Introduction
During vascular neurointervention procedures, the use of double antiaggregation therapy (mainly acetylsalicylic acid and clopidogrel) is required to minimize the risk of thromboembolic complications. Since clopidogrel is one of the most widely used agents in the perioperative management of patients undergoing neurointervention processes, knowing its mechanism of action and the factors that determine its activation and metabolism is of great importance to ensure the correct antiaggregation of these patients.
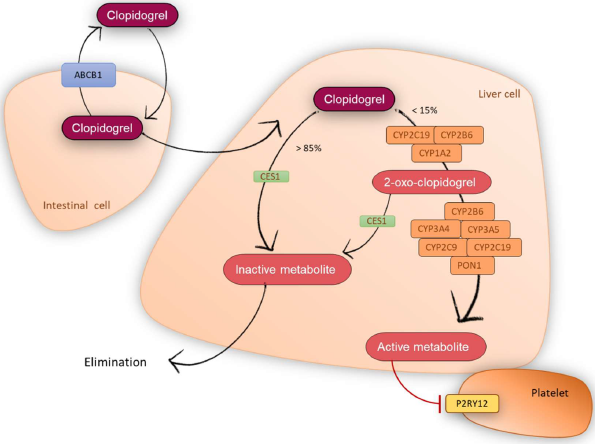
Clopidogrel is a prodrug that does not have an anti-aggregate effect by itself, but exerts its therapeutic effect once metabolized through the cytochrome P450 (CYP) enzymatic complex. Clopidogrel thiol active metabolite (R130964) specifically and irreversibly binds to the purinergic P2Y12 receptor of platelets (Figure 1), inhibiting ADP-mediated platelet activation and aggregation, throughout their lifespan of 7-10 days [1].
Figure 1: Clopidogrel metabolic pathway.
After both oral and intravenous clopidogrel dosing, anti-aggregation activity can be observed within two hours [2]. Moreover, after administration of multiple 75 mg daily doses of clopidogrel, the steady state is reached between days 3 and 7. Once reached, the average level of platelet inhibition is between 40% and 60% [3]. In general, after treatment discontinuation, platelet aggregation and bleeding time gradually return to baseline values within 5 days [3].
As shown in Figure 1, approximately 50% of the prodrug is absorbed, but 85% of it is hydrolyzed by the carboxylesterase 1 (CES1) to inactive forms (carboxylic acid) [4], while the remaining 15% will be accessible for transformation into the active metabolite [5]. Two consecutive oxidative processes occurred carried out by numerous CYP enzymes. First, the CYP2C19, CYP2B6 and CYP1A2 isoforms convert clopidogrel into 2-oxo-clopidogrel. Then, the isoforms CYP3A4, CYP3A5, CYP2B6, CYP2C9, CYP2C19 and the enzyme paraoxonase-1 (PON1) transform the 2-oxo-clopidogrel into its active metabolite [6,7]. However, 50% of 2-oxo-clopidogrel is metabolized by CES1 to an inactive compound, limiting the extent of active metabolite that is formed [8].
The rate of clopidogrel gastrointestinal absorption can vary between individuals, thus affecting the response onset [9,10]. The levels of expression of P-glycoprotein (P-gp), encoded by ABCB1, an ATP-dependent transporter, could affect its permeability and thus alter the oral bioavailability of clopidogrel [9,11].
The effect of clopidogrel diverges broadly among patients, so that between 5 and 56% of patients are poor responders or non-responders to clopidogrel [12], and, therefore, will be at higher risk of ischemic events after stent implantation [13-15]. This different response might be due in part to genetic variability, related to clopidogrel pharmacokinetics and mechanism of action. Therefore, we are going to review the main genes associated to the response to clopidogrel. Most of these studies have been performed in patients with acute coronary syndrome.
CYP2C19
Since CYP2C19 isoform is involved in the two metabolic steps and contributes to an estimated 45% of 2-oxoclopidogrel and 21% of the active metabolite [16], its genetic polymorphisms could significantly alter the response to clopidogrel treatment. Therefore, it has been the most studied enzyme of clopidogrel metabolism to date. Based on the CYP2C19 genotype, individuals are typically categorized into different phenotypes [17]: poor metabolizers (PM), subjects carrying two no-function alleles (i.e. CYP2C19 *2/*2, *2/*3 or *3/*3); intermediate metabolizers (IM), subjects who carry one no-function allele and one normal function allele, or subjects who carry one no-function allele and one increased-function allele (i.e. CYP2C19 *1/*2, *1/*3 or *2/*17); normal metabolizers (NM): subjects with two normal alleles (i.e. CYP2C19 *1/*1); rapid metabolizers (RM), subjects with one normal allele and one increased-function allele (i.e. CYP2C19 *1/*17) and ultra-rapid metabolizers (UM), subjects with two increased-function alleles (i.e. CYP2C19 *17/*17).
