Abstract
Due to their complex etiology and pathogenesis, hemangioma-related syndromes, such as LUMBAR and PHACE, present diagnostic and therapeutic challenges. Despite advances, standardized diagnostic criteria, screening protocols, and treatment options still need to be developed. We report a rare case of a 1-month-old patient with nonspecific symptoms, including failure to thrive and poor feeding, associated with lumbosacral ulcers. The diagnosis revealed overlapping features of LUMBAR and PHACE syndromes, highlighting the broad phenotypic spectrum and suggesting a potential common etiology. This case underscores the need for heightened clinical suspicion among general practitioners, dermatologists, and vascular physicians to manage these conditions. Propranolol is widely used for treating infantile hemangiomas, but due to its side effects, alternative therapies need to be explored. Emerging treatments, such as Atenolol, Timolol, Carteolol, and pulsed dye lasers, offer promise but require further investigation. However, the most crucial aspect is the need for ongoing research into the mechanisms of action, clinical applications, and side effects of current and alternative treatments. This research is essential for developing a comprehensive, safe management algorithm for both low- and high-risk infantile hemangiomas. It demands sustained engagement and commitment from the medical and research communities, ultimately improving patient outcomes and advancing medical knowledge.
Introduction
Vascular tumors are common birthmarks, with infantile hemangiomas (IH) being the most prevalent. Approximately 3–10% of term newborns have been affected, with an observed increase in incidence throughout recent years [1]. IHs are common benign endothelial cell tumors that have a distinct pattern of fast proliferation in the early months of life followed by a slow involution that could take years to finish [2]. The International Society for the Study of Vascular Anomalies states that IHs with an oval or round form denote the most typical morphology [3]. However, a new subtype of IH included in this list is infantile hemangioma with minimal or arrested growth (IH-MAG) [4]. The IH-MAGs are a very rare subset of IH with a recent increase in popularity among the literature distinguished by two unique features: its morphology consists of: (1) proliferative lesions (such papules or plaques) that cover less than 25% of the entire area, and (2) a proliferative phase that progresses almost unchanged or with very little growth [4].They are mostly fully formed at birth and do not fit the definition of congenital hemangiomas since they have different morphologic and immunostaining patterns. Although the exact etiology of hemangiomas remains enigmatic, the theory of the hypoxic uterine environment has gained the most traction [5]. This theory suggests that the development of hemangiomas may be influenced by the oxygen levels in the womb since recent studies find a higher incidence of placental infarctions in premature babies with hemangiomas and ischemic arterial malformations [6]. It is well known that hypoxia stimulates angiogenesis precursors, such as vascular endothelial growth factor (VEGF) and glucose transporter-1 (GLUT-1), in endothelial cells [6]. IH-MAGs, from a histological perspective, exhibit early-phase hemangioma characteristics but without the notable proliferation of typical hemangiomas. Based solely on histology, their characteristics bear a closer resemblance to congenital malformations, with the primary feature being dilated, thin-walled vessels located within the superficial dermis [7]. The positive GLUT-1 immunohistochemistry further confirms their unique nature. Endothelial cells, as per immunohistochemistry, display a double-contour appearance with cluster of differentiation- 34 (CD-34), in addition to being positive for GLUT-1, a striking similarity to the appearance of IHs during later stages of development, particularly during involution [7]. The erythrocyte-type glucose transporter protein GLUT-1 serves as a highly specific and vital diagnostic marker for IHs; it is negative for congenital hemangiomas and vascular malformations [7,8]. The positivity of GLUT-1 in these vascular lesions unequivocally identifies them as IHs, providing a key insight for diagnosis and treatment [7]. Some IHs, including IH-MAG, can be associated with extracutaneous anomalies such as PHACE syndrome (posterior fossa anomalies, upper body hemangiomas, arterial anomalies, cardiac anomalies, and eye anomalies) and LUMBAR syndrome (lower body hemangiomas, ulcerations/urogenital anomalies, myelopathies, bony deformities, anorectal malformations/arterial anomalies, and renal anomalies. Even though these two similar entities have their own diagnostic criteria, they can rarely exhibit clinically overlapping features, such as in our case. Therefore, it has been theorized about a continuum spectrum of the same entity instead of two different conditions [5,8-10]. It has been hypothesized that the embryological error may include a combination of postzygotic coding, noncoding, or somatic variants in oncogenes or tumor suppressor genes since they are involved in the pathogenesis of vascular and skin anomaly disorders between six to nine weeks of gestation [11]. Anatomic features can be associated with anomalies in the neural plate, crest, and adjacent cephalic mesoderm [12]. Although neuraxial IHs are rare in the general population, occurring in 1% of all patients with skin hemangiomas, several studies have described an increased frequency in patients with hemangioma syndromes [10]. Therefore, this type of IH requires close attention since it may represent a clinical finding of hemangiomas-related syndrome and, importantly, has no specific treatment, highlighting the urgent need for further research in this area.
