Abstract
Immature nervous systems are initially overpopulated with neurons. A crucial step of remodeling that removes inappropriate connections is required and performed predominantly by phagocytic glial cells. The Drosophila chemokine-like Orion tags axons to be eliminated. Could the way Orion is presented by neurons be the critical factor in modulating subsequent intracellular glial responses? Here we propose a model in which the mode of Orion presentation (soluble, GAG-bound, PS-bound, or EV-associated) might function as a switch in phagocytic signaling during neural remodeling.
Keywords
Chemokine-like Orion, Neuron, Glia, Infiltration, Phagocytosis, Neuronal remodeling
Abbreviations
MBs: Mushroom Bodies; APF: After Puparium Formation; MARCM: Mosaic Analysis with a Repressible Cell Marker; vCrz+: Ventral Corazonin-Expressing; PDF: Pigment Dispersing Factor; dda: Dendrites of class IV Dendritic Arborization; GAG: Glycosaminoglycan; ECM: Extracellular Matrix; PS: Phosphatidylserine; PM: Plasma Membrane; EVs: Extracellular Vesicles; VNC: Ventral Nerve Cord
Definitions
Chemokines: Family of chemoattractant cytokines, characterized by a CC, CXC, or CX3C motif, promoting the directional migration of cells within different tissues. Mammalian CX3CL1 (also known as fractalkine) is involved in neuron–glia communication.
Orion: a Drosophila CX3C chemokine-like protein required for axon debris elimination during development and injury.
Mushroom bodies: Structures in the brain of arthropods involved in olfactory learning and memory.
Phosphatidylserine: Phospholipid representing a neuronal ‘eat-me’ signal enabling phagocyte-mediated synapse pruning.
Glycosaminoglycans: Long, linear polysaccharides consisting of repeating disaccharide units which are essential components of the extracellular matrix and regulators of ligand-receptor complexes.
Extracellular vesicles: Lipid-bilayer composed vesicles released by cells and considered as mobile platforms capable to transfer functional proteins, facilitating cell to cell communication.
Glial infiltration: Glial penetration of compacted structures (e.g., MB bundles).
Glial phagocytosis: Glial engulfment and degradation of debris.
Introduction
Nervous systems are initially overpopulated with neurons and over wired. This is followed by a key period of remodeling within which a subset of inappropriate connections is removed to optimize adult functions. Neuronal elimination is also required after nervous system injury. In both cases, the clearance of resulting debris is performed predominantly by phagocytic glial cells [1–8] although other phagocytic cells such as epithelial cells can be involved in this mechanism [9]. Therefore, the selection of specific connections for elimination seems to involve an essential communication between neurons and glia [3]. The nervous system uses a diversity of molecules to identify engulfment targets. This includes chemokine signaling (CXCL12, CX3CL1, CCL2 and CXCL16) that is essential in guiding immune and glial responses during tissue remodeling and injury [10] and promotes the directional migration of cells in many systems. One of them, the Drosophila chemokine-like Orion, identified in our laboratory [11] has a major function in the fruit fly nervous system remodeling during development and injury [12,13]. However, major gaps exist concerning the way Orion, and chemokines in general, mediates communication between neurons and phagocytic cells. Phagocytosis involves several critical steps such as engulfment and digestion of the resulting material. In Drosophila mushroom bodies (MBs) neuronal remodeling, an additional step of axon-bundle infiltration is required since astrocyte-like glia (the sole infiltrating glial cells currently reported in MBs) surrounding MBs penetrate g axon bundles to initiate phagocytosis. Furthermore, it is important to point out that infiltration and phagocytosis are concomitant mechanisms that are difficult to dissociate because infiltrating glia gradually phagocytose neuronal debris. Orion represents a long-time missing ligand for glia and epithelial phagocytic cells in Drosophila and has emerged as a key regulator of glial infiltration into axon bundles and phagocytosis. Interestingly, a major role of actin in astrocytic infiltration has been recently reported in MB neuronal remodeling [14] but little is known about other molecular players in this mechanism. While the Orion role in recruiting glial extensions is established, the mechanisms determining the specificity of downstream signaling pathways in glia infiltration and phagocytosis remain unclear.
