Abstract
Bacterial chondronecrosis with osteomyelitis (BCO) is a leading cause of lameness in broiler chickens, which results in financial losses due to mortality and reduced growth. Due to the nature of the condition, it raises animal welfare and food safety concerns as well. The pathology in the commercial setting is derived from a combination of weak bone and bacterial translocation and infection. Multiple genetic, nutritional, and managerial strategies have been employed to help mitigate the negative consequences of BCO; however, without understanding of the underlying causes of onset and progression, any benefit of these interventions is likely tempered. Compounding our dearth of knowledge is the fact that current methods of identification rely on subjective gait scores for lameness that may not show changes until the problem is severe or require diagnosis of BCO at necropsy. Therefore, our research group has aimed to tackle both facets of this important poultry concern. Here, we highlight several of our recent findings regarding the proteomic signature of BCO bone, as well as two potentially non-invasive assessment tools, dual energy x-ray absorptiometry and computed tomography, that can aid in future study. Together, we believe that this information provides the framework for future investigations and for subsequent development of mechanism-based strategies to prevent this pathology as well as better tools to impact genetic selection against BCO.
Keywords
Bacterial chondronecrosis with osteomyelitis, Broilers, DXA, Computed tomography, Omics
Abbreviations
ACLY: ATP Citrate Lyase; ACTN1: Alpha-Actinin 1; ACTN4: Alpha-Actinin 4; APOA1: Apolipoprotein A1; BCO: Bacterial Chondronecrosis with Osteomyelitis; CT: Computed Tomography; DXA: Dual-energy X-ray Absorptiometry; FLNA: Filamin A; FLNB: Filamin B; HPX: Hemopexin; HU: Hounsfield Unit; ITGAV: Integrin Subunit α5; ITGB1: Integrin Subunit β1; OSTF1: Osteoblast Stimulating Factor 1
Introduction
Broiler chickens play an important role in worldwide meat production, as they are one of the most efficient animal protein sources, and support the livelihoods and food security of billions of people. Genetic selection in the poultry industry has made spectacular progress in terms of improved growth and efficiency; however, increased muscle mass being deposited in a shorter period of time has placed a strain on other organ systems, particularly the skeleton. These skeletal challenges largely affect the long bones of the leg, and can be phenotypically generalized as lameness, where a bird is reluctant to stand or walk, and moves with a noticeably altered gait [1]. Leg disorders in poultry are of great concern to the industry as they negatively impact both profitability and welfare. In terms of welfare, lameness has been associated with pain [1] and decreases in normal broiler behaviors such as dust bathing, foraging, and preening [2]. This leads to economic losses through decreased growth performance and increased mortality and culls from observed lameness, as well downgrades and condemnations at processing due to sub-clinical bone disorders [3]. Additionally, it is estimated over 10% of broilers are moderately to severely lame [4,5], highlighting the critical nature of this problem.
Though clinical lameness may have various origins, a leading cause is bacterial chondronecrosis with osteomyelitis (BCO). In this condition, opportunistic bacteria (primarily Staphylococcus species, although numerous pathogens have been isolated [6,7]) infiltrate clefts or microfractures in the growth plate of long bones, likely associated with rapid growth rate and body weight gain, shifts in center of gravity, and subsequent mechanical shear stress to the epiphyseal and physeal cartilage [8]. These clefts also disrupt local blood vessels, leading to focal ischemia and necrosis [8]. Despite knowledge of the consequences of BCO, mechanistic understanding of the pathology is limited, hindering the impact of interventions and strategies designed to alleviate this disorder.
Here we discuss several studies from our group, aimed at characterizing and identifying BCO at early and/or sub-clinical levels. With this knowledge, as an industry, we can be better equipped to design targeted strategies and interventions that may be most effective for maintaining health and welfare and improving BCO incidence in an agriculturally important species.