Case Presentation Overview
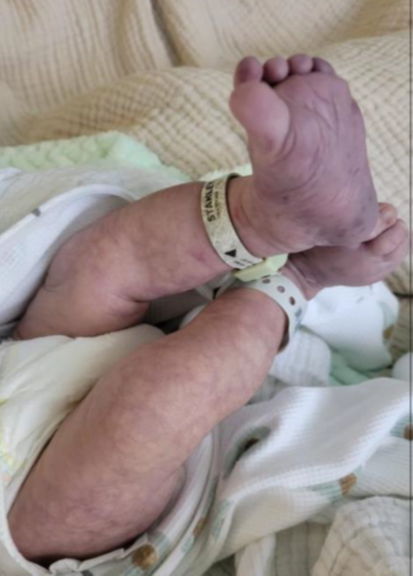
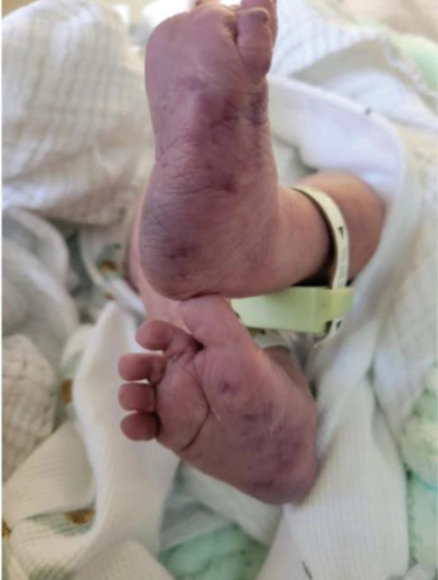
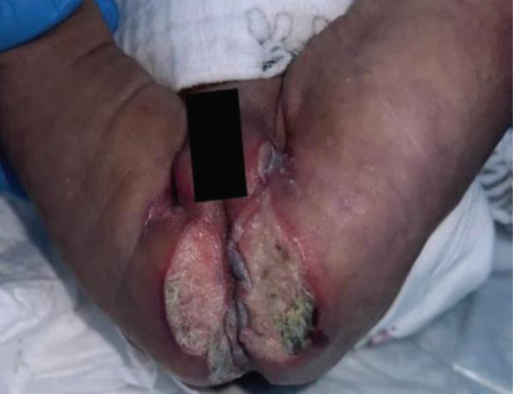
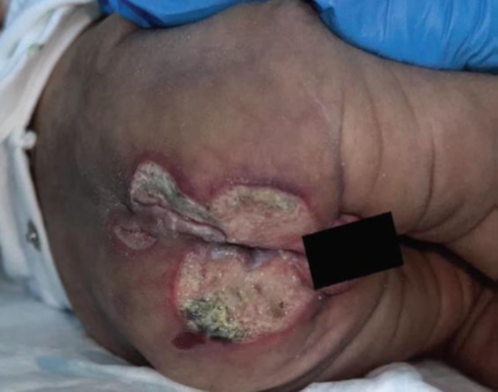
A 1-month-old female was admitted with a rare and intriguing case of worsening perianal and gluteal ulcers, which had developed over a week. She had a history of diffuse bluish discoloration with a telangiectatic pattern on her lower extremities and lumbar area (Figures 1 and 2), noted at birth but initially considered benign. Despite being born at full term without complications, she was hospitalized at 16 days of life for suspected neonatal sepsis. During her stay, she developed intergluteal erythema, which progressed into nonexudative ulcers within four days and was treated with topical wound care before being discharged. However, the lesions worsened, leading to her admission to our hospital. On evaluation, she had weak femoral pulses, hip dislocations, and mottled lower extremities with confluent ulcers (Figures 3 and 4). Imaging revealed extensive infantile hemangiomas in the perirectal, retroperitoneal, left adrenal, and left paraspinal areas, and vascular anomaly along with coarctation of the aorta, suggesting overlapping PHACE and LUMBAR syndromes. Treatment with propranolol for three months led to significant improvement, and she was discharged for continued care in her home state.
