Commentary
Salt sensitive hypertension is a major risk factor for stroke, heart failure, and end-stage renal disease [1]. The development of salt sensitive hypertension is involved in genetic and environmental factors. Excessive dietary salt intake is known as a main risk factor and one of the most important environmental determinants, exacerbates the salt sensitive hypertension by a renal redox metabolism [2,3]. The elevated blood pressure in the model of human salt sensitive forms of hypertension, Dahl slat sensitive (SS) rats, is anticipated by the metabolic imbalance of nitric oxide (NO) and reactive oxygen species (ROS) in the renal tissues [4-6], which the activity of fumarate dehydrogenase (FH) is considered a key factor in regulating the metabolic imbalance above.
FH, a key tricarboxylic acid (TCA) cycle enzyme, could effectively balance the NO and ROS content at the metabolic level is confirmed in the kidney, and improve the salt sensitive hypertension in SS rat strain [3,7]. In the previous proteomic analysis, an expression difference in FH was identified in the kidney of SS rat compared with salt-insensitive consomic SS-13BN (BN) rats [6]. Though the FH protein abundance is higher in the kidney of SS rats than it in BN rats, the expression difference in FH between two rat strains leads to a significant decrease in total FH activity in SS rat. Interestingly, high salt diet also weakens the total FH activity compared with the normal salt diet in SS rats [8]. The role of FH activity in regulating the metabolic imbalance of NO and ROS is further explored in SS rats.
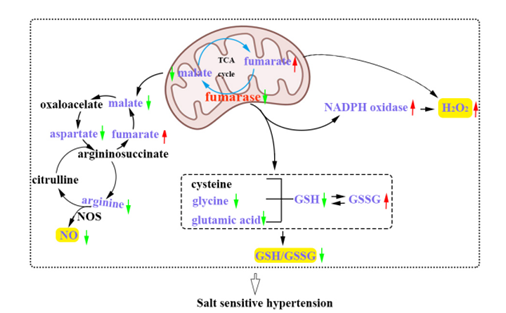
On the one hand, insufficient FH activity in kidney is confirmed to contribute to hypertension by increasing ROS level (Figure 1). Changes in activity leads to the changes in the level of substrate metabolic intermediaries, which in turn influence blood pressure regulation. In mitochondria, FH catalyzes the production of malate from fumarate in TCA cycle, the insufficiency of FH activity resulted in the excessive accumulation of fumarate in SS rat kidney [6]. The accumulation of fumarate in SS rat kidney elevated the blood pressure [6,7]. Study reported that dimethyl fumarate can ameliorate pulmonary hypertension by reducing oxidative stress damage and inflammation [9], and fumarate attenuated hypertension, renal injury and improved the redox state of the kidney in DOCA/salt hypertension by mechanisms involving selective reduction of L-arginine metabolism [10]. In our previous study, the mechanism of FH in regulating blood pressure in SS rats may be not identical. Administration of exogenous fumarate or its precursor contributed to salt sensitive hypertension by increasing the levels of ROS and H2O2, and this result has been confirmed in SS rats and the HK-2 cells [6,7]. Supplementation of exogenous fumarate may influence shared biological mechanisms in the study above, as both of them alter fumarate levels, but are limited in the magnitude, duration, and source (exogenous vs. endogenous) of the manipulations, the response in metabolic flux and signaling mechanisms may be different.
On the contrary, supplementation of malate significantly decreased the ROS level in the renal tissues of SS rats [7]. Importantly, proteomic analysis indicated that FH knockdown increased the NADPH oxidase (NOX) expression in HK-2 cells [11]. The NOX was the major source of excess superoxide in the renal medulla of SS rats, and its activity was also increased, suggesting that FH knockdown increased the NOX activity to participate in the development of salt sensitive hypertension. It is remarkably, however, that NOX may derive ROS through reducing FH expression and activity, increasing fumarate in mouse glomeruli [12]. Kristie reported that while administrating fumarase siRNA into the renal medullary interstitial in the Sprague-Dawley rats on the high-salt diet, the level of H2O2 increased and the blood pressure significantly increased [13]. However, overexpressing fumarase on the background of the SS rat (named as TgFh1 transgenic rats) decreased H2O2, finally attenuated the development of hypertension [13], which was a favorable evidence that FH is involved in the regulation of ROS in the development of salt sensitive hypertension.