Proteomic Analysis Identified Several Key Proteins in BCO
Using shotgun proteomics in a recent experiment described previously, we identified 222 differentially expressed proteins in the tibia of normal vs. BCO-affected broilers, where 158 were up-regulated and 64 were down-regulated in BCO as compared to normal bone [9]. Unsurprisingly, most of the proteins identified were involved in disorders such as bone injury and abnormality, connective tissue disorders, and inflammatory diseases, with top identified physiological functions including organismal survival, connective tissue development, and immune cell trafficking. Since our original report, we have further explored the potential function of some of the originally recognized proteins and have identified more possible links between these markers and bone health and disease. Of particular interest, two proteins we initially identified, ATP-citrate lyase (ACLY) and osteoclast stimulating factor 1 (OSTF1), as well as a cluster of proteins involved in extracellular interactions, have additional implications in bone health and disease.
ACLY is involved in bone remodeling through regulation of histone acetylation [10,11]. It has been shown that ACLY impacts both osteoblast [12] and osteoclast [10] differentiation through acetylation of β-catenin and Ras-related C3 botulinum toxin substrate 1 (Rac1). As ACLY uses mitochondrially-derived citrate as a substrate, and we have previously demonstrated mitochondrial dysfunction in BCO [13], and others have also shown mitochondrial dysfunction in human osteomyelitis [14], ACLY may be representative of the status of the mitochondria within the bone, indicating an additional avenue for targeted strategies to improve cellular health and reduce BCO. However, whether ACLY changes are an underlying cause or consequence of BCO are yet to be determined.
OSTF1 is an intracellular protein that is highly expressed by osteoclasts, and can indirectly enhance osteoclast formation and bone resorption [15], a process that is increased in BCO [16]. It has also recently been identified as a target for specific miRNA entities that are associated with osteonecrosis of the jaw in humans [17, 18], as well as a circulating marker in osteoporosis [19]. As OSTF1 is up-regulated in BCO bone, and we have shown evidence of dysregulation of the miRNA processing machinery in the same tissues [20], alteration of the miRNA milieu in BCO likely impacts the function of OSTF1 and other key factors in bone homeostasis in chicken. However, specific miRNA entities and their targets in bone of chicken have yet to be identified.
Several recent proteomics analyses of human osteonecrosis of the femur [21] and avascular necrosis of the femoral head [22] revealed some similarities with our findings in chicken BCO, particularly differential expression of hemopexin (HPX), apolipoprotein A1 (APOA1), filamins A and B (FLNA, FLNB), alpha-actinin 1 and 4 (ACTN1, ACTN4), and integrin subunit α5 and β1 (ITGAV, ITGB1) [21,22]. HPX has anti-inflammatory properties, through binding to heme released during hemolysis [23]. Its upregulation in BCO may be related to the breach of bone capillaries, where it acts as part of a survival mechanism to sequester heme to reduce it as a nutrient source for bacteria [24,25]. Little is known about direct effects of APOA1 in bone. In other models of bacterial infection, it shows anti-inflammatory properties, as it can bind and neutralize LPS [26]. It is plausible that this protein may play a similar role in bacterial infection within bone, but this has not been experimentally determined. Together, the filamin, alpha-actinin, and integrin proteins are involved in focal adhesion and extracellular interactions, functions that are crucial for maintaining cell signal transduction and intercellular communication [22]. Integrins function to establish cell-to-extra cellular matrix interactions [27], and link to the actin cytoskeleton through various proteins, including alpha-actinin and filamin [28], therefore it is not surprising that they are similarly regulated in BCO. These observed protein shifts may alter the ability of bone to maintain normal metabolic balance and impair the cellular response to stimuli (such as bacterial infection). ITGAV, in particular, has been shown to mediate S. aureus internalization into bone cells [29]. As we have previously shown that BCO in chicken can be a model for human osteomyelitis [20], further similarities between the two species may also prove mutually beneficial in identifying potential targets for detection and intervention. Indeed, several of these identified proteins have been implicated as either markers for human osteoporosis [19,30], or as targets for intervention in bone disorders [31]. What remains to be seen is whether they may function as markers for BCO in chicken. Further research into the correlation between bone and circulating proteins is warranted. Additionally, as activity of these protein nor the cause effect relationship between their expression and BCO cannot be determined by proteomics alone, targeted mechanistic studies are necessary.
