Abstract
Objective: The significant metastatic potential of uveal melanoma (UVM) lends to high mortality. Even with successful local tumor treatment, many patients will develop metastatic disease. The present study aims to elucidate the relationship between tumor-infiltrating immune cell (TIIC) diversity and survival to identify potential therapeutic targets and improve UVM prognosis.
Methods: Bulk deconvolution was used to determine the relative proportions of 22 hematopoietic TIIC from 80 UVM tumor samples. Cytolytic activity (CYT) was determined, and associated survival probabilities were mined using time-to-event data. Nominal P-values were subjected to FDR correction.
Results: High relative abundance of tumor-infiltrating naïve B cells, resting memory CD4+ T cells, and monocytes correlated with better overall and disease-free survival probability. Low relative abundance of CD8+ T cells correlated with better overall survival and disease-free survival probability. CYT correlated positively with relative abundance of naïve B cells, resting memory CD4+ T cells, and monocytes. CYT correlated negatively with relative abundance of CD8+ T cells.
Conclusion: Infiltrating naïve B cells, resting memory CD4+ T cells, monocytes, and CD8+ T cells are potential therapeutic targets in UVM that warrant further investigation. High CYT estimates associate with worse UVM survival outcomes.
Keywords
CD4+, UVM, Cytolytic activity, B cells, Infiltration
Introduction
Melanomas are a vast family of cancers derived from malignant melanocytes with significant locational diversity, including the skin, mucous membranes, and ocular region [1]. In a study of 84,836 cases of melanoma, Chang et al. found that up to 5% of such neoplasms are derived from the eye [1]. Among these cases, uveal melanoma (UVM) is the most common, accounting for up to 83% of all ocular melanomas [1,2]. Furthermore, as the most common primary ophthalmic malignancy in adults and with a median presentation age of 59 to 62 years of age, UVMs have a disproportionately high socio-economic impact [3]. This effect is compounded by the pathology’s high propensity for metastasis [4]. Currently, up to 50% of patients with UVM develop metastatic disease, most frequently affecting the liver [5]. In a retrospective medical record review of 8,033 eyes, Shields et al. identified increased age and tumor size as strong positive correlates with the probability of metastasis [6].
Modern treatment options for primary UVM aimed at mitigating metastatic disease include enucleation and radiation therapy. However, these approaches have been found to have limited and carriable success. In a 20-year retrospective chart review, Kujala et al. determined that 30-50% of patients who underwent enucleation and radiation therapy developed metastases, even after successful local treatment of the primary tumor [5]. Howeven after30-50% of patients willThis suggests the presence of dormant micro-metastases that form during early tumorigenesis [5,7]. Consequently, understanding the immunological underpinnings can help devise therapies for UVM. In this vein, tumor-infiltrating immune cells (TIICs) have demonstrated prognostic benefit across many cancer types [8]. Existing studies investigating TIIC show that an increased abundance of infiltrating T cells is associated with worse clinical outcomes in UVM [9-11]. However, these experiments utilized immunohistochemistry as their method of detection, limiting the accuracy of their analysis. In order to detect TIIC in small quantities and elucidate complex and related cell types, a different approach is necessary [12].
CIBERSORTx (https://cibersortx.stanford.edu/) is a well-established deconvolution tool to accurately estimate cell-type abundances without single-cell transcriptome data. This algorithm can be applied to investigate TIIC diversity via in silico quantification, supporting downstream clinical correlate analysis [13]. This method has improved clinical efficacy and consistently replicates conventional flow cytometry results, enabling researchers to rapidly elucidate tissue cell expression [12,14-15]. Improving UVM treatment options requires a strong understanding of the immunogenic landscape, particularly for treatment-resistant populations. Here we utilize CIBERSORTx to quantify 22 leukocytes and explore their individual contributions to overall and disease-free UVM survival. This evaluation contributes to a growing body of literature demonstrating application of an effective alternative analysis to immunohistochemistry while also identifying potential immunological therapeutic targets to improve UVM patient outcomes.
Methods
Data acquisition
Level 3 bulk mRNASeq data and corresponding survival data representing eighty (80) UVM patients were obtained from the Broad Institute GDAC Firehose portal (https://gdac.broadinstitute.org/). The gene-level transcripts per kilobase million (TPM) values, defined as 1e6 * scaled RSEM estimates, were used for downstream matrix deconvolution.