CYP2C19 IM-PM phenotypes have been associated with a hyporesponse to clopidogrel, since lower levels of active metabolite will be achieved with a non-functional enzyme. Consequently, these patients showed a diminished efficacy and a higher risk of vascular adverse events, including stent thrombosis [18-21].
In contrast, the presence of the increased-function *17 allele lead to increased platelet inhibition and hyperesponse to clopidogrel, as increased formation of active metabolite will result in increased antiplatelet efficacy [22-25]. As a consequence, these patients are at higher risk of bleeding [20,26,27].
In this regard, in 2011 and subsequently in 2013, the Clinical Pharmacogenetics Implementation Consortium (CPIC) made a series of therapeutic recommendations based on the CYP2C19 genotype for the treatment of acute coronary syndromes with clopidogrel [28]. Briefly, NM and RM-UM patients may take standard doses of clopidogrel as indicated in the drug label. However, it is recommended that IM and PM patients receive an alternative antiplatelet therapy, such as prasugrel or ticagrelor.
The two oxidative steps necessary for the formation of the active metabolite require the activity of several cytochrome P450 enzymes, many of which are quite polymorphic. Although current evidence shows a greater contribution of CYP2C19, several studies have shown that other enzymes might be also relevant in the response to clopidogrel.
CYP2C8/CYP2C9
A genome wide association study identified 13 polymorphisms within the CYP2C18-CYP2C19-CYP2C9-CYP2C8 cluster associated with a decreased response to clopidogrel [29]. However, there is controversy between the association of the most studied CYP2C9 alleles (*2 and *3) and the effect of clopidogrel. While some authors have found no significant relationship [23] others associate the presence of the *3 allele with a higher incidence of stent thrombosis [30].
CYP3A4/CYP3A5
CYP3A4 reduced activity was associated with an increased risk of stent thrombosis in patients with acute coronary syndrome treated with clopidogrel [31]. In addition, one study postulates that the role of CYP3A4/5 in clopidogrel metabolism may be more relevant than previously called [32].
CYP1A2
Regarding the influence of CYP1A2 polymorphisms in the response to clopidogrel, there is no clear evidence. It has been described that the increased-function CYP1A2*1F allele is not associated with an increased incidence of cardiac adverse events in patients with coronary artery disease [33]. In contrast, Cresci et al. describe an increased risk of bleeding events associated with the reduced-function CYP1A2*1C allele in African-American populations with acute myocardial infarction [34].
CYP2B6
The reduced-function CYP2B6*5 and *9 alleles, have not been associated with a different response to clopidogrel in patients with coronary artery disease [33,35,36].
PON1
Specifically, two polymorphisms in PON1 coding region, L55M and Q192R, have been associated with lower PON1 activity and concentration [37-39]. PON1 has been proposed as one of the crucial enzymes for clopidogrel bioactivation since it participates in the metabolism of 2-oxo-clopidogrel to its active metabolite. In patients with coronary artery disease treated with clopidogrel, Q192R Q/Q genotype was associated to an increased risk of stent thrombosis [7].
CES1
Since CES1 is responsible for metabolizing 85% of clopidogrel, genetic variations that affect the expression and/or activity of CES1 may be of great relevance to clopidogrel treatment, since they will increase the amount of active metabolite that is formed. Indeed, the CES1 G143E polymorphism, with a frequency of 1%, was associated with a decreased protein functionality [40]. Lewis et al. found that, in line with the expected, carriers of the mutated allele showed increased levels of active metabolite and improved response to clopidogrel in patients with coronary heart disease [41].
ABCB1
The most commonly studied variant in ABCB1 is C3435T. Several studies have evaluated the influence of C3435T polymorphism on clopidogrel treatment. Taubert et al. described lower levels of clopidogrel and its metabolite in patients carrying the T/T genotype, probably as a result of increased intestinal flow due to increased expression of P-gp [9]. However, this is contrary to what would be expected since the T allele has been described to reduce the expression of the protein [42-45] and, therefore, it results in increased absorption and decreased elimination. If this is the case, patients carrying the T/T genotype should achieve higher concentrations of clopidogrel and its active metabolite.
P2RY12
P2RY12 is the gene that encodes for the P2Y12 G-protein-coupled receptor. It is involved in platelet aggregation and is one of the target molecules for the treatment of thromboembolisms. Multiple polymorphisms have been described in this gene, although its involvement in the functionality of the receptor requires further research. In particular, some polymorphisms have been associated with increased platelet reactivity in clopidogrel treatment [46,47]. However, these associations have not been replicated and the level of evidence is low.