Imaging for Early Identification of BCO
The sub-clinical nature of the BCO bone pathology creates issues for diagnosis and the use of behavioral traits for genetic selection. As chickens are a prey species, they may also mask signs of pain until it is severe [32], making gait scoring for lameness inadequate. Indeed, the standard system for identification and scoring of BCO severity (Figure 1) relies on necropsy. To compound the difficulty in assessment, subclinical incidence (presence of lesions without overt lameness) has also been documented [33]. Together, these aspects of BCO pathology and diagnosis have impacted its persistence in broilers; therefore, improved means of identifying and measuring BCO are of paramount importance in improving genetic selection and designing effective preventative measures and treatments.
Figure 1. Progression stage and scoring of proximal femoral (A-D) and tibial degeneration (E-H) leading to BCO in broilers. The severity of BCO lesions in tibia and femur is assessed by a scoring scheme on a scale of 0 to 2+. (A) Normal proximal femur with white cap of epiphyseal cartilage (e); (B) Normal proximal tibia cut to show the epiphysis (e) with the physis/growth plate (p) fully supported by struts of trabecular bone in the metaphyseal zone (m); (C) Femoral head separation (FHS: epiphyseolysis) with the epiphysis remaining in the socket when the femur was disarticulated, revealing the underlying surface of the growth plate or physis (p) and an early region of necrosis (n); (D) Proximal tibia cut to reveal bacterial sequestrae (s) and a necrotic void (nv) within the metaphysis; (E) Terminal femoral head necrosis in which the femoral epiphysis and part of the physis (p) remained attached to the acetabulum during disarticulation, revealing a large necrotic void (nv) within the metaphysis; (F-H) Secondary centers of ossification (*), bacterial infiltration and sequestrae (s), necrotic voids (nv), copious fibrinonecrotic exudate (fe) and microfractures below the growth plate (arrows) provide macroscopic evidence of bone damage associated with terminal osteomyelitis.
Imaging technologies, once prohibitive due to cost and time constraints, have increasing potential within the poultry industry, as technology advances and the cost of equipment decreases. Recently, we explored the use of two non-invasive measures to identify BCO in broiler chickens. Used frequently in human medicine for body composition [34,35] and bone density analyses [36], dual-energy x-ray absorptiometry (DXA) has more recently been validated, in a research setting, for similar whole-body measures in poultry [37,38]; however, research regarding imaging of individual body parts or specific locations is lacking. We have used a small, high-resolution DXA (iNSiGHT DXA, Scintica, London, ON, Canada) which utilizes a cone beam x-ray which passes through the sample, where the attenuation of the x-ray is recorded by the detector below the sample, and provides the composition in the x, y, and z plane, producing a 2D x-ray image. This technology has allowed the imaging and analysis of chicken leg bones, particularly the femoral head. Through the use of DXA, we were able to identify the presence of necrosis in femurs that would macroscopically be considered normal via current BCO scoring methodologies (Greene, et al, 2024, under review). These proximal femur heads had intact bone and articular cartilage caps, but with DXA scan, an area of hyposignal density was identified that, upon typical dissection, showed areas of necrosis within the metaphysis. These areas had the same appearance as that of bone scored 1 or 2 (Figure 1). Using DXA could help identify sub-clinical BCO, an important asset that is currently lacking for both production and scientific research. However, as the high-resolution DXA is relatively compact, there may be a limit to the size of the bird that can be imaged. Regardless, with additional longitudinal research, this technology and analysis could likely be employed to identify young birds with sub-clinical BCO and remove them from breeding programs early on.