Figures 1 and 2: Bluish telangiectatic pattern from the lumbar area to bilateral lower extremities associated with dark bluish-purple discoloration in the soles at one day of life.
Figure 3 and 4: Erythematous, purulent, and confluent ulcers at the lumbar, gluteal, and genital areas at one month of life.
Discussion
Diagnostic criteria and screening
At the time of our case report publication, no consensus was established for diagnosing LUMBAR syndrome [5]. Since this condition is rarer than PHACE syndrome, there are still unanswered questions regarding the natural history, outcome, diagnostic criteria, screening, and management guidelines [13]. Recently, however, a consensus was reached to establish diagnostic criteria for LUMBAR syndrome. The diagnostic criteria included the following: (1) the presence of a segmental or patterned infantile hemangioma in the lumbosacral, sacrococcygeal, or pelvic cutaneous regions; and (2) at least one additional criterion involving the urogenital, spinal, bony, anorectal, arterial or renal organ systems [13]. In accordance with the recent consensus, our patient would have fulfilled the following criteria: presence of a segmental IH since birth, bilateral hip dysplasia with limited acetabular development and computer tomography angiography (CTA) remarkable for decreased caliber at the lower abdominal and bilateral iliac branches in CTA [5]. Since prompt diagnosis is critical to decrease the risk of serious complications, Le?aute?-Labre?ze developed the Infantile Hemangioma Referral Score (IHReS) tool for IHs [14]. An easy-to-use validated screening tool with a sensitivity of ∼97% to identify patients who require a prompt referral to a specialist [5]. Recent studies have demonstrated that IHReS has a sensitivity reaching 100% and could refer all patients who need treatment when compared with Hemangioma Severity Scale (HSS) [15]. For LUMBAR syndrome, it has been proposed that all patients presenting with either segmental IH of any lumbosacral or perineal region under three months of age or simply having a characteristic criterion should undergo ultrasound imaging of the spine, abdomen, and pelvis with color Doppler [5,16]. A spinal MRI can be performed for better visualization after three to four months of age [13]. The establishment of formal criteria and some screening recommendations is a promising step that can improve overall awareness between physicians and pave the way for future studies. These studies can further our understanding and help establish formal screening and management guidelines for this rare condition.