Computing tumor-infiltrating immune cell fractions
CIBERSORTx, a well-established RNA deconvolution algorithm, was used to quantify immune cell proportions. A curated leukocyte gene signature matrix (LM22), comprised of 547 immune cell type-specific genes, was used to estimate the relative abundance of 22 mature human hematopoietic populations. CIBERSORTx was run at 1000 permutations with bulk-mode batch correction.
Statistical analysis
Deconvolution results were considered statistically significant at CIBERSORTx p-value <0.05, derived from Monte Carlo sampling across all LM22 gene sets. Kaplan-Meier (KM) analysis utilized median immune cell relative fraction to assess for distinct survival probabilities. Overall survival (OS) was defined as the length of time from date of diagnosis to date of any-cause death. Disease-free survival (DFS) was defined as the length of time post-treatment that a patient survives without cancer recurrence. Cell types with median relative fraction of 0% were excluded from downstream analysis. Q-values <0.01 were deemed statistically significant. Cytolytic activity (CYT), a relative measure of cancer immunity, was defined as the log-average of GZMA and PRF1 mRNA expression levels [16]. Kendall rank correlation was evaluated with hierarchical clustering using R package ‘corrplot’ [17]. All analyses were conducted using R version 4.1.2.
Results
UVM TIIC landscape
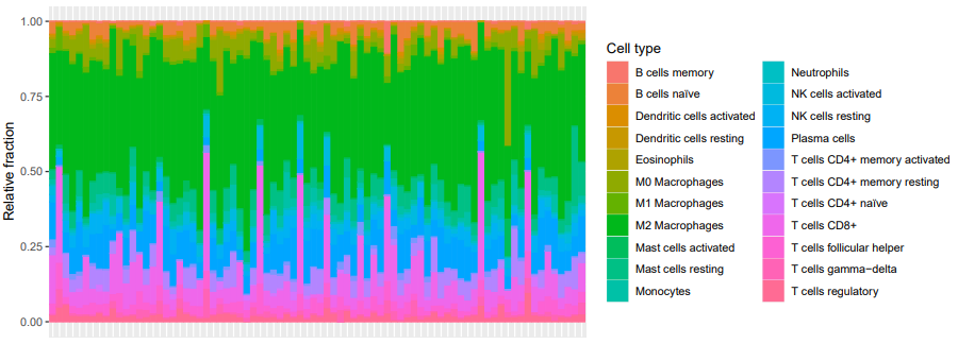
Batch-corrected deconvolution was achieved using an established omics approach (Figure 1). M2 macrophages represented, on average, 41% of the tested immune cell population; B cells accounted for <5% of the total TIIC population; eosinophils and neutrophils combined to represent 0.5% of the tumor immune cell environment. See Table 1 for a tabulation of the 22-TIIC deconvolution output.
Figure 1:
|
Tumor-infiltrating Immune Cell Type |
Relative abundance (%) |
CIBERSORT p-value |
|
Naïve B cells |
3.570133 |
0 |
|
Memory B cells |
0.297648 |
0 |
|
Plasma cells |
9.528872 |
0 |
|
CD8+ T cells |
10.91962 |
0 |
|
Naïve CD4+ T cells |
0.297768 |
0.004 |
|
Resting memory CD4+ T cells |
4.235605 |
0 |
|
Activated memory CD4+ T cells |
0.520088 |
0.006 |
|
Follicular helper T cells |
3.475006 |
0.005 |
|
Regulatory T cells |
2.291469 |
0.006 |
|
Gamma-delta T cells |
0.97832 |
0 |
|
Resting NK cells |
3.082866 |
0 |
|
Activated NK cells |
2.682618 |
0 |
|
Monocytes |
2.844411 |
0 |
|
M0 Macrophages |
5.468126 |
0 |
|
M1 Macrophages |
1.525249 |
0.001 |
|
M2 Macrophages |
41.04857 |
0 |
|
Resting dendritic cells |
0.710383 |
0 |
|
Activated dendritic cells |
0.196516 |
0.013 |
|
Resting mast cells |
5.461177 |
0 |
|
Activated mast cells |
0.348891 |
0 |
|
Eosinophils |
0.401973 |
0 |
|
Neutrophils |
0.114694 |
0.003 |
TIIC associations with survival
The association of isolated tumor-infiltrating leukocyte populations with overall survival was assessed by KM estimation to identify the greatest contributors to UVM patient outcomes (Figure 2, top row). Levels of naïve B cells, resting memory CD4+ T cells, and monocytes in the tumor microenvironment correlated positively with better probabilities of overall survival. Higher proportion of CD8+ T cells were negatively related with UVM overall survival outcomes. Next, we analyzed disease-free progression among the TIIC subpopulation with significant overall survival interaction to explore whether similar trends are obtained (Figure 2, bottom row). All TIICs with previously identified contributions to overall survival retained their directionality and remained statistically significant.