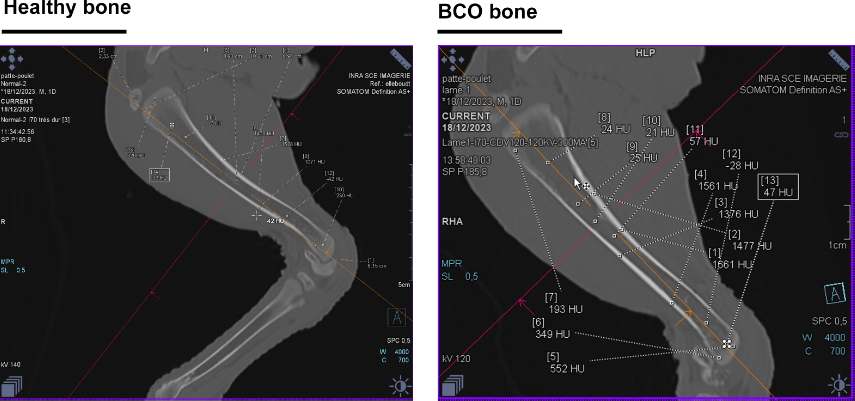
The use of computed tomography (CT) scan technology in poultry research is not new [39,40], but the manner in which it is analyzed and applied can be improved. We have recently ameliorated the time-lapse use and shown that chicken legs can be imaged via CT scan within 5 minutes (comprising installation, acquisition, and image generation), where differences in morphology and density can be visualized and quantified. Though BCO primarily affects the proximal femur and tibia, CT technology allows for monitoring of multiple bone parameters. For example, long bones that are more circular in cross-section are more resistant to torsion [41], a property that may impact the incidence of micro-fractures and clefts that allow BCO to develop. Indeed, in a pilot study comparing cross-sectional dimensions of tibia from 49-day old lame as compared to normal birds, the ratio of the long axis to the short axis was ~1.27 for the lame as compared to ~1.18 for the normal, indicating more elliptical bone in the lame birds (Figure 2). As this is based on a small observational pilot sample (n=4), this hypothesis should be tested with a fully designed experiment. Larger studies correlating bone shape and BCO incidence across different ages as well as breeds/strains of chicken could present the potential for a future tool for non-invasive diagnosis or early detection and identification of at-risk birds, as well as in generating a metric basis for genetic selection. Additionally, Hounsfield unit (HU) values across cortical bone, trabecular bone, and the bone marrow of broilers are distinct and in a similar range to that reported for bone of other species (Figure 3). Importantly, if this technology could be employed to observe the same birds longitudinally, it is possible that early necrosis events could be determined by monitoring decrease in HU values over time [42]. This would be of particular importance to breeding programs, as bone susceptibility could be directly assessed on the primary breeder, as opposed to on siblings, as is currently the case for BCO due to the terminal nature of diagnosis. Again, however, larger studies are necessary to correlate the values with BCO incidence/severity and potentially establish diagnostic ranges of values. Indeed, as it is the analysis proposed here that is novel, retroactive analyses could be performed on data collected from CT scans of chicken bone to date. Currently, the largest drawback to the use of CT in poultry is cost of the machine and its operation. At this time, it is likely limited to a research setting, but that does not diminish the information on BCO and bone health that may be obtained.
Figure 2. Representative computed tomography (CT) images of cross-sections of tibia from (A) normal and (B) lame broilers. White cross-hairs indicate the length of the long- and short-axis of the tibia.
Figure 3. Representative computed tomography (CT) images of longitudinal sections of tibia from (A) normal and (B) lame broilers. HU measured across different areas of bone are indicated by dotted lines.
Conclusion
Lameness in broilers poses a critical welfare, health, and economic challenge to the poultry industry that must be solved. Genetic, nutritional, and managerial strategies to mitigate the impact of lameness have been employed, but are only partially efficacious, as current measures rely on end-point or terminal diagnoses. In order to prevent or delay the onset of BCO and lameness, early diagnosis through non-invasive means is of utmost importance.
Conflict of Interest
The authors declare no conflicts of interest.
Funding
These studies were supported by grants from the University of Arkansas Chancellor’s Innovation Funds (003226-00001A to SD), Cobb-Vantress Inc. (FY2020 to SD), and USDA-AFRI Sustainable Agriculture Systems (2020-69012-31823 to SD).
References
2. Vasdal G, Moe R, De Jong I, Granquist E. The relationship between measures of fear of humans and lameness in broiler chicken flocks. Animal. 2018;12(2):334-9.
3. Pines M, Reshef R. Poultry bone development and bone disorders. In: Scanes CG, editor. Sturkie's Avian Physiology (Sixth Edition). San Diego: Academic Press; 2015. p. 367-77.
4. Opengart K, Bilgili S, Warren G, Baker K, Moore J, Dougherty S. Incidence, severity, and relationship of broiler footpad lesions and gait scores of market-age broilers raised under commercial conditions in the southeastern United States. J Appl Poult Res. 2018;27(3):424-32.