Clopidogrel pharmacogenetics in patients undergoing neurointervention
In spite of the vast amount of publications investigating the influence of polymorphisms on the effect of clopidogrel, most of them focus on patients with cardiological disorders, especially those with acute coronary syndrome undergoing percutaneous coronary intervention. Cerebrovascular disorders treated with neurointerventional procedures have not been investigated in depth. For this type of patients, the treatment with clopidogrel and acetylsalicylic acid is also the first therapeutic option. Moreover, extrapolation of evidence from one group of patients to others is not the appropriate approach given the specificity of the medical condition and treatment, which may influence the therapeutic outcome [48].
Up to 9% of patients undergoing neurointervention procedures develop thromboembolic complications [49]. Dual antiplatelet therapy decreases the occurrence of vascular adverse events by up to 75-80% in patients undergoing percutaneous coronary intervention, an effect that would be similar in the cohort of patients undergoing neurointervention [49]. However, there is a subgroup of clopidogrel hyporesponders estimated at 5-30% in the cardiovascular population and up to 66% in patients undergoing neurointervention [49].
Bearing in mind that patients undergoing neurointerventional procedures represent a quite heterogeneous cohort of patients, and that there are no standardized guidelines for neurovascular conditions, it is essential to enlarge our knowledge of the effect of clopidogrel on this cohort. Following this path, several publications studied the effect of clopidogrel in relation to the different metabolizing phenotypes:
Zhu et al. conducted a study of the association between CYP2C19 polymorphisms and the clinical efficacy of clopidogrel therapy in 241 Asian patients after ischemic stroke, who underwent carotid artery stenting. They found that patients carrying CYP2C19*2 and *3 alleles were more likely to suffer a subsequent ischemic event than those individuals with a wild-type genotype [50]. The CYP2C19*17 allele was not analyzed in this study.
González et al. analyzed the influence of CYP2C19 phenotypes on the response to clopidogrel in 209 patients who underwent stent placement in the carotid artery and who had high platelet reactivity during treatment. They found a statistically significant lower antiaggregant effect in IM/PM patients compared to UM and NM. In addition, the number of non-responders was significantly higher among the IM/PM group [35].
Ge et al. measured the effects of clopidogrel on the aggregometry of 215 patients with intracranial aneurysms treated by stent assisted coils embolization. They found that carriers of the CYP2C19*2 and *3 alleles, especially PM, had a higher risk of clopidogrel resistance, while the NM phenotype was significantly associated with the incidence of bleeding events [51]. The CYP2C19*17 allele was not included in this study.
Colley et al. published a review on the involvement of the CYP2C19*2 allele in neurointervention procedures, showing that this allele is associated with a hyporesponse to clopidogrel [49].
Lin et al. analyzed the response to clopidogrel in 108 patients undergoing neurointervention for intracranial aneurysms or stenosis. They found no association with the CYP2C19*2 and *3 alleles, however, they concluded that being a carrier of the CYP2C19*17 allele, contrary to the expected, is related to the incidence of ischemic events, regardless of the effect of clopidogrel. However, they mention the need to confirm the influence of this allele on the clinical outcome of patients undergoing endovascular treatment [52].
All these studies suggest that the presence of no-function alleles is associated with clopidogrel resistance and the incidence of ischemic events, which is in line with studies in patients with acute coronary syndrome. However, the influence of the CYP2C19*17 allele is not as clear, even contradictory to what might be expected.
Our recent research tried to confirm these results and to overcome the limitations of these studies. Our group published first in March 2019 and, second in June 2019, a retrospective observational study in 123 and 144 patients, respectively, subjected to percutaneous neurointervention who were treated with clopidogrel [53-55]. In the first study, we aimed to evaluate the influence of CYP2C19 phenotype on clopidogrel response. In the second, we also evaluate the influence of the main polymorphisms of the transporter ABCB1, other CYP enzymes (CYP1A2, CYP2B6, CYP2C8, CYP2C9, CYP2C19, CYP3A4 and CYP3A5), PON1, CES1 and P2RY12, including 21 new cases.