Types of treatments
Pharmacological agents, like propranolol and steroids, have been widely used as the first-line therapy for the treatment of infantile hemangiomas [17,18]. In the case of the patient presented in our case report, propranolol, a non-selective beta-blocker, was used intravenously and orally to properly manage IH throughout the hospital stay and upon discharge, respectively [4]. This agent's proposed mechanism of action includes inhibiting both beta-1 and beta-2 adrenoreceptors and suppressing vascular endothelial growth factor (VEGF) and vascular endothelial growth factor receptor-2 (VEGFR-2) expression [17,19,20]. Indications for using propranolol include periocular, nasal, lip, auricular, intracranial, and intraspinal IH [15]. Routes of administration include intravenously, oral, or intralesional. At this moment oral propranolol has been accepted as the first-line treatment due to its excellent safety record. Although intralesional propranolol hydrochloride has fewer side effects than those of oral administration, its effectiveness was still insufficient [21]. Oral propranolol side effects include hypoglycemia, bradycardia, hypotension, bronchospasm, and electrolyte disturbances. It can cross the blood-brain barrier (BBB), leading to potential neurocognitive problems and sleep disturbances. However, it's important to note that recent studies have demonstrated that these side effects are temporary [22], providing reassurance about their long-term impact. No neurocognitive deterioration was developed during infancy when using propranolol, and, if any, may be resolved by brain plasticity [23]. Sleep disturbances can be managed by giving the medication earlier [24]. Given these previously mentioned side effects, researchers have investigated other treatments such as Atenolol, Timolol, Carteolol, and pulsed dye lasers (PDL) [17]. Atenolol is a beta-1 selective beta-blocker that does not cross the BBB [18]. Therefore, it has been shown to have a similar efficacy with fewer side effects [18] and can be used if the patient has contraindications or has unbearable side effects from propranolol [20]. When IHs are superficial, timolol maleate is the topical agent of choice. Another non-selective beta-blocker has a 99% effectiveness on superficial IH compared to the 90% effectiveness of topical propranolol [22]. Side effects of timolol include eczema, ulcers, skin rashes, and erythema [25]. We can use Carteolol, another non-selective beta-blocker for IHs in the head, neck, periorbital, and cervical areas [17]. Side effects include erythema and scarring [17]. Before propranolol revolutionized the treatment of IHs, corticosteroids were the first line due to their antiangiogenic effect. However, it's important to note that they do come with a set of side effects, including Cushing-like manifestations, adrenal suppression, weight gain, behavioral disturbances, and growth disorders [17,20,26]. Various studies have compared the efficacy of propranolol and oral corticosteroids, with propranolol emerging as the more effective and less side effect-prone option [20]. Another study found that the combined use of corticosteroids and propranolol initially yielded benefits, but these advantages dissipated after six months [20]. Energy-based medical devices like laser treatment are another treatment for remaining hemangiomas, high-risk lesion management, contraindications, or patients at risk of side effects from propranolol. Among the several types, pulsed dye laser (PDL) is the most commonly used laser for cutaneous vascular lesions [27,28]. Its benefit resides in selective photo-thermolysis, which induces thrombosis within the vessels without damaging the dermis [27]. PDL, the gold standard for treating IH-related residual telangiectasias, is highly effective, instilling confidence in its use [29]. Despite their high efficacy, studies have demonstrated that PDL alone is not superior to propranolol. In fact, its utility alone is limited to more superficial cases [27]. The combination of laser therapy with other treatments like beta-blockers provides better outcomes than any other modality alone [27-29]. Blistering, crusting, pigmentation changes, and atrophic scarring are possible side effects after using the PDL [29]. To reduce the risk of side effects, several ointments have been utilized; for example, panthenol ointment is applied to the affected areas to lessen skin irritation and inflammation and hasten the healing of epidermal wounds, and povidone-iodine solution is advised when blistering or crusting is present [27]. Although alternative therapies have increased the number of available options, it is crucial to keep investigating current and alternative treatments, specifically their mechanisms of action, clinical use, and short-term and long-term side effects, to stay at the forefront of medical advancements.