5. Granquist EG, Vasdal G, De Jong IC, Moe RO. Lameness and its relationship with health and production measures in broiler chickens. Animal. 2019;13(10):2365-72.
6. Jiang T, Mandal RK, Wideman RF, Jr., Khatiwara A, Pevzner I, Min Kwon Y. Molecular survey of bacterial communities associated with bacterial chondronecrosis with osteomyelitis (BCO) in broilers. PloS One. 2015;10(4):e0124403.
7. Mandal RK, Jiang T, Al-Rubaye AA, Rhoads DD, Wideman RF, Zhao J, et al. An investigation into blood microbiota and its potential association with Bacterial Chondronecrosis with Osteomyelitis (BCO) in broilers. Sci Rep. 2016;6:25882.
8. Wideman RF. Bacterial chondronecrosis with osteomyelitis and lameness in broilers: a review. Poult Sci. 2016;95(2):325-44.
9. Cook J, Greene ES, Ramser A, Mullenix G, Dridi JS, Liyanage R, et al. Comparative- and network-based proteomic analysis of bacterial chondronecrosis with osteomyelitis lesions in broiler’s proximal tibiae identifies new molecular signatures of lameness. Sci Rep. 2023;13(1):5947.
10. Guo Q, Kang H, Wang J, Dong Y, Peng R, Zhao H, et al. Inhibition of ACLY leads to suppression of osteoclast differentiation and function via regulation of histone acetylation. J Bone Miner Res. 2021;36(10):2065-80.
11. Da W, Jiang W, Tao L. ROS/MMP-9 mediated CS degradation in BMSC inhibits citric acid metabolism participating in the dual regulation of bone remodelling. Cell Death Disc. 2024;10(1):77.
12. Shares BH, Busch M, White N, Shum L, Eliseev RA. Active mitochondria support osteogenic differentiation by stimulating β-catenin acetylation. J Biol Chem. 2018;293(41):16019-27.
13. Ferver A, Greene E, Wideman R, Dridi S. Evidence of mitochondrial dysfunction in bacterial chondronecrosis with osteomyelitis–affected broilers. Front Vet Sci. 2021;8:640901.
14. Mendelsohn DH, Niedermair T, Walter N, Alt V, Rupp M, Brochhausen C. Ultrastructural evidence of mitochondrial dysfunction in osteomyelitis patients. Int J Mol Sci. 2023;24(6):5709.
15. Vermeren M, Lyraki R, Wani S, Airik R, Albagha O, Mort R, et al. Osteoclast stimulation factor 1 (Ostf1) KNOCKOUT increases trabecular bone mass in mice. Mamm Genome. 2017;28:498-514.
16. Wideman RF, Prisby RD. Bone circulatory disturbances in the development of spontaneous bacterial chondronecrosis with osteomyelitis: a translational model for the pathogenesis of femoral head necrosis. Front Endocrinol (Lausanne). 2012;3:183.
17. Caserta S, Stagno F, Gangemi S, Allegra A. Highlights on the effects of non-coding RNAs in the osteonecrosis of the jaw. Int J Mol Sci. 2024;25(3):1598.
18. Mohd Yunus SS, Soh HY, Abdul Rahman M, Peng X, Guo C, Ramli R. MicroRNA in medication related osteonecrosis of the jaw: a review. Front Physiol. 2023;14:1021429.
19. Yang C, Ren J, Li B, Jin C, Ma C, Cheng C, et al. Identification of gene biomarkers in patients with postmenopausal osteoporosis. Mol Med Rep. 2019;19(2):1065-73.
20. Greene E, Flees J, Dhamad A, Alrubaye A, Hennigan S, Pleimann J, et al. Double-stranded RNA is a novel molecular target in osteomyelitis pathogenesis: A translational avian model for human bacterial chondronecrosis with osteomyelitis. Am J Pathol. 2019;189(10):2077-89.
21. Zhao G, Liu Y, Zheng Y, An M, Zhang J, Zhang J, et al. Exploring molecular mechanisms of intra-articular changes in osteonecrosis of femoral head using DIA proteomics and bioinformatics. J Orth Surg Res. 2024;19(1):13.