In immune systems, the mode of chemokine presentation: whether soluble, membrane-bound, or matrix-tethered, has profound consequences on receptor clustering, internalization, and downstream signaling [15]. Such differences can influence cell behavior, migration guidance or effector functions.
This raises the intriguing question: could the way Orion is presented be the critical factor in modulating receptor engagement and subsequent intracellular glial responses leading either to MB axon bundle infiltration or phagocytosis? To follow up to this question we will focus on the different sources of Orion currently reported, the Orion presentation mode associated with them, as well as the related cell signaling responses in phagocytes. Finally, we will comment about the dual role of Orion in infiltration and phagocytosis.
Different Sources of Orion in Neuronal Remodeling
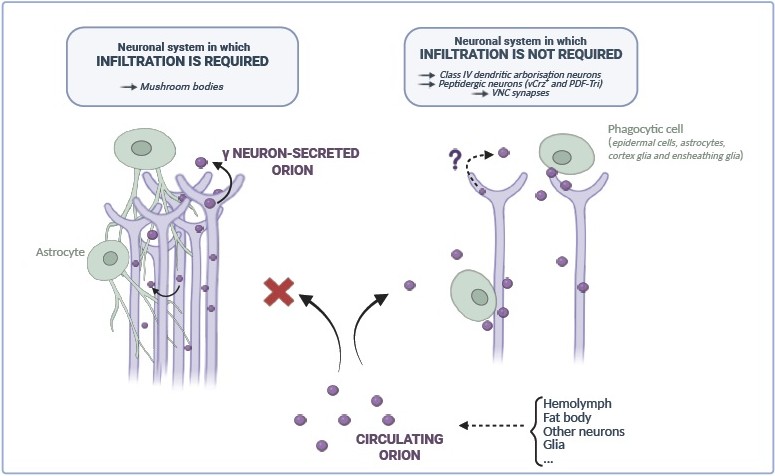
Orion is expressed by g neurons early in pupae, and by epithelial cells, fat body, trachea, several types of glial cells, and other structures at larval stages [11,12]. All these cells represent putative sources of Orion and might participate in neuronal remodeling. Orion contains a signal peptide sequence allowing to engage a protein secretion pathway. We showed that in Drosophila MBs Orion secretion by g neurons are compulsory for pruning [11]. Thus, in an orion-/- context, supplying Orion in g axons restore adult g axon remodeling phenotypes (including axon fragmentation and debris clearance). This pruning restauration lets to suppose a 6 h after puparium formation (APF) MB axon-bundle glia infiltration rescue. Furthermore, orion-/- mosaic analysis with a repressible cell marker (MARCM)-induced single clones are perfectly pruned. This indicates that the role of Orion is non g axon autonomous (but g axon-bundle autonomous). Therefore, one can suggest that Orion secreted by closely positioned orion+/- axons is able to act on orion-/- axon remodeling. Consequently, Orion secreted by axons could also be a source of Orion for neighbors g axons. However, providing Orion in glia does not restore g axon pruning mutant phenotypes, suggesting that Orion is required specifically in neurons. Why does an external source of Orion fail to rescue the g axon mutant pruning phenotype? Even though we do not have the precise answer to this question, one could speculate that since g axon bundles are composed of about three hundred compacted axons, glia-secreted Orion is unable to appropriately access it most probably due to MBs bundle spatial constraints.