In addition, FH activity was also confirmed to associate with antioxidant system, including antioxidant enzymes and small molecular weight antioxidants. Insufficient FH activity weakened glutathione metabolism in FH knockdown HK-2 cells [7]. The GSH synthetic substrates, including glutamate and glycine, significantly decreased along with the FH knockdown in HK-2 cell, which resulted in the GSH content decreased even the NADPH content increased compared with the normal control cells. The decreased GSH/GSSG ratio due to the decreased GSH content caused by insufficient FH activity meant a decreased antioxidant capacity. However, conflicting results showed that activities of antioxidant molecules, such as Glucose-6-phosphate 1-dehydrogenase, Glucose-6-phosphate 1-dehydrogenase, Glutathione reductase, Glutathione S-transferase and Glutathione peroxidase, are increased in FH knockdown HK-2 cells [7], which was thought to be a compensatory response process to maintain the normal metabolism. These findings from previous studies suggest that FH exerts antihypertensive function through regulating the redox metabolism (Figure 1).
On the other hand, FH-related metabolites regulate the blood pressure through changing the NO content, especially the NO synthesis substrates (Figure 1). As a potent vasodilator, NO is synthesized as a byproduct in the conversion of L-arginine to L-citrulline, a major intracellular source of cellular NO, which is catalyzed by nitric oxide synthase (NOS) [14]. Though FH is not directly involved in NO synthesis, the production of NO can be limited by the capacity of the kidney to regenerate L-arginine through the citrulline-NO pathway in the kidney. Study has showed that the levels of L-arginine and citrulline in the renal tissues of SS rats significantly decreased compared with BN rats [5]. Many studies also have showed that the defectiveness of L-arginine-NO pathway is closely linked to NO deficiency [15,16]. FH-related metabolites, malate, was converted to oxaloacetate, which can be converted to aspartate. The insufficiency of FH activity resulted in the decreased malate in the kidney of SS rats, which meant that the levels of L-arginine and aspartate both decreased due to the decreased level of malate. This result was further confirmed in HK-2 and HUVECs cells after the FH was knockdown [5]. The reduction of L-arginine and aspartate, the key NO synthetic intermediates in arginine-citrulline-NO pathway, suggested the NO content decreased and final contributed to hypertension [17]. However, the arginine-citrulline-NO pathway was restored by the supplementation of malate or aspartate in the SS rats. Malate supplementation in SS rats attenuated salt sensitive hypertension [4,5]. The levels of L-arginine and aspartate significantly increased in renal tissues while supplementing the malate, and final increased the NO content [5], this result was also confirmed after aspartate supplementation in SS rats. These results showed that FH promoted NO production by the malate-arginine-citrulline-NO pathway (Figure 1).
In SS rats, the thick ascending limb eNOS is the major source of renal NO, eNOS dysfunction can result in hypertension [18]. However, FH overexpression in SS rats with eNOS haploinsufficiency effectively blunt the development of hypertension through restoring malate-arginine-citrulline-NO pathway [19]. FH overexpression increased the level of L-arginine and L-arginine/citrulline ratio in the renal outer medulla of SS rats with the eNOS heterozygous mutation [19]. These findings suggest that the insufficiency of FH activity in SS rats may contribute to the development of salt sensitive hypertension by weakening the malate-arginine-citrulline-NO pathway.
In conclusion, the current mechanism of FH in regulating salt sensitive hypertension is partly elucidated, which is involving in metabolic regulation of NO and ROS. However, other mechanisms by which FH participates in the development salt-sensitive hypertension need to be further explored.
Figure 1: Metabolic imbalance of nitric oxide and reactive oxygen species due to insufficient fumarase activity contributes to the development of salt sensitive hypertension in the kidney of SS rats; GSH, Reduced Glutathione; GSSG, Oxidized Glutathione; NOS, Nitric Oxide Synthase.
Acknowledgements
This study was supported by the National Natural Science Foundation of China (Grant Nos. 82070751).
Disclosure
The authors declare that they have no conflicts of interest.
References
2. Zheng X, Zhao X, Jin Y, Zhou L, Yang P, Ahmad H, et al. High salt diet contributes to hypertension by weakening the medullary tricarboxylic acid cycle and antioxidant system in Dahl salt-sensitive rats. Biochimie. 2021 Feb 1;181:154-61.