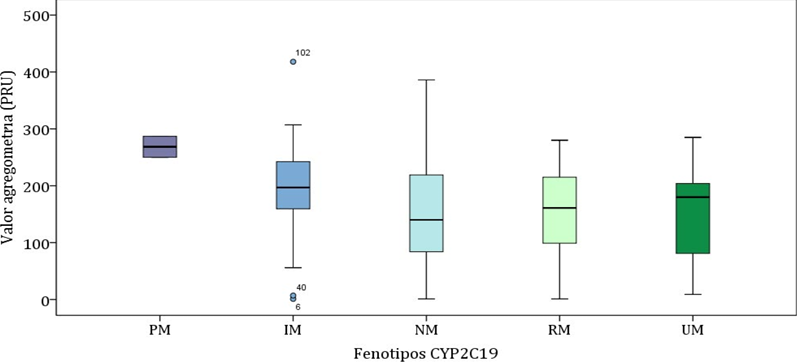
Our results confirm that CYP2C19 is the most relevant enzyme in the formation of clopidogrel active metabolite. Indeed, as expected, the CYP2C19*2 allele was associated with a hyporesponse to clopidogrel in patients after percutaneous neurointervention [53]. Moreover, the CYP2C19*17 allele resulted a protective factor for the incidence of ischemic events but a risk factor for the incidence of bleeding events [55]. Only two PM subjects (with genotype *2/*2) showed a significantly higher aggregometry value (268.5 ± 26.2 PRU) with respect to the rest of the groups (196.2 ± 84.9 PRU in IM, 140.3 ± 89.2 PRU in NM, 158.8 ± 73.6 PRU in RM and 151.8 ± 108.0 PRU in UM; p=0.013) (Figure 2). Moreover, regarding the type of clinical events related to the CYP2C19 phenotype, the prevalence of ischemic events was lower in the RM-UM group (2.3%) compared to IM-PM (10.8%) and NM (15.9%), p=0.060 [53-55]. On the other hand, the highest prevalence of bleeding events was in the RM-UM group (15.9%) compared to NM (7.9%) and IM-PM (2.7%), p=0.109 [53, 55].
Figure 2: Aggregometry value according to CYP2C19 phenotype.
After a multivariate analysis, age, concomitant treatment with proton-pump inhibitors (PPIs) and phenotype CYP2C19 IM-PM were predictors of worse response to clopidogrel associated with a higher value of aggregometry. Moreover, CYP2C19 RM-UM phenotype was a protective factor, and the duration of treatment a risk factor, for the development of any ischemic event. Finally, with regard to the prediction of bleeding events, the phenotype CYP2C19 RM-UM was the only risk factor [54].
Regarding other genes, we observed that the mutated haplotype of ABCB1 was a predictor of better response to clopidogrel, so P-glycoprotein seems to play a relevant role in its absorption and/or distribution [54]. Furthermore, subjects carrying CES1 G143E polymorphism showed an increased antiaggregant effect, suggesting a higher formation of active metabolite. Finally, polymorphisms in other CYP450 enzymes, PON1 and P2RY12 seemed not to influence the effect of clopidogrel after a neurointervention.
In addition, as our study also analyzed the influence of the concomitant use of PPIs, we observed that its use decreases the response to clopidogrel, especially in patients carrying a CYP2C19*2 allele or patients with a wild-type genotype. This is due to the inhibition of CYP2C19 by PPIs.
Despite having found no significant association between IM-PM phenotype and the incidence of ischemic events, we believe that CPIC therapeutic recommendations in patients with acute coronary syndrome with IM-PM phenotype should also be applied in patients undergoing neurointerventional procedures (Table 1), for all the reasons mentioned above. Moreover, we suggest that therapeutic recommendations should be extended to RM-UM patients to prevent the risk of bleeding complications (Table 1).
As a conclusion from our research, we could highly recommend a routine prior genotyping of CYP2C19 for all patients to be treated with clopidogrel, the prescription of an antiaggregate other than clopidogrel in patients carrying the CYP2C19*2 or *17 allele and to avoid the concomitant use of PPIs and clopidogrel.
According to our knowledge, no further research has been performed on this issue in the last few months. However, we would like to recommend further perspectives. First, our study should be extended with a larger sample size in order to establish significant correlations that allow the design of a treatment algorithm. It should be based on the phenotype of the patients, concomitant treatments and other factors to be taken into account, such as age and sex.
Subsequently, it would be advisable to carry out a randomized clinical trial with three treatment arms: one in which patients are treated according to usual clinical practice, without available pharmacogenetic information, where decisions to change treatment or adjust doses are made according to the clinician's criteria; another treatment arm where the patient is genotyped prior to the initiation of treatment with clopidogrel, so that the adjustment of the treatment to be prescribed and the doses to be administered are guided by pharmacogenetic information (mainly CYP2C19) and the use of concomitant medication, applying the treatment algorithm. Finally, a third treatment arm where some of the alternatives to clopidogrel treatment are administered (e.g. ticagrelor). It would be useful to check whether pharmacogenetic-guided treatment, is cost-effective with respect to the administration of another antiplatelet to all patients, regardless of their genotype. Thus, it could be tested if this therapeutic individualization reduces the incidence of adverse events, improves the response to clopidogrel and avoids an over cost to the national health system. The sample size should be large in order to find subjects with minority alleles of low-frequency polymorphisms, which makes collaboration between research groups from various centres necessary.