Ventral corazonin-expressing (vCrz+) neurons and pigment dispersing factor (PDF)-Tri neurons, die by apoptosis during Drosophila metamorphosis [16] and early after fly eclosion [17,18] respectively. Intriguingly, intrinsic neuronal expression of Orion in these neurons is not sufficient to rescue orion-/- pruning phenotypes. Only a general expression of Orion in all neurons [13] or even in glia (our unpublished results) rescues these phenotypes. Thus, in paradigms involving remodeling of spatially open systems in which phagocyte infiltration is not required (opposite to compacted MB bundles), different sources of Orion might be possible such as surrounding neurons, glia, or epithelial cells (see below). Interestingly, in the case of the dendrites of class IV dendritic arborization (dda) neuron remodeling, providing Orion in the fat body is sufficient to rescue dda laser-induced uncleared debris at larval stages in an orion-/- context [12]. Unexpectedly, the absence of Orion in dda neurons does not affect dda remodeling in this paradigm, in accordance with the low amount of Orion found in these neurons. The major source of Orion either for dda developmental pruning or for dda debris elimination after injury is still to be elucidated, but most probably, hemolymph fluid-circulating Orion secreted from several sources is the one required in this system. Overall, potential sources of Orion and associated glia infiltration requirements are represented in Figure 1.
Figure 1. Putative Orion sources and glia infiltration requirements. (Created with Biorender.com).
Orion Presentation to Glia
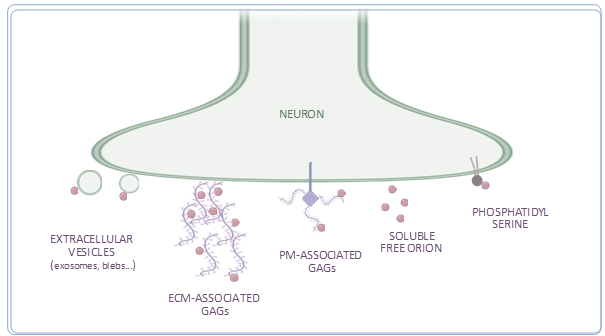
Orion displays three putative glycosaminoglycan (GAG) binding sites [11]. One of them, named GAG3 domain, located close to the C-terminal end of this protein, seems to have a major function in MB pruning. Accordingly, Orion is presented to glial cells via GAGs to induce MB remodeling. Are Orion-GAG binding sites only required for MB glia infiltration, for glia phagocytosis or both? Overall, GAGs have a key function in ligand signaling (as for Wnt and Hedgehog) as they are required either for cell-to-cell morphogen diffusion or to concentrate and cluster ligands at the cell surface [19,20]. GAGs might allow Orion monomers clustering as observed in MB-bundle 3D rendering [11]. Since GAGs are often present at the extracellular matrix (ECM) [21], Orion might bind ECM GAGs to pave the way for glial process infiltration. In this case, an additional molecule, potentially phosphatidylserine (PS), must be required to tag axons for elimination (see below). Orion could also associate to axon membrane-linked proteoglycans, composed by a core protein and long GAG chains, which might display not only a signal for infiltration, but also a required phagocytic tag. Unfortunately, the precise cellular location of Orion-binding GAGs has not been elucidated either in MBs or in other remodeling paradigms and they may represent a key component to reveal new related signaling pathways. One interesting experiment to mimic Orion binding to plasma membrane (PM)-associated GAGs would be to express a PM-tethered Orion in orion-/- MB axons and assess whether this Orion presentation form is sufficient to induce both glia infiltration and phagocytosis. Whether Orion as a soluble free protein has a function in neuronal remodeling by itself is currently unknown.
Therefore, we propose at least two manners of Orion presentation to glia in MBs: Orion non associated to PM able to travel at least from one axon to another and Orion bound to GAG-exposing axons or to GAG-containing ECM.
In the dda laser injury paradigm, it was shown that Orion bridges PS-exposing neurons and the phagocytic receptor Draper leading to phagocytosis [12]. Here dda neurons display Orion via PS to phagocytic epithelial cells. Interestingly, presenting a PM-tethered Orion, in which Orion is coupled to the transmembrane domain CD2 (Orion-CD2), to phagocytic epithelial cells was sufficient to induce dda elimination in the absence of PS in a wild type orion context. In the absence of wild type Orion in this paradigm, one can wonder whether phagocytic digestion would occur normally in “Orion-CD2-eating” phagocytic epithelial cells and whether the fate and viability of these cells would be altered. Therefore, for dda neuron elimination, we propose the requirement of soluble Orion travelling in hemolymph fluids surrounding dda neurons that could bind to dda-exposed PS to tag dendrites to be phagocytosed by epidermal cells.
