Abstract
Pregabalin is an antiepileptic and analgesic drug, commercialized under the name of Lyrica and other names, generally used to treat neuropathic pain. The determination of pregabalin was quantified in tissues and fluids samples by using Gas chromatography coupled with mass spectrometry, isolation and precipitation protein by Ammonium Sulfate method. The limit of detection (LOD) is 200 ng and the limit of quantification (LOQ) in is 400 ng. Ibuprofen was used as internal standard, this methods has been applied in two cases of pregabalin.
Objective: To determine pregabalin in two cases study tissues and blood by using method isolation and precipitation of proteins by Ammonium Sulfate extraction, quantified by GC-MS.
Keywords
Pregabalin, GC-MS, Ammonium Sulfate, Human Tissues and Blood
Introduction
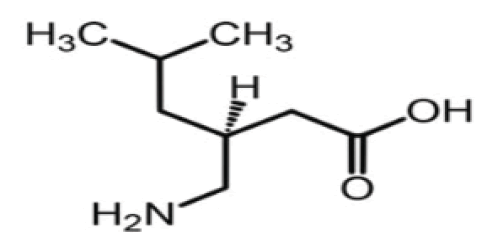
Pregabalin is the (S)-(+)-isomer of 3-isobutyl-GABA and it is available as hard capsules containing doses ranging from 25 to 300mg, famous trade name is Lyrica among others names, Freely soluble in water and both basic and acidic solutions Figure 1.
Figure 1: Structure of S-enantiomer of pregabalin.
Pregabalin was approved for medical use in the United States in 2004 [1]. It was developed as a successor togabapentin. It is available as a generic medication in a number of countries but not the United States as of 2018 [2].
Pregabalin is structurally analogous to the inhibitory neurotransmitter gamma-aminobutyric acid (GABA). Pregabalin acts as a modulator of voltage-gated calcium channels in the CNS.
The molecule exerts its effect on voltage gated calcium channels by binding presynaptically to the alpha-2-delta subunit. Since voltage gated calcium channels are widely distributed throughout the central nervous system, pregabalin is able to modulate the release of several excitatory neurotransmitters such as glutamate, substance-P, norepinephrine and calcitonin gene related peptide. This results in inhibition of overexcited neurons, which ultimately returns them to a normal state of function [3,4].
It has a beneficial effect on sleep and sleep architecture, characterized by the enhancement of slowwave sleep [5].
Pregabalin is mainly renally excreted with 98% of the drug eliminated unchanged in the urine, while less than 0.1% of the drug is eliminated through the fecal route. Further, based on preclinical studies, pregabalin does not appear to undergo racemization to the R enantiomer in the body [6].
The N-methylated derivative of pregabalin, the major metabolite of pregabalin found in urine, the Cytochrome P450 no activity and in interaction is little identify information.
Pregabalin is absorbed from the intestines by an active transport process mediated via the large neutral amino acid transporter 1 (LAT1, SLC7A5), a transporter for amino acids such as L-leucine and L-phenylalanine [7-9].
Materials and Methods
Instrumentation
1. Vortex(FALC).
2. Gas chromatography (Agilent 6890) coupled to a mass spectrometry detector (Agilent5973), Column of GC-MS is Agilent HP-5-MS; 0.25mm x 60m x 0.25um capillary, 60m x 250 um nominal.
3. Helium gas type and pressure: 39.8 psi and flow 1.5ml/min, the average velocity: 32 cm/sec.
4. Standard and reagents.
Pregabalin supplied from Epico- Egypt Company, Ammonium sulfate, HCL, Ethyl acetate-Sigma Company.
Procedure
The extract converted to alkaline by ammonia then shaking with ethyl acetate solvent was used then dried under nitrogen evaporator. Ibuprofen was used as internal standard. The samples injected 2uL of each samples into GC-MS by using methanol HPLC grade.
Result and Discussions
The extraction of drugs from tissues requires the tissue matrix be broken down to release drugs into accessible solvent of extraction. This can be achieved by cutting tissues and homogenization. Ammonium Sulfate method is direct solvent extraction are used for protein precipitation.
The chosen solvent should ideally extract as much of the target analyte as possible excellent solubility. Protein Precipitation is the process in which protein is separated from tissues or any extra contaminants that may be mixed with suitable solvents and pH.