3. Tian Z, Liang M. Renal metabolism and hypertension. Nature Communications. 2021 Feb 11;12(1):1-2.
4. Zheng X, Li X, Chen M, Yang P, Zhao X, Zeng L, et al. The protective role of hawthorn fruit extract against high salt-induced hypertension in Dahl salt-sensitive rats: impact on oxidative stress and metabolic patterns. Food & Function. 2019;10(2):849-58.
5. Hou E, Sun N, Zhang F, Zhao C, Usa K, Liang M, et al. Malate and aspartate increase L-arginine and nitric oxide and attenuate hypertension. Cell Reports. 2017 May 23;19(8):1631-9.
6. Tian Z, Liu Y, Usa K, Mladinov D, Fang Y, Ding X, et al. Novel role of fumarate metabolism in dahl-salt sensitive hypertension. Hypertension. 2009 Aug 1;54(2):255-60.
7. Zheng X, Chen M, Li X, Yang P, Zhao X, Ouyang Y, et al. Insufficient fumarase contributes to hypertension by an imbalance of redox metabolism in Dahl salt-sensitive rats. Hypertension Research. 2019 Nov;42(11):1672-82.
8. Zheng X, Zhou L, Jin Y, Zhao X, Ahmad H, OuYang Y, et al. β-Aminoisobutyric acid supplementation attenuated salt-sensitive hypertension in Dahl salt-sensitive rats through prevention of insufficient fumarase. Amino Acids. 2022 Feb;54(2):169-80.
9. Grzegorzewska AP, Seta F, Han R, Czajka CA, Makino K, Stawski L, et al. Dimethyl Fumarate ameliorates pulmonary arterial hypertension and lung fibrosis by targeting multiple pathways. Scientific Reports. 2017 Feb 2;7(1):1-4.
10. Edosuyi O, Choi M, Igbe I, Oyekan A. Fumarate exerted an antihypertensive effect and reduced kidney injury molecule (KIM)-1 expression in deoxycorticosterone acetate-salt hypertension. Clinical and Experimental Hypertension. 2021 Aug 18;43(6):555-64.
11. Taylor NE, Glocka P, Liang M, Cowley Jr AW. NADPH oxidase in the renal medulla causes oxidative stress and contributes to salt-sensitive hypertension in Dahl S rats. Hypertension. 2006 Apr 1;47(4):692-8.
12. You YH, Quach T, Saito R, Pham J, Sharma K. Metabolomics reveals a key role for fumarate in mediating the effects of NADPH oxidase 4 in diabetic kidney disease. Journal of the American Society of Nephrology. 2016 Feb 1;27(2):466-81.
13. Usa K, Liu Y, Geurts AM, Cheng Y, Lazar J, Baker MA, et al. Elevation of fumarase attenuates hypertension and can result from a nonsynonymous sequence variation or increased expression depending on rat strain. Physiological Genomics. 2017 Sep 1;49(9):496-504.
14. Kone BC. Nitric oxide synthesis in the kidney: isoforms, biosynthesis, and functions in health. InSeminars in Nephrology. 2004;24(4):299-315.
15. Taddei S, Virdis A, Mattei P, Ghiadoni L, Sudano I, Salvetti A. Defective L-arginine–nitric oxide pathway in offspring of essential hypertensive patients. Circulation. 1996 Sep 15;94(6):1298-303.
16. Schlaich MP, Parnell MM, Ahlers BA, Finch S, Marshall T, Zhang WZ, et al. Impaired L-arginine transport and endothelial function in hypertensive and genetically predisposed normotensive subjects. Circulation. 2004 Dec 14;110(24):3680-6.
17. Li Q, Yon JY, Cai H. Mechanisms and consequences of eNOS dysfunction in hypertension. Journal of Hypertension. 2015 Jun;33(6):1128.
18. Hong NJ, Gonzalez-Vicente A, Saez F, Garvin JL. Mechanisms of decreased tubular flow-induced nitric oxide in Dahl salt-sensitive rat thick ascending limbs. American Journal of Physiology-Renal Physiology. 2021 Sep 1;321(3):F369-77.
19. Xue H, Geurts AM, Usa K, Wang F, Lin Y, Phillips J, et al. Fumarase overexpression abolishes hypertension attributable to endothelial NO synthase haploinsufficiency in dahl salt-sensitive rats. Hypertension. 2019 Aug;74(2):313-22.