Whether or not GAGs are required in the dda paradigm or whether PS is required for MB pruning is still unknown. Do GAGs and PS have a redundant role in presenting Orion to phagocytes during neuronal remodeling? In a recent model, it was proposed that chemokine-presenting apoptotic blebs guide phagocytes toward apoptotic cells for their elimination [22]. This model proposes that chemokines interact with the surface of apoptotic blebs via both GAGs and membrane exposed PS. In fact, a gradient of presumably GAG-binding chemokines is first exposed on apoptotic blebs and guides phagocytes toward apoptotic cells. The authors also found that cells undergoing apoptosis in vivo then switch chemokine-tethering mechanisms, down-regulating cell surface GAGs and up-regulating PS. Importantly, unlike GAG-bound chemokines, PS-bound chemokines retain their ability to activate phagocytic receptors, providing key differences between the two molecules. Therefore, they propose that as tissue apoptotic level rises because of injury or infection, PS may be increasingly favored over GAGs as a mediator of phagocytosis. Thus, this model is also noteworthy to consider in MBs even though PS has not been reported yet in this paradigm. Interestingly, the presence of blebs was also reported during metamorphosis along single g axon clones using the MARCM technique [23], which raises the question: are GAGs and PS acting sequentially in these g axon blebs at pruning to present Orion to phagocytic astrocytes, as in apoptotic blebs? A conceivable experiment would be to deplete GAGs in MB and determine if it can be compensated by PS.
Remarkably, in two independent works, one in vitro in S2 drosophila cell cultures [24] and a second one in vivo using Drosophila larval hemolymph [25] it was reported that Orion associates with extracellular vesicles (EVs). These cellular compartments circulating in biological fluids have different sizes and cellular origins (apoptotic bodies, micro vesicles or blebs and exosomes) [26] and represent an interesting system to transport and amplify protein effects [27]. Furthermore, it was shown that EVs stimulate phagocytosis in vitro [28] and that they opsonize apoptotic debris via PS [29]. Whether EV-associated Orion is required for neuronal remodeling is not documented. However, EVs as mobile Orion presentation platforms could potentially amplify infiltration and/or phagocytosis. Consequently, they should also be considered when studying Orion presentation to phagocytes. Overall, proposed presentation forms of Orion to phagocytes are represented in Figure 2.
Figure 2. Suggested presentation forms of Orion to phagocytic cells. (Created with Biorender.com).
It could also be interesting to determine how Orion is presented in neurons that die by apoptosis and might require Orion presentation in both soma and neurites, such as the vCrz+ and the PDF-Tri neurons.
Orion Receptor Activation
Orion is presented by neurons to several types of phagocytic targets such as astrocyte-like glia, cortex glia, ensheathing glia, and epidermal cells [11–13]. In particular, the presence of glial extensions is quite specific to astrocytes. Thus, Orion may induce infiltration likely when pruning is mediated by astrocytes, and phagocytosis in other targets (without excluding astrocytes). Whether Orion signals via the same receptor for infiltration and phagocytosis in astrocytes is not known, but it is noteworthy to point out that a same receptor can induce different signaling pathways [30].
The draper (drpr) gene (MEGF10 homologue in vertebrates and receptor of C1q [31]), the most studied phagocytic receptor [32], is a known receptor for Orion in dda neurons [12] and maybe also in MBs. Drpr behaves as a multifunctional protein referred to as a “hydra” [33] as it participates in all stages of corpse clearance: damage detection, corpse engulfment, and corpse processing in phagocytes. These stages involve different cellular pathways such as cytoskeletal rearrangements and phagosome maturation. Some of the molecular actors of these pathways have been identified [34,35]. However, if Drpr is the unique Orion receptor, able to activate Orion-mediated MBs glia infiltration, debris engulfment and phagocytic digestion: What makes the transition between each mechanism possible? Could it just be chronological?
Interestingly, unlike Drpr, Orion is not required in paradigms like wing and olfactory receptor neuron injury [13]. Thus, Draper signaling pathways mediated by other ligands must be also considered.
