Abstract
Background: Cutis verticis gyrata (CVG) is characterized by excessive skin folding on the scalp and can be classified into primary essential, primary nonessential, and secondary. While most cases are asymptomatic, some patients seek treatment due to cosmetic concerns. The primary objective of this case study was to evaluate the efficacy of intradermal hyaluronidase for cutis verticis gyrata of the scalp in the presented patient.
Methods: The patient underwent four injection sessions over the course of 20 weeks, separated by 6-8 weeks. The hyaluronidase solution was injected directly into the folds of concern in the parietal and occipital scalp. Baseline and follow up photographs were taken to monitor progress as shown in provided figures.
Results: At the end of the injection treatments, the patient received a total of 1950 U of hyaluronidase distributed into the areas of concern. While the patient did report subjective improvement, image comparison from baseline to follow up evaluation after treatment showed mild augmentation of patient’s grooves and folds after 20 weeks of treatment.
Conclusion: Cutis verticis gyrata represents a versatile disease that may carry systemic consequences but that could also be primary essential to the skin. While the cosmetic nature is difficult to treat, intradermal hyaluronidase offers a safe alternative to surgical intervention. Further studies may be warranted to highlight its potential role in the treatment of CVG.
Keywords
Cutis verticis gyrata, Scalp folding, Intradermal, Hyaluronidase, Cosmetic, Injection, Aesthetic
Introduction
Cutis verticis gyrata (CVG) is a rare condition, characterized by hypertrophic skin folds and grooves that lead to a cerebriform appearance of the scalp. CVG is also referred to as paquidermia verticis gyrata, cutis verticis plicata, and “bulldog” scalp syndrome [1]. CVG can be classified into primary and secondary, with the primary form being further classified into primary essential and primary nonessential, with the latter also known as cutis verticis gyrata-intellectual disability [1]. While primary nonessential CVG is associated with neurologic and ophthalmologic pathologies, primary essential CVG lacks any comorbidities [2]. Secondary CVG (as the name implies) is a result of an underlying medical condition that leads to distortion in scalp structure [2].
Although the etiology of primary essential CVG is unclear, hormonal imbalance has been hypothesized as one of the potential causes due to male predominance and postpubertal onset [1]. Pathology of the disorder ranges from normal skin to thickened connective tissue with hyperplasia of adnexal structures and an increase in collagen and trapping of glands [1]. While primary essential CVG is benign, it can be associated with discomfort and pruritus, likely stemming from accumulation of cutaneous secretions in the grooves of the scalp [3]. Quality of life can be affected due to cosmetic concerns. CVG typically presents as symmetric, redundant folds on the scalp with deep furrows and convolutions most commonly running from anterior to posterior [1]. The primary essential form is a diagnosis of exclusion, making ruling out underlying systemic disease of utmost priority.
Standard treatments for CVG include addressing any underlying disorders or comorbidities (as is the case with primary nonessential and secondary CVG), local symptomatic care and scalp hygiene (to lessen associated discomfort), as well as surgical resection and grafting. The type of surgical repair depends on the size, location of the folds and patient preference [1]. Studies have shown that partial resection with immediate closure is the safest and most effective technique to correct CVG surgically. There is sparse evidence in the literature on the use of hyaluronidase injections in CVG, but those patients who have undergone this treatment modality have had substantial results [3].
Case Presentation
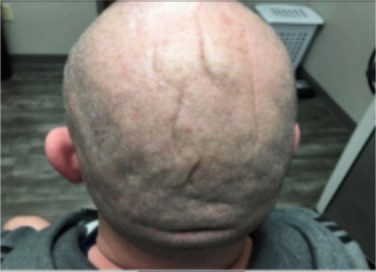
We present a case of primary essential CVG in a 40 year old male and no significant medical history. Onset was noted about 15 years ago, however the patient was being seen for laser treatment of telangiectasias prior and mentioned the dissatisfactory appearance of his scalp. Physical exam noted prominent skin folds and grooves primarily affecting the parietal and occipital scalp (Figure 1). There was no history of trauma or scalp treatments. Primary nonessential and secondary CVG were ruled out via patient-reported medical history and a symptom-based review of systems, as the patient is healthy and asymptomatic. The patient was counseled on his treatment options and was made aware that surgery is the only current accepted treatment. However, the patient was informed of the potentially beneficial option to attempt hyaluronidase injections into the scalp to improve the redundant grooves and folds that can be cosmetically disfiguring. The patient opted for treatment with hyaluronidase injections, after giving informed consent on the risk of contour defects and dimpling of the skin.
