to H. T.).
References
2. Robson MG. Toll-like receptors and renal disease. Nephron Experimental Nephrology. 2009;113(1):e1-7.
3. Kawai T, Adachi O, Ogawa T, Takeda K. Akira S. TLR signaling. Cell Death Differ. 2006;13:816-25.
4. Patel MC, Shirey KA, Pletneva LM, Boukhvalova MS, Garzino-Demo A, et al. Novel drugs targeting Toll-like receptors for antiviral therapy. Future Virology. 2014 Sep;9(9):811-29.
5. Conti F, Spinelli FR, Truglia S, Miranda F, Alessandri C, Ceccarelli F, et al. Kidney expression of toll like receptors in lupus nephritis: quantification and clinicopathological correlations. Mediators of Inflammation. 2016 Aug 21;2016.
6. Anders HJ. Pseudoviral immunity–a novel concept for lupus. Trends in Molecular Medicine. 2009 Dec 1;15(12):553-61.
7. Hagberg N, Rönnblom L. Systemic lupus erythematosus–a disease with a dysregulated type I interferon system. Scandinavian Journal of Immunology. 2015 Sep;82(3):199-207.
8. Tanaka H, Imaizumi T. Inflammatory chemokine expression via Toll-like receptor 3 signaling in normal human mesangial cells. Clinical and Developmental Immunology. 2013 Jan 1;2013.
9. Lorenz G, Lech M, Anders HJ. Toll-like receptor activation in the pathogenesis of lupus nephritis. Clinical Immunology. 2017 Dec 1;185:86-94.
10. Anders HJ, Saxena R, Zhao MH, Parodis I, Salmon JE, Mohan C. Lupus nephritis. Nature Reviews Disease Primers. 2020 Jan 23;6(1):7.
11. Murakami S, Morioka T, Nakagawa Y, Suzuki Y, Arakawa M, Oite T. Expression of adhesion molecules by cultured human glomerular endothelial cells in response to cytokines: comparison to human umbilical vein and dermal microvascular endothelial cells. Microvascular Research. 2001 Nov 1;62(3):383-91.
12. Hägele H, Allam R, Pawar RD, Anders HJ. Double-stranded RNA activates type I interferon secretion in glomerular endothelial cells via retinoic acid-inducible gene (RIG)-1. Nephrology Dialysis Transplantation. 2009 Nov 1;24(11):3312-8.
13. Liu Q, Imaizumi T, Kawaguchi S, Aizawa T, Matsumiya T, Watanabe S, et al. Toll-like receptor 3 signaling contributes to regional neutrophil recruitment in cultured human glomerular endothelial cells. Nephron. 2018;139:349-58.
14. Hirono K, Imaizumi T, Aizawa T, Watanabe S, Tsugawa K, Shiratori T, et al. Endothelial expression of fractalkine (CX3CL1) is induced by Toll-like receptor 3 signaling in cultured human glomerular endothelial cells. Modern Rheumatology. 2019 Nov 1:1-8.
15. Aizawa T, Imaizumi T, Hirono K, Watanabe S, Tsugawa K, Tanaka H. Chloroquine attenuates TLR3-mediated plasminogen activator inhibitor-1 expression in cultured human glomerular endothelial cells. Clinical and Experimental Nephrology. 2019 Apr 2;23(4):448-54.
16. Liu Q, Imaizumi T, Aizawa T, Hirono K, Kawaguchi S, Watanabe S, et al. Cytosolic sensors of viral RNA are involved in the production of interleukin-6 via toll-like receptor 3 signaling in human glomerular endothelial cells. Kidney and Blood Pressure Research. 2019;44(1):62-71.
17. Wörnle M, Roeder M, Sauter M, Merkle M, Ribeiro A. Effect of dsRNA on mesangial cell synthesis of plasminogen activator inhibitor type 1 and tissue plasminogen activator. Nephron Experimental Nephrology. 2009;113(2):e57-65.
18. Bertsias G, Tektonidou M, Amoura Z, Aringer M, Bajema I, Berden JH, et al. Joint European League Against Rheumatism and European Renal Association-European Dialysis and Transplant Association (EULAR/ERA-EDTA) recommendations for the management of adults and paediatric lupus nephritis. Annals of the Rheumatic Diseases. 2012;71:1771-82.
19. Imaizumi T, Hayakari R, Matsumiya T, Yoshida H, Tsuruga K, Watanabe S, et al. Chloroquine attenuates TLR3/IFN-β signaling in cultured normal human mesangial cells: A possible protective effect against renal damage in lupus nephritis. Modern Rheumatology. 2017 Nov 2;27(6):1004-9.
20. Cunha C, Alexander S, Ashby D, Lee J, Chusney G, Cairns TD, et al. Hydroxycloroquine blood concentration in lupus nephritis: a determinant of disease outcome?. Nephrology Dialysis Transplantation. 2018 Sep 1;33(9):1604-10.