As a final consideration, we would point out that although the implementation of pharmacogenetic results in the routine practice is difficult, it is also possible. As a result of our research, the radiologists of our hospital are more aware of the need to include genotype as an additional variable to be considered in prescribing. Therefore, we believe that multi-disciplinary teamwork is necessary to develop therapeutic guidelines that clinicians can understand and apply, so that pharmacogenetic information can be useful for the actual prescribers.
|
Phenotype |
Genotypes |
Consequences |
Clopidogrel therapeutic recommendation |
|
Rapid and Ultrarapid metabolizer (RM-UM) (~5-30% of patients) |
*1/*17, *17/*17 |
Increased risk of bleeding |
Alternative antiplatelet therapy (if no contraindication) |
|
Normal metabolizer (NM) (~35-50% of patients) |
*1/*1 |
Normal platelet inhibition; normal residual platelet aggregation |
Clopidogrel - label recommended dosage and administration |
|
Intermediate metabolizer (IM) (~18-45% of patients) |
*1/*2, *1/*3, *2/*17 |
Reduced platelet inhibition, increased risk for ischemic events |
Alternative antiplatelet therapy (if no contraindication) |
|
Poor metabolizer (PM) (~2-15% of patients) |
*2/*2, *2/*3, *3/*3 |
Reduced platelet inhibition, increased risk for ischemic events |
Alternative antiplatelet therapy (if no contraindication) |
Conflicts of Interests
F.A.S. has been consultant or investigator in clinical trials sponsored by the following pharmaceutical companies: Abbott, Alter, Chemo, Cinfa, FAES, Farmalíder, Ferrer, GlaxoSmithKline, Galenicum, Gilead, Italfarmaco, Janssen-Cilag, Kern, Normon, Novartis, Servier, Silverpharma, Teva and Zambon. M.S.R declares no conflicts of interest.
Source of Funding
There has been no involvement of funding sources in the manuscript writing or in the decision to submit the article for publication.
Acknowledgments
We thank all the personnel from the Clinical Pharmacology Department of Hospital Universitario de La Princesa for their great support.
References
2. Herbert JM, Frehel D, Vallee E, Kieffer G, Gouy D, Berger Y, et al. Clopidogrel, a novel antiplatelet and antithrombotic agent. Cardiovasc Drug Rev 1993;11:180-198.
3. Agencia Española de Medicamentos y Productos sanitarios (AEMPS). Ficha técnica del clopidogrel. Ministerio de Sanidad, Política Social e Igualdad. Revisada: octubre de 2016.
4. Lins R, Broekhuysen J, Necciari J, Deroubaix X. Pharmacokinetic profile of 14C-labeled clopidogrel. Semin Thromb Hemost. 1999;25 Suppl 2:29-33.
5. Ferri N, Corsini A, Bellosta S. Pharmacology of the new P2Y12 receptor inhibitors: insights on pharmacokinetic and pharmacodynamic properties. Drugs. 2013 Oct;73(15):1681-709.
6. Sangkuhl K, Klein TE, Altman RB. Clopidogrel pathway. Pharmacogenet Genomics. 2010 Jul;20(7):463-5.
7. Bouman HJ, Schömig E, van Werkum JW, Velder J, Hackeng CM, Hirschhäuser C, et al. Paraoxonase-1 is a major determinant of clopidogrel efficacy. Nat Med. 2011 Jan;17(1):110-6.
8. Trenk D, Hochholzer W. Genetics of platelet inhibitor treatment: Genetics of platelet inhibitor treatment. Br J Clin Pharmacol. 2014 Apr;77(4):642-53.
9. Taubert D, von Beckerath N, Grimberg G, Lazar A, Jung N, Goeser T, et al. Impact of P-glycoprotein on clopidogrel absorption. Clin Pharmacol Ther. 2006 Nov;80(5):486-501.
10. Cairns JA, Eikelboom J. Clopidogrel resistance: more grist for the mill. J Am Coll Cardiol. 2008 May 20;51(20):1935-7.
11. Floyd CN, Passacquale G, Ferro A. Comparative pharmacokinetics and pharmacodynamics of platelet adenosine diphosphate receptor antagonists and their clinical implications. Clin Pharmacokinet. 2012 Jul 1;51(7):429-42.