Figure 2:
TIIC correlation and spatial representation of cytolytic activity
Because CYT is understood to associate variably with survival outcomes across different cancer types, we sought to investigate its interplay with distinct UVM TIIC subpopulations, which, to the authors knowledge represents a first-use case (Figure 3A). Among the set of TIICs which revealed positive correlation with UVM survival probability, CYT correlated negatively. Further, CYT correlated positively with relative fraction of CD8+ T cells, which reported negative association with UVM survival probability. Principal component (PC) analysis of the first two PC axes representing the (Figure 3B). See Table 2 for the Kendall rank correlation coefficient results.
Figure 3.
|
Tumor-infiltrating Immune Cell Type |
tau-B value |
Survival association |
|
CD8+ T cells |
0.620 (Strong) |
Negative |
|
Naïve B cells |
-0.614 (Strong) |
Positive |
|
Resting memory CD4+ T cells |
-0.415 (Strong) |
Positive |
|
Monocytes |
-0.288 (Moderate) |
Positive |
Discussion
Transcriptome analysis revealed a complex TIIC landscape with a predominance of M2 macrophages (Figure 1). M2 macrophages are produced by alternative activation of tumor-associated macrophages (TAMs) and promote tumor progression and metastasis [18]. Therefore, targeting TAM activation has proven as an effective tumor therapy [19]. Conversely, classically activated M1 macrophages exhibit anti-tumor behavior [20]. Among the examined UVM population, M1 macrophages observed a low relative TIIC abundance of <2%, whereas M2 macrophages represented 41% of the overall TIIC landscape (Figure 1, Table 1). In existing literature, plasticity between M1 and M2 macrophages has been found to be influenced by the tissue microenvironment. Consequently, microenvironmental modification has been identified as a potential therapeutic target [21].
Furthermore, score-based systems have been created to assess how the genomic landscape of neoplasms influences and is influenced by anti-tumor activity. The cytolytic activity score (CYT) has been documented as such a metric. CYT approximates for cancer immunity based on mRNA expression of two cytotoxic granules: granzyme A and perforin [16,22-23]. High CYT routinely implicates better survival due to activation of effector T and NK cells, but exception exists [24]. In the selected primary UVM population, cytolytic activity negatively correlates with the abundance of tumor-infiltrating naïve B cells (strong), resting memory CD4+ T cells (strong), and monocytes (moderate) (Figure 3B, Table 2). Cytolytic activity positively correlates with the abundance of CD8+ T cells, consistent with differentiation into cytotoxic T lymphocytes that produce cytotoxic granules.
Despite this association, our results demonstrate that a high density of CD8+ T cells decreases overall survival in UVM (Figure 2). This negative prognosis is consistent with previous literature that documents worse clinical outcomes with greater T-cell infiltration [9-11]. Staibano et al., suggest that CD8+ function may be impaired in UVM [25]. Building off this conclusion, McKenna and Previte hypothesized that CD8+ T cells with limited functionality accumulate and signal M2 macrophages that exhibit pro-tumor activity [26]. Looking beyond the primary tumor, T cells and macrophages represent the predominant infiltrate in liver metastases [27,28]. Our results also signal that overall survival is positively influenced by the increased presence of naïve B cells, resting memory CD4+ T cells, and monocytes (Figure 2). Monocyte heterogeneity yields some subtypes with pro-tumor properties, facilitating angiogenesis and metastasis, within both the classical and non-classical subsets [29]. Alternatively, anti-tumoral monocytes play roles in tumor cytotoxicity, metastatic prevention, recruitment of NK cells, and inhibition of Tregs [29]. Similar to TAMs, monocytes exhibit a high degree of plasticity that may serve as a target with the goal of enhancing antitumoral immunity [30].