Conclusion
Hemangioma-related syndromes such as LUMBAR and PHACE continue to intrigue us, with many aspects beyond their etiology and pathogenesis remaining enigmatic. The ongoing debate and active investigation in areas such as diagnostic criteria, screening protocols, and short-term and long-term therapeutic options are crucial. The recent expert consensus for the diagnostic criteria for LUMBAR syndrome is a significant step forward, facilitating and standardizing physicians' evaluations and enhancing our understanding of its phenotypic variability. Despite this advance, it is of utmost importance that general practitioners, dermatologists, and vascular physicians maintain high suspicion and utilize standard tools for screening infantile hemangiomas. This responsibility and diligence are key in identifying potential cases and being vigilant about potential overlapping features. While propranolol remains the most widely used treatment for infantile hemangiomas, the emergence of alternative therapies is a promising development that is expanding the therapeutic landscape. This expansion offers hope for improved patient outcomes and a more comprehensive approach to management. However, it is crucial that we continue our investigations into treatment options for infantile hemangiomas. The urgency and importance of this ongoing research cannot be overstated. By doing so, we can create standardized guidelines that will empower physicians to know when to start therapy, which therapy to use based on the IHs' characteristics, when to combine different treatment modalities, and when to stop the treatments since there is no specific consensus about these topics. This commitment to ongoing research will ensure comprehensive assessment, effective management, and the prevention of serious complications, ultimately improving patient outcomes.
References
2. Bruckner AL, Frieden IJ. Infantile hemangiomas. J Am Acad Dermatol. 2006 Oct;55(4):671-82.
3. Feygin T, Goldman-Yassen AE, Licht DJ, Schmitt JE, Mian A, Vossough A, et al. Neuroaxial Infantile Hemangiomas: Imaging Manifestations and Association with Hemangioma Syndromes. AJNR Am J Neuroradiol. 2021 Aug;42(8):1520-27.
4. Bae KN, Shin K, Kim HS, Kim BS, Kim MB, Ko HC. Infantile Hemangiomas with Minimal and Arrested Growth: Clinical Features and Treatment Outcomes with 0.5% Topical Timolol Maleate. Ann Dermatol. 2021 Oct;33(5):448-55.
5. Berríos LR, Rodríguez BC, Sadurní MB, Martinez KJ, Santiago CM, González NK. Beyond Infantile Hemangiomas: A Glimpse into Overlapping Rare Syndromes Emphasizing the Vigilant Screening for PHACE and LUMBAR Syndromes. Case Rep Pediatr. 2024 Apr 18;2024:7501793.
6. Colonna V, Resta L, Napoli A, Bonifazi E. Placental hypoxia and neonatal haemangioma: clinical and histological observations. Br J Dermatol. 2010 Jan;162(1):208-9.
7. Ma EH, Robertson SJ, Chow CW, Bekhor PS. Infantile Hemangioma with Minimal or Arrested Growth: Further Observations on Clinical and Histopathologic Findings of this Unique but Underrecognized Entity. Pediatr Dermatol. 2017 Jan;34(1):64-71.
8. Pahl KS, McLean TW. Infantile Hemangioma: A Current Review. J Pediatr Hematol Oncol. 2022 Mar 1;44(2):31-9.
9. Davenport R, Su JC, Nathalie J, Richmond CM, Yang Tan T, Robertson SJ. Clinical overlap of PHACE and LUMBAR syndromes. Pediatr Dermatol. 2022 Sep;39(5):752-6.
10. Feygin T, Goldman-Yassen AE, Licht DJ, Schmitt JE, Mian A, Vossough A, et al. Neuroaxial Infantile Hemangiomas: Imaging Manifestations and Association with Hemangioma Syndromes. AJNR Am J Neuroradiol. 2021 Aug;42(8):1520-7.
11. Shayegan LH, Frieden IJ, Davies OM, Drolet BA, Siegel DH, Chamlin SL, et al. The Coexistence of Upper and Lower Body Segmental Infantile Hemangiomas: Patterns and Implications. Journal of Vascular Anomalies. 2022 Sep 1;3(3):e043.
12. Winter PR, Itinteang T, Leadbitter P, Tan ST. PHACE syndrome--clinical features, aetiology and management. Acta Paediatr. 2016 Feb;105(2):145-53.