12. Serebruany VL, Steinhubl SR, Berger PB, Malinin AI, Bhatt DL, Topol EJ. Variability in platelet responsiveness to clopidogrel among 544 individuals. J Am Coll Cardiol. 2005 Jan;45(2):246-51.
13. Gurbel PA, Bliden KP, Guyer K, Cho PW, Zaman KA, Kreutz RP, et al. Platelet reactivity in patients and recurrent events post-stenting: results of the PREPARE POST-STENTING Study. J Am Coll Cardiol. 2005 Nov 15;46(10):1820-6.
14. Bonello L, Tantry US, Marcucci R, Blindt R, Angiolillo DJ, Becker R, et al. Consensus and future directions on the definition of high on-treatment platelet reactivity to adenosine diphosphate. J Am Coll Cardiol. 2010 Sep 14;56(12):919-33.
15. Matetzky S, Shenkman B, Guetta V, Shechter M, Beinart R, Bienart R, et al. Clopidogrel resistance is associated with increased risk of recurrent atherothrombotic events in patients with acute myocardial infarction. Circulation. 2004 Jun 29;109(25):3171-5.
16. Kazui M, Nishiya Y, Ishizuka T, Hagihara K, Farid NA, Okazaki O, et al. Identification of the human cytochrome P450 enzymes involved in the two oxidative steps in the bioactivation of clopidogrel to its pharmacologically active metabolite. Drug Metab Dispos Biol Fate Chem. 2010 Jan;38(1):92-9.
17. Caudle KE, Dunnenberger HM, Freimuth RR, Peterson JF, Burlison JD, Whirl-Carrillo M, et al. Standardizing terms for clinical pharmacogenetic test results: consensus terms from the Clinical Pharmacogenetics Implementation Consortium (CPIC). Genet Med Off J Am Coll Med Genet. 2017 Feb;19(2):215-23.
18. Mega JL, Simon T, Collet J-P, Anderson JL, Antman EM, Bliden K, et al. Reduced-function CYP2C19 genotype and risk of adverse clinical outcomes among patients treated with clopidogrel predominantly for PCI: a meta-analysis. JAMA. 2010 Oct 27;304(16):1821-30.
19. Giusti B, Gori AM, Marcucci R, Saracini C, Sestini I, Paniccia R, et al. Cytochrome P450 2C19 loss-of-function polymorphism, but not CYP3A4 IVS10 + 12G/A and P2Y12 T744C polymorphisms, is associated with response variability to dual antiplatelet treatment in high-risk vascular patients. Pharmacogenet Genomics. 2007 Dec;17(12):1057-64.
20. Zabalza M, Subirana I, Sala J, Lluis-Ganella C, Lucas G, Tomás M, et al. Meta-analyses of the association between cytochrome CYP2C19 loss- and gain-of-function polymorphisms and cardiovascular outcomes in patients with coronary artery disease treated with clopidogrel. Heart. 2012 Jan 15;98(2):100-8.
21. Sibbing D, Stegherr J, Latz W, Koch W, Mehilli J, Dörrler K, et al. Cytochrome P450 2C19 loss-of-function polymorphism and stent thrombosis following percutaneous coronary intervention. Eur Heart J. 2009 Apr;30(8):916-22.
22. Sibbing D, Gebhard D, Koch W, Braun S, Stegherr J, Morath T, et al. Isolated and interactive impact of common CYP2C19 genetic variants on the antiplatelet effect of chronic clopidogrel therapy. J Thromb Haemost JTH. 2010 Aug;8(8):1685-93.
23. Mega JL, Close SL, Wiviott SD, Shen L, Hockett RD, Brandt JT, et al. Cytochrome p-450 polymorphisms and response to clopidogrel. N Engl J Med. 2009 Jan 22;360(4):354-62.
24. Frére C, Cuisset T, Gaborit B, Alessi M-C, Hulot J-S. The CYP2C19*17 allele is associated with better platelet response to clopidogrel in patients admitted for non-ST acute coronary syndrome. J Thromb Haemost JTH. 2009 Aug;7(8):1409-11.
25. Tiroch KA, Sibbing D, Koch W, Roosen-Runge T, Mehilli J, Schömig A, et al. Protective effect of the CYP2C19 *17 polymorphism with increased activation of clopidogrel on cardiovascular events. Am Heart J. 2010 Sep;160(3):506-12.