Is Orion Involved Only in Glia Infiltration in MBs or in Both Infiltration and Phagocytosis?
Orion is involved in the triggering of glia infiltration in MB bundles [11]. At 24 h APF, degenerated MB axons are already fragmented into small parts and astrocyte-like glia surround and phagocytose them. We showed that in orion-/-, even though astrocytes also surround small axon pieces, they do not eliminate them [11]. At this developmental stage, since neuronal debris are accessible for glia, one can assume that infiltration is no longer required. Therefore, in MB-surrounding astrocytes Orion might also be involved in phagocytosis, otherwise, debris digestion would take place at this developmental period in orion-/-. Alternatively, in MB-surrounding astrocytes, signaling pathways could sequentially activate infiltration and phagocytosis and therefore lack of infiltration might lead to absence of phagocytosis. Thus, based on the current data we cannot definitively conclude about this issue in MBs, but since Orion is required for phagocytosis in other systems (see below) and due to the presence of non-phagocyted axon debris contacting glial extensions in orion-/- we propose that Orion directly triggers both infiltration and phagocytosis in MBs. Consequently, we are unable to dissect the role of Orion in glia infiltration independently of phagocytosis in orion-/-.
Intriguingly, in 6 h APF MB-surrounding astrocytes of wild type and orion-/- Drpr level seems comparable and high [11]. In the ventral nerve cord (VNC), it was shown that glial transformation from a larval astrocyte to a phagocyte correlates with increased Drpr expression level [7]. As, low level of Drpr in astrocytes block phagocytosis and expression of Drpr leads to phagocytosis, we might conclude that astrocytes with high level of Drpr, as these surrounding orion-/- MBs are potentially phagocytic. Thus, are orion-/- MB-surrounding astrocytes unable to infiltrate and phagocytose being potentially phagocytic?
We also examined the role of Orion in synapse remodeling in the VNC [13], taking advantage of the fact that astrocyte processes are already present in this region at larval stages [36] before neuronal remodeling occurs. Interestingly, we showed that Orion is involved in the overall transformation of astrocytes into phagocytes. This was based on morphological characterization of astrocyte-like glia of orion-/- 6 h APF pupae that display long processes and a lack of phagocytic vacuoles as in larval stages. Therefore, these observations indicate that Orion is required for synapse phagocytosis.
We also explored other neuronal remodeling paradigms, including vCrz+ and PDF-Tri neurons, in which glial infiltration is not required since glial cells surround vCrz+ and PDF-Tri neurites and cell bodies before pruning. Here we revealed the role of Orion in phagocytosis not only via astrocytes but also ensheathing and cortex glia [13]. Therefore, our results suggest that Orion activates phagocytosis in three different types of glial cells and point to Orion as an activator not only of glial infiltration but also phagocytosis. Since these peptidergic neurons die by apoptosis, their cell body is also cleared by glia. Thus, the vCrz+ and PDF-Tri study extends the role of Orion to neuronal cell body phagocytosis and opens the question of Orion presentation by cell bodies to phagocytic cells.
Conclusion
In conclusion, the presentation of chemokines represents a critical step to allow phagocytic migration and initiation of phagocytosis. Infiltration, when required, appears to be a key step to trigger the phagocytic response. The chemokine-like Orion represents a crucial ligand to dissect the mechanisms by which glial cells penetrate axon bundles and clear cellular debris during development and after injury.
We hypothesize that the manner Orion is presented to phagocytes might represent the potential “hidden switch” in phagocytic signaling pathway activation during neuronal remodeling leading to infiltration and phagocytosis.
Future research will be essential to unravel the distinct but interconnected processes of glial infiltration and phagocytosis. Key questions remain, notably how chemokine signaling orchestrates glial activation, infiltration, and the transition toward effective phagocytosis. Critical next steps include testing if forced tethering of Orion to GAGs or PS biases phagocyte responses toward infiltration or phagocytosis, respectively, and determining if EV-associated Orion integrates these modalities.
References
2. Awasaki T, Ito K. Engulfing action of glial cells is required for programmed axon pruning during Drosophila metamorphosis. Curr Biol. 2004 Apr 20;14(8):668–77.