The most popular ones is Salt Induced Precipitation (“Salting Out”) with ammonium sulfate. The first methods of “salting out” a protein can occur with a number of different types of neutral salts but ammonium sulfate is preferred salt because it is high on the Hofmeister series and has a high solubility rate. Protein interacts with the salt as opposed to the water, which leads to less interaction between the water and the protein’s solvent layer, which in turn leads to more hydrophobic patches being exposed and to encourage those patches to interact with one another. This leads the proteins to aggregate and precipitate.
Validation studies
1. Linearity of the method was constructed for pregabalin reference standard solutions by plotting the concentrations of the compound versus peak area response. The linearity was evaluated by linear regression analysis, which was calculated by the least square regression method [10].
The parameters LOD and LOQ were determined on the basis of response and slope of the regression equation.
2. Limit Of Detection (LOD): several calibrators were injected in different concentrations (1500 ng, 1000 ng, 500 ng, 200 ng, 100ng) a peak at 200 ng concentration fulfilled the criteria of acceptance for LOD of liquid-liquid extraction concentration as peak noise ratio ≥ 3:1(determined by peak height).
3. Selectivity: 4 blank samples were prepared and injected and no significant signals were detected in the same retention time of analyte.
4. Sensitivity: several calibrators were injected in different concentrations (1500 ng, 1000 ng, 500 ng, 200 ng, 100 ng) the response was found to be directly proportional to concentration.
5. Limitation: samples with concentration less than 400 ng in matrix liquid-liquid extraction did not show signal with accepting the method.
In this study determined and quantified pregabalin methods and validated, and applied by extraction pregabalin from two cases the first old man 43years his wife gives pregabalin drugs to sleep after that she die him (Asphyxia), pregabalin was extracted from tissues, the second case is youth girl 27 years abuse pregabalin drug (Figures 2 & 3).
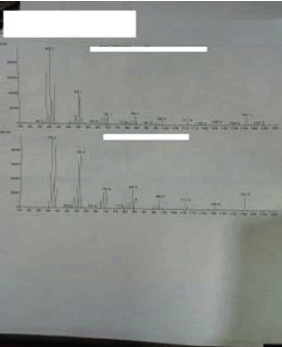
Figure 2: Ion Fragmentation of Pregabalin m/z (41, 55, 70, 84, 98, 110,141).
Figure 3: Ion Fragmentation of Pregabalin m/z (41, 55, 70, 84, 98, 110, 141).
Conclusion
In conclusion, the proposed GC-MS method a simple, accurate and reproducible method for routine analysis Pregabalin in blood and tissues such as liver.
References
2. Generic Lyrica.
3. Rajappa GC, Vig S, Bevanaguddaiah Y, Anadaswamy TC. Efficacy of pregabalin as premedication for post-operative analgesia in vaginal hysterectomy. Anesthesiology and Pain Medicine. 2016 Jun; 6(3).
4. Gajraj NM. Pregabalin: its pharmacology and use in pain management. Anesthesia & Analgesia. 2007 Dec 1; 105(6):1805-15.
5. Bandelow B, Wedekind D, Leon T. Pregabalin for the treatment of generalized anxiety disorder: a novel pharmacologic intervention. Expert Review of Neurotherapeutics. 2007 Jul 1; 7(7):769-81.
6. Rodríguez J, Castaneda G, Munoz L. Direct determination of pregabalin in human urine by nonaqueous CE-TOFMS. Electrophoresis. 2013 May; 34(9-10):1429-36.
7. Calandre EP, Rico-Villademoros F, Slim M. Alpha2delta ligands, gabapentin, pregabalin and mirogabalin: a review of their clinical pharmacology and therapeutic use. Expert review of neurotherapeutics. 2016 Nov 1; 16(11):1263-77.
8. Sills GJ. The mechanisms of action of gabapentin and pregabalin. Current opinion in pharmacology. 2006 Feb 1; 6(1):108-13.
9. Dickens D, Webb SD, Antonyuk S, Giannoudis A, Owen A, Rädisch S, et al. Transport of gabapentin by LAT1 (SLC7A5). Biochemical pharmacology. 2013 Jun 1; 85(11):1672-83.
10. Daraghmeh N, Al-Omari M, Badwan AA, Jaber AM. Determinaton of sildenafil citrate and related substances in the commercial products and tablet dosage form using HPLC. Journal of pharmaceutical and biomedicalanalysis. 2001 Jun 1; 25(3-4):483-92.