26. Sibbing D, Koch W, Gebhard D, Schuster T, Braun S, Stegherr J, et al. Cytochrome 2C19*17 allelic variant, platelet aggregation, bleeding events, and stent thrombosis in clopidogrel-treated patients with coronary stent placement. Circulation. 2010 Feb 2;121(4):512-8.
27. Li Y, Tang H-L, Hu Y-F, Xie H-G. The gain-of-function variant allele CYP2C19*17: a double-edged sword between thrombosis and bleeding in clopidogrel-treated patients. J Thromb Haemost JTH. 2012 Feb;10(2):199-206.
28. Scott SA, Sangkuhl K, Stein CM, Hulot J-S, Mega JL, Roden DM, et al. Clinical Pharmacogenetics Implementation Consortium guidelines for CYP2C19 genotype and clopidogrel therapy: 2013 update. Clin Pharmacol Ther. 2013 Sep;94(3):317-23.
29. Shuldiner AR, O’Connell JR, Bliden KP, Gandhi A, Ryan K, Horenstein RB, et al. Association of cytochrome P450 2C19 genotype with the antiplatelet effect and clinical efficacy of clopidogrel therapy. JAMA. 2009 Aug 26;302(8):849-57.
30. Harmsze A, van Werkum JW, Bouman HJ, Ruven HJ, Breet NJ, Ten Berg JM, et al. Besides CYP2C19*2, the variant allele CYP2C9*3 is associated with higher on-clopidogrel platelet reactivity in patients on dual antiplatelet therapy undergoing elective coronary stent implantation. Pharmacogenet Genomics. 2010 Jan;20(1):18-25.
31. Rytkin E, Mirzaev KB, Grishina EA, Smirnov VV, Ryzhikova KA, Sozaeva ZA, et al. DoCYP2C19andABCB1gene polymorphisms and low CYP3A4 isoenzyme activity have an impact on stent implantation complications in acute coronary syndrome patients? Pharmacogenomics Pers Med. 2017;10:243-5.
32. Ford NF. The Metabolism of Clopidogrel: CYP2C19 Is a Minor Pathway. J Clin Pharmacol. 2016;56(12):1474-83.
33. Viviani Anselmi C, Briguori C, Roncarati R, Papa L, Visconti G, Focaccio A, et al. Routine assessment of on-clopidogrel platelet reactivity and gene polymorphisms in predicting clinical outcome following drug-eluting stent implantation in patients with stable coronary artery disease. JACC Cardiovasc Interv. 2013 Nov;6(11):1166-75.
34. Cresci S, Depta JP, Lenzini PA, Li AY, Lanfear DE, Province MA, et al. Cytochrome p450 gene variants, race, and mortality among clopidogrel-treated patients after acute myocardial infarction. Circ Cardiovasc Genet. 2014 Jun;7(3):277-86.
35. González A, Moniche F, Cayuela A, García-Lozano JR, Torrecillas F, Escudero-Martínez I, et al. Effect of CYP2C19 Polymorphisms on the Platelet Response to Clopidogrel and Influence on the Effect of High Versus Standard Dose Clopidogrel in Carotid Artery Stenting. Eur J Vasc Endovasc Surg Off J Eur Soc Vasc Surg. 2016 Feb;51(2):175-86.
36. Saydam F, Değirmenci İ, Birdane A, Özdemir M, Ulus T, Özbayer C, et al. The CYP2C19*2 and CYP2C19*17 Polymorphisms play a Vital Role in Clopidogrel Responsiveness after Percutaneous Coronary Intervention: A Pharmacogenomics Study. Basic Clin Pharmacol Toxicol. 2017 Jul;121(1):29-36.
37. Humbert R, Adler DA, Disteche CM, Hassett C, Omiecinski CJ, Furlong CE. The molecular basis of the human serum paraoxonase activity polymorphism. Nat Genet. 1993 Jan;3(1):73-6.
38. Garin MC, James RW, Dussoix P, Blanché H, Passa P, Froguel P, et al. Paraoxonase polymorphism Met-Leu54 is associated with modified serum concentrations of the enzyme. A possible link between the paraoxonase gene and increased risk of cardiovascular disease in diabetes. J Clin Invest. 1997 Jan 1;99(1):62-6.
39. Costa LG, Cole TB, Vitalone A, Furlong CE. Measurement of paraoxonase (PON1) status as a potential biomarker of susceptibility to organophosphate toxicity. Clin Chim Acta Int J Clin Chem. 2005 Feb;352(1–2):37-47.
40. Zhu H-J, Patrick KS, Yuan H-J, Wang J-S, Donovan JL, DeVane CL, et al. Two CES1 gene mutations lead to dysfunctional carboxylesterase 1 activity in man: clinical significance and molecular basis. Am J Hum Genet. 2008 Jun;82(6):1241-8.