3. Boulanger A, Dura JM. Neuron-glia crosstalk in neuronal remodeling and degeneration: Neuronal signals inducing glial cell phagocytic transformation in Drosophila. Bioessays. 2022 May;44(5):e2100254.
4. Hakim Y, Yaniv SP, Schuldiner O. Astrocytes play a key role in Drosophila mushroom body axon pruning. PLoS One. 2014 Jan 21;9(1):e86178.
5. Lee SY, Chung WS. The roles of astrocytic phagocytosis in maintaining homeostasis of brains. J Pharmacol Sci. 2021 Mar;145(3):223–7.
6. Luo P, Chu SF, Zhang Z, Xia CY, Chen NH. Fractalkine/CX3CR1 is involved in the cross-talk between neuron and glia in neurological diseases. Brain Res Bull. 2019 Mar;146:12–21.
7. Tasdemir-Yilmaz OE, Freeman MR. Astrocytes engage unique molecular programs to engulf pruned neuronal debris from distinct subsets of neurons. Genes Dev. 2014 Jan 1;28(1):20–33.
8. Watts RJ, Schuldiner O, Perrino J, Larsen C, Luo L. Glia engulf degenerating axons during developmental axon pruning. Curr Biol. 2004 Apr 20;14(8):678–84.
9. Han C, Song Y, Xiao H, Wang D, Franc NC, Jan LY, et al. Epidermal cells are the primary phagocytes in the fragmentation and clearance of degenerating dendrites in Drosophila. Neuron. 2014 Feb 5;81(3):544–60.
10. Trettel F, Di Castro MA, Limatola C. Chemokines: Key Molecules that Orchestrate Communication among Neurons, Microglia and Astrocytes to Preserve Brain Function. Neuroscience. 2020 Jul 15;439:230–40.
11. Boulanger A, Thinat C, Züchner S, Fradkin LG, Lortat-Jacob H, Dura JM. Axonal chemokine-like Orion induces astrocyte infiltration and engulfment during mushroom body neuronal remodeling. Nat Commun. 2021 Mar 23;12(1):1849.
12. Ji H, Wang B, Shen Y, Labib D, Lei J, Chen X, et al. The Drosophila chemokine-like Orion bridges phosphatidylserine and Draper in phagocytosis of neurons. Proc Natl Acad Sci U S A. 2023 Jun 13;120(24):e2303392120.
13. Perron C, Carme P, Rosell AL, Minnaert E, Ruiz-Demoulin S, Szczkowski H, et al. Chemokine-like Orion is involved in the transformation of glial cells into phagocytes in different developmental neuronal remodeling paradigms. Development. 2023 Oct 1;150(19):dev201633
14. Marmor-Kollet N, Berkun V, Cummings G, Keren-Shaul H, David E, Addadi Y, et al. Actin-dependent astrocytic infiltration is a key step for axon defasciculation during remodeling. Cell Rep. 2023 Feb 28;42(2):112117.
15. Schumann K, Lämmermann T, Bruckner M, Legler DF, Polleux J, Spatz JP, et al. Immobilized chemokine fields and soluble chemokine gradients cooperatively shape migration patterns of dendritic cells. Immunity. 2010 May 28;32(5):703–13.
16. Choi YJ, Lee G, Park JH. Programmed cell death mechanisms of identifiable peptidergic neurons in Drosophila melanogaster. Development. 2006 Jun;133(11):2223–32.
17. Gatto CL, Broadie K. Fragile X mental retardation protein is required for programmed cell death and clearance of developmentally-transient peptidergic neurons. Dev Biol. 2011 Aug 15;356(2):291–307.
18. Helfrich-Förster C. Development of pigment-dispersing hormone-immunoreactive neurons in the nervous system of Drosophila melanogaster. J Comp Neurol. 1997 Apr 14;380(3):335–54.
19. Matusek T, Marcetteau J, Thérond PP. Functions of Wnt and Hedgehog-containing extracellular vesicles in development and disease. J Cell Sci. 2020 Sep 28;133(18):jcs209742.