13. Metry D, Copp HL, Rialon KL, Iacobas I, Baselga E, Dobyns WB, et al. Delphi Consensus on Diagnostic Criteria for LUMBAR Syndrome. J Pediatr. 2024 Sep;272:114101.
14. Léauté-Labrèze C, Baselga Torres E, Weibel L, Boon LM, El Hachem M, van der Vleuten C, et al. The Infantile Hemangioma Referral Score: A Validated Tool for Physicians. Pediatrics. 2020 Apr;145(4):e20191628.
15. Qiu T, Yang K, Dai S, Chen S, Ji Y. Analysis of Therapeutic Decisions for Infantile Hemangiomas: A Prospective Study Comparing the Hemangioma Severity Scale with the Infantile Hemangioma Referral Score. Children (Basel). 2022 Nov 28;9(12):1851.
16. Valdebran M, Wine Lee L. Hemangioma-related syndromes. Curr Opin Pediatr. 2020 Aug;32(4):498-505.
17. Macca L, Altavilla D, Di Bartolomeo L, Irrera N, Borgia F, Li Pomi F, et al. Update on Treatment of Infantile Hemangiomas: What's New in the Last Five Years? Front Pharmacol. 2022 May 26;13:879602.
18. Pathman L, Simpson J, Penington AJ, Phillips RJ. Evaluating the Use of Atenolol for the Treatment of Infantile Hemangiomas. Journal of Vascular Anomalies. 2022 Dec 1;3(4):e048.
19. Tang YJ, Zhang ZZ, Chen SQ, Chen SM, Li CJ, Chen JW, et al. Effect of topical propranolol gel on plasma renin, angiotensin II and vascular endothelial growth factor in superficial infantile hemangiomas. J Huazhong Univ Sci Technolog Med Sci. 2015 Oct;35(5):759-62.
20. Lamy S, Lachambre MP, Lord-Dufour S, Béliveau R. Propranolol suppresses angiogenesis in vitro: inhibition of proliferation, migration, and differentiation of endothelial cells. Vascul Pharmacol. 2010 Nov-Dec;53(5-6):200-8.
21. Fei Q, Lin Y, Chen X. Treatments for infantile Hemangioma: A systematic review and network meta-analysis. EClinicalMedicine. 2020 Aug 18;26:100506.
22. Tiemann L, Hein S. Infantile Hemangioma: A Review of Current Pharmacotherapy Treatment and Practice Pearls. J Pediatr Pharmacol Ther. 2020;25(7):586-99.
23. Hermans MM, Rietman AB, Schappin R, de Laat PCJ, Mendels EJ, Breur JMPJ, et al. Long-term neurocognitive functioning of children treated with propranolol or atenolol for infantile hemangioma. Eur J Pediatr. 2023 Feb;182(2):757-67.
24. Giachetti A, Díaz MS, Boggio P, Posadas Martínez ML. Early propranolol treatment of infantile hemangiomas improves outcome. An Bras Dermatol. 2023 May-Jun;98(3):310-5.
25. Filoni A, Ambrogio F, De Marco A, Pacifico A, Bonamonte D. Topical beta-blockers in dermatologic therapy. Dermatol Ther. 2021 Jul;34(4):e15016.
26. Yuan SM, Zhang M, Guo Y, Cui L, Hong ZJ, Jiang HQ. Intralesional injection of diprospan is effective for infantile hemangioma. J Craniofac Surg. 2015 Mar;26(2):422-4.
27. Ziad K, Badi J, Roaa Z, Emily AH. Laser treatment of infantile hemangioma. J Cosmet Dermatol. 2023 Jun;22 Suppl 2:1-7.
28. Xu W, Zhao H. Management of infantile hemangiomas: Recent advances. Front Oncol. 2022 Nov 29;12:1064048.
29. Shah SD, Mathes EF, Baselga E, Frieden IJ, Powell J, Garzon MC, et al. Multicenter retrospective review of pulsed dye laser in nonulcerated infantile hemangioma. Pediatr Dermatol. 2023 Jan;40(1):28-34.