41. Lewis JP, Horenstein RB, Ryan K, O’Connell JR, Gibson Q, Mitchell BD, et al. The functional G143E variant of carboxylesterase 1 is associated with increased clopidogrel active metabolite levels and greater clopidogrel response. Pharmacogenet Genomics. 2013 Jan;23(1):1-8.
42. Ameyaw MM, Regateiro F, Li T, Liu X, Tariq M, Mobarek A, et al. MDR1 pharmacogenetics: frequency of the C3435T mutation in exon 26 is significantly influenced by ethnicity. Pharmacogenetics. 2001 Apr;11(3):217-21.
43. Kim RB, Leake BF, Choo EF, Dresser GK, Kubba SV, Schwarz UI, et al. Identification of functionally variant MDR1 alleles among European Americans and African Americans. Clin Pharmacol Ther. 2001 Aug;70(2):189-99.
44. Sakaeda T, Nakamura T, Horinouchi M, Kakumoto M, Ohmoto N, Sakai T, et al. MDR1 genotype-related pharmacokinetics of digoxin after single oral administration in healthy Japanese subjects. Pharm Res. 2001 Oct;18(10):1400-4.
45. Kurata Y, Ieiri I, Kimura M, Morita T, Irie S, Urae A, et al. Role of human MDR1 gene polymorphism in bioavailability and interaction of digoxin, a substrate of P-glycoprotein. Clin Pharmacol Ther. 2002 Aug;72(2):209-19.
46. Szymezak J, Moreau C, Loriot M-A, Durand E, Van Viet H, Desnos M, et al. High on-clopidogrel platelet reactivity: genotyping can help to optimize antiplatelet treatment. Thromb Res. 2011 Jul;128(1):92-5.
47. Sridharan K, Kataria R, Tolani D, Bendkhale S, Gogtay NJ, Thatte UM. Evaluation of CYP2C19, P2Y12, and ABCB1 polymorphisms and phenotypic response to clopidogrel in healthy Indian adults. Indian J Pharmacol. 2016 Aug;48(4):350-4.
48. Rakicevic L, Nestorovic A. Pharmacogenetics of Clopidogrel Therapy and Neurointerventional Procedures: We Need Precision Data for Precision Medicine. Clin Pharmacol Ther [Internet]. 2018 Jun 19 [cited 2018 Jul 16]; Available from: http://doi.wiley.com/10.1002/cpt.1105
49. Colley R, Yan B. Genetic determinations of variable responsiveness to clopidogrel and implications for neurointerventional procedures. Interv Neurol. 2012 May;1(1):22–30.
50. Zhu W-Y, Zhao T, Xiong X-Y, Li J, Wang L, Zhou Y, et al. Association of CYP2C19 Polymorphisms with the Clinical Efficacy of Clopidogrel Therapy in Patients Undergoing Carotid Artery Stenting in Asia. Sci Rep. 2016 03;6:25478.
51. Ge H, Lv X, Ren H, Jin H, Jiang Y, He H, et al. Influence of CYP2C19 genetic polymorphisms on clinical outcomes of intracranial aneurysms treated with stent-assisted coiling. J Neurointerventional Surg. 2017 Oct;9(10):958-62.
52. Lin M, Todaro M, Chan J, Churilov L, Zhu WS, Ramdave S, et al. Association between CYP2C19 Polymorphisms and Outcomes in Cerebral Endovascular Therapy. AJNR Am J Neuroradiol. 2016 Jan;37(1):108-13.
53. Saiz-Rodríguez M, Romero-Palacián D, Villalobos-Vilda C, Caniego JL, Belmonte C, Koller D, et al. Influence of CYP2C19 Phenotype on the Effect of Clopidogrel in Patients Undergoing a Percutaneous Neurointervention Procedure. Clin Pharmacol Ther. 2019;105(3):661-71.
54. Saiz-Rodríguez M, Belmonte C, Caniego JL, Koller D, Zubiaur P, Bárcena E, et al. Influence of CYP450 Enzymes, CES1, PON1, ABCB1, and P2RY12 Polymorphisms on Clopidogrel Response in Patients Subjected to a Percutaneous Neurointervention. Clin Ther. 2019 Jun;41(6):1199-1212.e2.
55. Saiz-Rodríguez M, Belmonte C, Abad-Santos F. CYP2C19 Ultrarapid Phenotype as a Risk Predictor of Subsequent Events During Clopidogrel Treatment in Patients Undergoing a Percutaneous Neurointervention. Clin Pharmacol Ther. 2019;105(5):1074-5.