The extent to which these findings can be generalized and interpreted is limited by certain experimental shortcomings. The cohort consisted of only 80 primary UVM samples with survival data available from the Broad Institute GDAC Firehose. A larger dataset would conceivably account for additional diversity in TIIC proportions. Additionally, the CIBERSORTx algorithm is unable to account for spatial tissue and cytoarchitecture. While CIBERSORTx can competently differentiate between monocytes and macrophages, the algorithm cannot distinguish different monocyte subsets. Subset classification is essential to understand the possible mechanisms of monocyte involvement in overall UVM survival. Still, the authors confer that the findings reported in the current study lend strong support for further omics investigation of CYT and isolated TIIC populations in the context of primary UVM.
Conclusion
Deconvolution algorithms have allowed the rapidly characterization and contextualization of the tumor microenvironment. Among the analyzed 22-member set of leukocytes, M2 macrophages were most abundant in the UVM tumor environment. KM results indicated that overall survival probabilities correlated with greater relative abundance of naïve B cells, monocytes, and resting memory CD4+ T cells. Infiltrating CD8+ T cells correlate with lower overall survival. Interestingly, cytolytic activity estimates positively correlated with levels of CD8+ T cells, and negatively correlated with levels of naïve B cells, resting memory CD4+ T cells, and monocytes. These data suggest that high CYT associates significantly with worse survival outcomes through abundance estimates of individual TIICs, supporting its prognostic value for UVM. The authors hope the findings shared here inspire future UVM research towards the benefit of identifying potential immunotherapy targets.
References
2. Jager MJ, Shields CL, Cebulla CM, Abdel-Rahman MH, Grossniklaus HE, Stern MH, et al. Uveal melanoma. Nature Reviews Disease Primers. 2020 Apr 9;6(1):1-25.
3. Kujala E, Mäkitie T, Kivela¨ T. Very long-term prognosis of patients with malignant uveal melanoma. Investigative Ophthalmology & Visual science. 2003 Nov 1;44(11):4651-9.
4. Slater K, Hoo PS, Buckley AM, Piulats JM, Villanueva A, Portela A, et al. Evaluation of oncogenic cysteinyl leukotriene receptor 2 as a therapeutic target for uveal melanoma. Cancer and Metastasis Reviews. 2018 Sep;37(2):335-45.
5. Carvajal RD, Schwartz GK, Tezel T, Marr B, Francis JH, Nathan PD. Metastatic disease from uveal melanoma: treatment options and future prospects. British Journal of Ophthalmology. 2017 Jan 1;101(1):38-44.
6. Shields CL, Furuta M, Thangappan A, Nagori S, Mashayekhi A, Lally DR, Kelly CC, Rudich DS, Nagori AV, Wakade OA, Mehta S. Metastasis of uveal melanoma millimeter-by-millimeter in 8033 consecutive eyes. Archives of Ophthalmology. 2009 Aug 1;127(8):989-98.
7. Eskelin S, Pyrhönen S, Summanen P, Hahka-Kemppinen M, Kivelä T. Tumor doubling times in metastatic malignant melanoma of the uvea: tumor progression before and after treatment. Ophthalmology. 2000 Aug 1;107(8):1443-9.
8. Paijens ST, Vledder A, de Bruyn M, Nijman HW. Tumor-infiltrating lymphocytes in the immunotherapy era. Cellular & Molecular Immunology. 2021 Apr;18(4):842-59.
9. Whelchel JC, Farah SE, McLean IW, Burnier MN. Immunohistochemistry of infiltrating lymphocytes in uveal malignant melanoma. Investigative Ophthalmology & Visual science. 1993 Jul 1;34(8):2603-6.
10. De Cruz Jr PO, Specht CS, McLean IW. Lymphocytic infiltration in uveal malignant melanoma. Cancer. 1990 Jan 1;65(1):112-5.
11. Durie FH, Campbell AM, Lee WR, Damato BE. Analysis of lymphocytic infiltration in uveal melanoma. Investigative Ophthalmology & Visual Science. 1990 Oct 1;31(10):2106-10..