22. Huang J, Hu F, Alolga RN, Yin X. Comprehensive proteomic characterization of articular cartilage from femoral head necrosis patients. Front Biosci. 2022;27(6):181.
23. Bakker W, Melgert B, Faas M. Hemopexin: anti-inflammatory, pro-inflammatory, or both? J Leukocyte Biol. 2010;87(1):1-2.
24. Pishchany G, Haley KP, Skaar EP. Staphylococcus aureus growth using human hemoglobin as an iron source. JoVE. 2013;72:e50072.
25. Bornside GH, Bouis Jr PJ, Cohn Jr I. Hemoglobin and Escherichia coli, a lethal intraperitoneal combination. J Bacteriol. 1968;95(5):1567-71.
26. Li Y, Dong J-B, Wu M-P. Human ApoA-I overexpression diminishes LPS-induced systemic inflammation and multiple organ damage in mice. Eur J Pharmacol. 2008;590(1-3):417-22.
27. Hynes RO. Integrins: bidirectional, allosteric signaling machines. Cell. 2002;110(6):673-87.
28. Visser R, Rico-Llanos GA, Pulkkinen H, Becerra J. Peptides for bone tissue engineering. J Controll Release. 2016;244:122-35.
29. Foster TJ, Geoghegan JA, Ganesh VK, Höök M. Adhesion, invasion and evasion: the many functions of the surface proteins of Staphylococcus aureus. Nat Rev Microbiol. 2014;12(1):49-62.
30. Sun X, Wu X. Association of apolipoprotein A1 with osteoporosis: a cross-sectional study. BMC Musculoskel Dis. 2023;24(1):157.
31. Marie PJ. Targeting integrins to promote bone formation and repair. Nat Rev Endocrinol. 2013;9(5):288-95.
32. Livingston A. Physiological basis for pain perception in animals. Vet Anaesth Analg. 1994;21(2):73-7.
33. Wideman RF, Hamal KR, Stark JM, Blankenship J, Lester H, Mitchell KN, et al. A wire-flooring model for inducing lameness in broilers: evaluation of probiotics as a prophylactic treatment. Poult Sci. 2012;91(4):870-83.
34. Guss CE, McAllister A, Gordon CM. DXA in children and adolescents. J Clin Densitom. 2021;24(1):28-35.
35. Albanese CV, Diessel E, Genant HK. Clinical applications of body composition measurements using DXA. J Clin Densitom. 2003;6(2):75-85.
36. Goel H, Binkley N, Boggild M, Chan WP, Leslie WD, McCloskey E, et al. Clinical use of trabecular bone score: The 2023 ISCD official positions. J Clin Densitom. 2024;27(1):101452.
37. Swennen Q, Janssens GP, Geers R, Decuypere E, Buyse J. Validation of dual-energy x-ray absorptiometry for determining in vivo body composition of chickens. Poult Sci. 2004;83(8):1348-57.
38. Schallier S, Li C, Lesuisse J, Janssens GPJ, Everaert N, Buyse J. Dual-energy X-ray absorptiometry is a reliable non-invasive technique for determining whole body composition of chickens. Poult Sci. 2019;98(6):2652-61.
39. Casey-Trott T, Heerkens JLT, Petrik M, Regmi P, Schrader L, Toscano MJ, et al. Methods for assessment of keel bone damage in poultry. Poult Sci. 2015;94(10):2339-50.
40. Scholz AM, Bünger L, Kongsro J, Baulain U, Mitchell AD. Non-invasive methods for the determination of body and carcass composition in livestock: dual-energy X-ray absorptiometry, computed tomography, magnetic resonance imaging and ultrasound: invited review. Animal. 2015;9(7):1250-64.
41. Dumont ER. Bone density and the lightweight skeletons of birds. Proc Biol Sci. 2010;277(1691):2193-8.
42. Kubon S, Lawson McLean A, Eckardt N, Neumeister A, Dinc N, Senft C, et al. Early detection of aseptic bone necrosis post-cranioplasty: A retrospective CT analysis using Hounsfield units. J Cranio Maxill Surg. 2024;S1010-5182(24)00048-9