Figure 1. Baseline.
Methods
Prior to treatment, risks and benefits of the proposed therapy were discussed. Per literature review, there is minor risk of anaphylaxis to hyaluronidase thus appropriate precautions were available at the clinic on injection day. Prior to the first injection, patient was advised to avoid anti-inflammatory medications (NSAIDs, aspirin), anti-histamines, mast-cell stabilizers, anti-oxidants, and Vitamin C as these may antagonize hyaluronidase. During the patient’s first treatment session, 1 mL of hyaluronidase (150 USP/mL) was mixed with 1 mL of bacteriostatic saline to provide more volume to inject into the affected areas. A total volume of 2 mL was injected evenly throughout the occipital and parietal scalp, where the majority of the unsatisfactory grooves resided. The mixture was injected in a longitudinal fashion into the folds along either side of the groove. Patient followed up 6 weeks later for a second treatment. In light that the patient tolerated the diluted hyaluronidase well with no complications, it was then decided to inject a total of 4 mL of undiluted hyaluronidase (150 USP/mL), distributed evenly into the folds of concern. This regimen was repeated six weeks later, and a final time when the patient followed up for a fourth treatment 8 weeks thereafter, using the same concentration and volume of undiluted hyaluronidase (150 USP/mL). Baseline photographs were taken (Figure 1) and were repeated at each follow-up visit to track progress. Comparisons were made using the posterior view which was able to capture all of the concerning folds and grooves.
Results
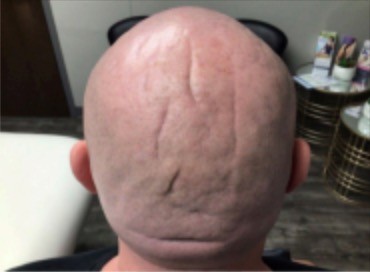
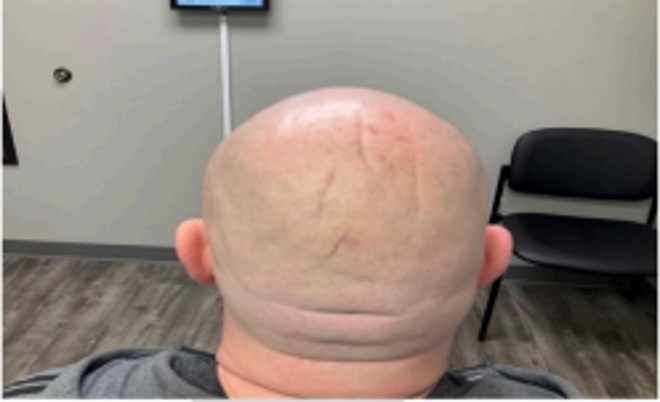
After four injection sessions, patient electively decided to discontinue. Patient received a total of 1,950 units of hyaluronidase distributed evenly into the folds adjacent to concerning grooves over a period of 20 weeks. Patient saw little to no improvement after first injection of only 150 IU of hyaluronidase (Figure 2). After the second treatment utilizing increased amounts of hyaluronidase, patient reported mild subjective improvement (Figure 3). Further photographs are included demonstrating progress after three injections, and then five months later when the patient returned for routine follow-up (Figures 4 and 5). The final photograph in Figure 5 marks about 11 months since the start of the injections. Patient was shown the before and after photographs and reported mild improvement in overall appearance. From the viewers’ standpoint, the therapy provided minimal improvement to grooves and folds of concern.
Figure 2. Post Treatment #1.
Figure 3. Post Treatment #2.
Figure 4. Post Treatment #3.
Figure 5. Six months after treatment #4.