12. Chen B, Khodadoust MS, Liu CL, Newman AM, Alizadeh AA. Profiling tumor infiltrating immune cells with CIBERSORT. In: Cancer systems biology. New York: Humana Press. 2018; pp. 243-259
13. Newman AM, Steen CB, Liu CL, Gentles AJ, Chaudhuri AA, Scherer F, et al. Determining cell type abundance and expression from bulk tissues with digital cytometry. Nature Biotechnology. 2019 Jul;37(7):773-82.
14. Tang G, Yin W. Development of an immune infiltration-related prognostic scoring system based on the genomic landscape analysis of glioblastoma multiforme. Frontiers in Oncology. 2020 Feb 18;10:154.
15. Yang S, Liu T, Cheng Y, Bai Y, Liang G. Immune cell infiltration as a biomarker for the diagnosis and prognosis of digestive system cancer. Cancer Science. 2019 Dec;110(12):3639-49.
16. Rooney MS, Shukla SA, Wu CJ, Getz G, Hacohen N. Molecular and genetic properties of tumors associated with local immune cytolytic activity. Cell. 2015 Jan 15;160(1-2):48-61.
17. Wei T, Simko V, Levy M, Xie Y, Jin Y, Zemla J. Package ‘corrplot’. Statistician. 2017 Oct 16;56(316):e24.
18. Ngambenjawong C, Gustafson HH, Pun SH. Progress in tumor-associated macrophage (TAM)-targeted therapeutics. Advanced Drug Delivery Reviews. 2017 May 15;114:206-21.
19. Yin M, Li X, Tan S, Zhou HJ, Ji W, Bellone S, et al. Tumor-associated macrophages drive spheroid formation during early transcoelomic metastasis of ovarian cancer. The Journal of Clinical Investigation. 2016 Nov 1;126(11):4157-73.
20. Pan Y, Yu Y, Wang X, Zhang T. Tumor-associated macrophages in tumor immunity. Frontiers in Immunology. 2020 Dec 3;11:583084.
21. Boutilier AJ, Elsawa SF. Macrophage polarization states in the tumor microenvironment. International Journal of Molecular Sciences. 2021 Jun 29;22(13):6995.
22. Balli D, Rech AJ, Stanger BZ, Vonderheide RH. Immune Cytolytic Activity Stratifies Molecular Subsets of Human Pancreatic CancerImmune Cytolytic Activity and Subsets of Pancreatic Cancer. Clinical Cancer Research. 2017 Jun 15;23(12):3129-38.
23. Narayanan S, Kawaguchi T, Yan L, Peng X, Qi Q, Takabe K. Cytolytic activity score to assess anticancer immunity in colorectal cancer. Annals of Surgical Oncology. 2018 Aug;25(8):2323-31.
24. Roufas C, Chasiotis D, Makris A, Efstathiades C, Dimopoulos C, Zaravinos A. The expression and prognostic impact of immune cytolytic activity-related markers in human malignancies: a comprehensive meta-analysis. Frontiers in Oncology. 2018 Feb 21;8:27.
25. Staibano S, Mascolo M, Tranfa F, Salvatore G, Mignogna C, Bufo P, et al. Tumor infiltrating lymphocytes in uveal melanoma: a link with clinical behavior?. International Journal of Immunopathology and Pharmacology. 2006 Jan;19(1):205873920601900117.
26. McKenna KC, Previte DM. Influence of CD8+ T regulatory cells on intraocular tumor development. Frontiers in Immunology. 2012 Sep 28;3:303.
27. Krishna Y, McCarthy C, Kalirai H, Coupland SE. Inflammatory cell infiltrates in advanced metastatic uveal melanoma. Human Pathology. 2017 Aug 1;66:159-66.
28. Grossniklaus HE. Understanding uveal melanoma metastasis to the liver: the Zimmerman effect and the Zimmerman hypothesis. Ophthalmology. 2019 Apr 1;126(4):483-7.
29. Olingy CE, Dinh HQ, Hedrick CC. Monocyte heterogeneity and functions in cancer. Journal of Leukocyte Biology. 2019 Aug;106(2):309-22.
30. Ugel S, Canè S, De Sanctis F, Bronte V. Monocytes in the tumor microenvironment. Annual Review of Pathology: Mechanisms of Disease. 2021 Jan 24;16:93-122.