20. Takada S, Fujimori S, Shinozuka T, Takada R, Mii Y. Differences in the secretion and transport of Wnt proteins. J Biochem. 2017 Jan;161(1):1–7.
21. Silva JC, Carvalho MS, Han X, Xia K, Mikael PE, Cabral JMS, et al. Compositional and structural analysis of glycosaminoglycans in cell-derived extracellular matrices. Glycoconj J. 2019 Apr;36(2):141–54.
22. Pontejo SM, Murphy PM. Chemokines act as phosphatidylserine-bound "find-me" signals in apoptotic cell clearance. PLoS Biol. 2021 May 26;19(5):e3001259.
23. Lee T, Lee A, Luo L. Development of the Drosophila mushroom bodies: sequential generation of three distinct types of neurons from a neuroblast. Development. 1999 Sep;126(18):4065–76.
24. Thomas RE, Vincow ES, Merrihew GE, MacCoss MJ, Davis MY, Pallanck LJ. Glucocerebrosidase deficiency promotes protein aggregation through dysregulation of extracellular vesicles. PLoS Genet. 2018;14(9):e1007694.
25. Linnemannstöns K, Karuna M P, Witte L, Choezom D, Honemann-Capito M, Lagurin AS, et al. Microscopic and biochemical monitoring of endosomal trafficking and extracellular vesicle secretion in an endogenous in vivo model. J Extracell Vesicles. 2022 Sep;11(9):e12263.
26. Arif S, Moulin VJ. Extracellular vesicles on the move: Traversing the complex matrix of tissues. Eur J Cell Biol. 2023 Dec;102(4):151372.
27. Jahnke K, Staufer O. Membranes on the move: The functional role of the extracellular vesicle membrane for contact-dependent cellular signalling. J Extracell Vesicles. 2024 Apr;13(4):e12436.
28. Bahrini I, Song JH, Diez D, Hanayama R. Neuronal exosomes facilitate synaptic pruning by up-regulating complement factors in microglia. Sci Rep. 2015 Jan 23;5:7989.
29. Patil M, Saheera S, Dubey PK, Kahn-Krell A, Kumar Govindappa P, Singh S, et al. Novel Mechanisms of Exosome-Mediated Phagocytosis of Dead Cells in Injured Heart. Circ Res. 2021 Nov 12;129(11):1006–20.
30. Fambrough D, McClure K, Kazlauskas A, Lander ES. Diverse signaling pathways activated by growth factor receptors induce broadly overlapping, rather than independent, sets of genes. Cell. 1999 Jun 11;97(6):727–41.
31. Iram T, Ramirez-Ortiz Z, Byrne MH, Coleman UA, Kingery ND, Means TK, et al. Megf10 Is a Receptor for C1Q That Mediates Clearance of Apoptotic Cells by Astrocytes. J Neurosci. 2016 May 11;36(19):5185–92.
32. MacDonald JM, Beach MG, Porpiglia E, Sheehan AE, Watts RJ, Freeman MR. The Drosophila cell corpse engulfment receptor Draper mediates glial clearance of severed axons. Neuron. 2006 Jun 15;50(6):869–81.
33. Davidson AJ, Wood W. Phagocyte Responses to Cell Death in Flies. Cold Spring Harb Perspect Biol. 2020 Apr 1;12(4):a036350.
34. Kurant E, Axelrod S, Leaman D, Gaul U. Six-microns-under acts upstream of Draper in the glial phagocytosis of apoptotic neurons. Cell. 2008 May 2;133(3):498–509.
35. Ziegenfuss JS, Biswas R, Avery MA, Hong K, Sheehan AE, Yeung YG, et al. Draper-dependent glial phagocytic activity is mediated by Src and Syk family kinase signalling. Nature. 2008 Jun 12;453(7197):935–9.
36. Stork T, Sheehan A, Tasdemir-Yilmaz OE, Freeman MR. Neuron-glia interactions through the Heartless FGF receptor signaling pathway mediate morphogenesis of Drosophila astrocytes. Neuron. 2014 Jul 16;83(2):388–403.