Discussion
CVG is a condition of the scalp characterized by disfiguring grooves and folds that resemble the gyri of the brain. There are primary and secondary variants, based on if there is associated underlying disease. Primary CVG is further classified into essential and non-essential subtypes, with non-essential associated with neuropsychiatric and ophthalmological abnormalities. Secondary CVG has been associated with cerebriform intradermal nevus, acromegaly, syphilis, scleromyxedema, amyloidosis, diabetes mellitus, malignancy, intracerebral aneurysm, anabolic steroid use, and connective tissue disorders [1]. Despite the frequently asymptomatic nature of CVG, patients may have a significant degree of discomfort accompanied by odor, pruritus, irritation and uncomfortable sensations in the area [1]. While the hair generally remains intact, there may be some hair texture changes in certain patients. Of utmost importance in this condition is to rule out an underlying cause that may reveal a primary nonessential or secondary form [1]. While surgical intervention might be the best treatment option for those with major psychological implications or infectious complications, patients may initially opt for a less invasive alternative. Welborn et al. first presented improvement of CVG with hyaluronidase injections, resulting in minimal side effects [4]. Local injections of hyaluronidase can result in side effects such as injection site reactions and pruritus. In our patient, no side effects occurred with the hyaluronidase injections [3].
Hyaluronidase is a natural non-ATP requiring enzyme that catalyzes the degradation of hyaluronan, or hyaluronic acid, the main component of connective tissue [5]. Hyaluronic acid is a substance that restricts fluid movement and reduces the volume of injectable drugs into the subcutaneous space [5]. Hyaluronidase temporarily and reversibly depolymerizes hyaluronan by cleaving its glycosidic bonds, allowing fluids to better travel through the extracellular matrix [5]. The primary use of hyaluronidase in injectable practices is its capacity to degrade hyaluronic acid filler to correct complications following injection [6]. Decreasing hyaluronic acid in the area of skin overgrowth may lead to decreased skin elasticity in those areas [4]. This breakdown process is the one that may be responsible for clinical improvement of the scalp after subcutaneous injection of hyaluronidase for CVG patients [6].
Besides supportive care and hygiene protocols, hyaluronidase is the only reported non-invasive option available for patients with CVG according to current medical literature. While literature on intradermal hyaluronidase for CVG remains sparse, the available reports have shown cosmetic improvement in treated patients [3,4]. Our patient’s experience of mild improvement aligns with these prior findings, further supporting hyaluronidase as a safe and potentially effective alternative to surgical intervention. However, variability in patient response has been noted, and in our case, only minimal clinical improvement was observed, underscoring the need for additional research. A limitation of our study was the lack of laboratory and imaging studies to definitively exclude secondary CVG, relying instead on patient history and review of systems.
Conclusion
Primary essential CVG is a rare skin disorder that may affect patient quality of life due to the disfiguring appearance of the scalp. The purpose of this case report is to explore the potential use of hyaluronidase injections for the treatment of CVG. Although hyaluronidase injections remain a valid alternative to the more invasive approach of surgical intervention, this treatment method alone may not provide satisfactory results depending on the patient’s expectations.
Acknowledgements
This research was supported (in whole or in part) by HCA Healthcare and/or an HCA Healthcare affiliated entity. The views expressed in this publication represent those of the author(s) and do not necessarily represent the official views of HCA Healthcare or any of its affiliated entities.
Conflicts of Interest
None disclosed.
Author Contributions
All authors contributed to the conception and design of the work, acquisition and interpretation of data, and drafting or revising of the manuscript. All authors approved the final version of the manuscript and agree to be accountable for all aspects of the work.
References
2. Yang JJ, Sano DT, Martins SR, Tebcherani AJ, Sanchez AP. Primary essential cutis verticis gyrata - case report. An Bras Dermatol. 2014 Mar-Apr;89(2):326-8.
3. Patrick C, Maria H E, Mohammed M E. Hyaloronidase as a treatment for cutis verticis gyrata. Plastic Surgery Case Studies. 2021 Jun 18;7:2513826X211022221.
4. Welborn M, Dallo C, Altmeyer M. Cutis verticis gyrata improved with injected hyaluronidase treatments. Dermatol Arch. 2019;3(1):74-6.
5. King M, Convery C, Davies E. This month's guideline: The Use of Hyaluronidase in Aesthetic Practice (v2.4). J Clin Aesthet Dermatol. 2018 Jun;11(6):E61-8.
6. Gilson RL, Zafar Gondal A. Hyaluronidase. [Updated 2021 Jun 8]. StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing