Abstract
A series of studies suggested that hypertension is significantly associated with the risk of gallstone disease (GSD). However, due to differences in study design, population stratification, sample size, and diagnostic criteria for GSD, the results of each study are inconsistent, limiting the strength and application of these pieces of evidence. We previously conducted a cross-sectional study incorporating over 300,000 population, and the results showed hypertension was significantly associated with the risk of GSD. However, it was a single-center study, and the external generalization of research results is limited to a certain extent. Thus, we conducted another multi-center study and a systematic review and meta-analysis to verify further the relationship between hypertension and the risk of GSD.
Keywords
Gallstone diseases, Hypertension, Multi-center study, Systematic review and meta-analysis
Introduction
Gallstone disease (GSD) is a common digestive system disease whose prevalence varies greatly depending on race and geographic location [1,2]. The plurality of GSD in western developed countries fluctuates from 10% to 20% and about 2% to 8% in Asian countries [3,4]. In addition, observational studies have reported that GSD is a significant risk factor for type 2 diabetes [5,6], gastrointestinal cancer [7], ischemic heart disease [8], and other diseases [9,10]. Its high prevalence and complication rate have caused severe medical and economic burdens on patients [11,12]. Identifying the risk factors for GSD to enhance and further intervention for high-risk groups is essential to reduce the burden of GSD further.
High blood pressure may play a critical role in forming gallstones [13]. Previous observational studies have shown that hypertension is significantly associated with GSD risk [4,14-20]. However, due to differences in study design, population stratification, sample size, and diagnostic criteria for GSD, the results of each study are inconsistent, limiting the strength and application of these pieces of evidence. We surveyed more than 300,000 subjects to explore the association between hypertension and GSD [21]. The results showed hypertension was correlated with GSD risk, and the association was not altered when stratified by sex and the severity of hypertension. Although with a relatively large sample size, it was just a single-center study, and the results need to be verified widely.
In this study, we intend to confirm further the relationship between hypertension and the risk of GSD by performing a multi-center cross-sectional study based on a survey of the health checkup population of four hospitals in China. Then, we conducted a systematic review and meta-analysis with trial sequential analysis (TSA) to verify these associations.
Methods
Published study
The published research analyzed the association between blood pressure and GSD risk by the logistic regression. During the analysis, the subjects were first divided into hypertension and non-hypertension to study the association between hypertension and gallstone disease. Then, according to the severity of hypertension, they were split into groups with normal blood pressure, high blood pressure, grade one, grade two, and grade three hypertension, respectively, to explore the association between hypertension and the risk of gallstone disease and the dose-response relationship. Finally, the association between GSD and systolic hypertension and diastolic hypertension per 5 mmHg increase was investigated.
Current study
The study recruited participants who underwent health checkups at four Chinese hospitals between January 2015 and May 2020. The four hospitals were as follows: the People’s Hospital of Kaizhou District of Chongqing, the First Affiliated Hospital of Chongqing Medical University, Beijing Xiaotangshan Hospital, and Tianjin Medical University Cancer Institute and Hospital. Cholecystectomy was defined as a history of the gallbladder removal operation. GSD was diagnosed as the presence of gallstones and/or cholecystectomy [22].
The systematic review and meta-analysis followed the guidelines of the Meta-Analysis of Observational Studies in Epidemiology (MOOSE) [23]. We systematically searched the Embase and Medline database to identify relevant English publications from inception to March 2021. Additionally, we retrieved China National Knowledge Infrastructure (CNKI) database, the Wanfang Data Knowledge Service Platform, and the Chinese Scientific Journal Database (VIP database) for eligible studies in Chinese. The keywords include “gallstone” OR “gallstone disease” OR “cholelithiasis” AND “hypertension” OR “blood pressure” OR “systolic blood pressure (SBP)” OR “diastolic blood pressure (DBP)” (PROSPERO; registration ID: CRD42021247589).
In the multi-center cross-sectional study, in each center, the relationship between hypertension (DBP/SBP) and GSD risk was assessed by multivariable logistic regression analysis. Subsequently, we pooled the regression results from each hospital using the meta-analysis method. In addition, we conducted subgroup analyses by subtype of GSD (gallstones or cholecystectomy), age (< 40, 40-60, > 60 years), and gender (male or female). Finally, we performed TSA to verify the reliability of the study results [24]. The evidence is considered sufficient and conclusive if the Z-curve passes through the TSA monitoring boundary or required information size (RIS) boundary. Otherwise, the association is inconclusive, and more studies are needed to verify the results further. All statistical analyses were performed using SPSS 26.0 (IBM, USA). Meta-analysis was performed by Stata 16 (Stata, College Station, TX). The statistically significant level was two-tailed and was set at p <0.05.
Results
A total of 633948 participants were enrolled in this study. Seventeen thousand thirty-eight subjects enrolled in the First Affiliated Hospital of Chongqing Medical University, 372,289 subjects in People’s Hospital of Kaizhou District of Chongqing, 80,681 subjects in Beijing Xiaotangshan Hospital, and 10940 participants in Tianjin Medical University Cancer Institute and Hospital.
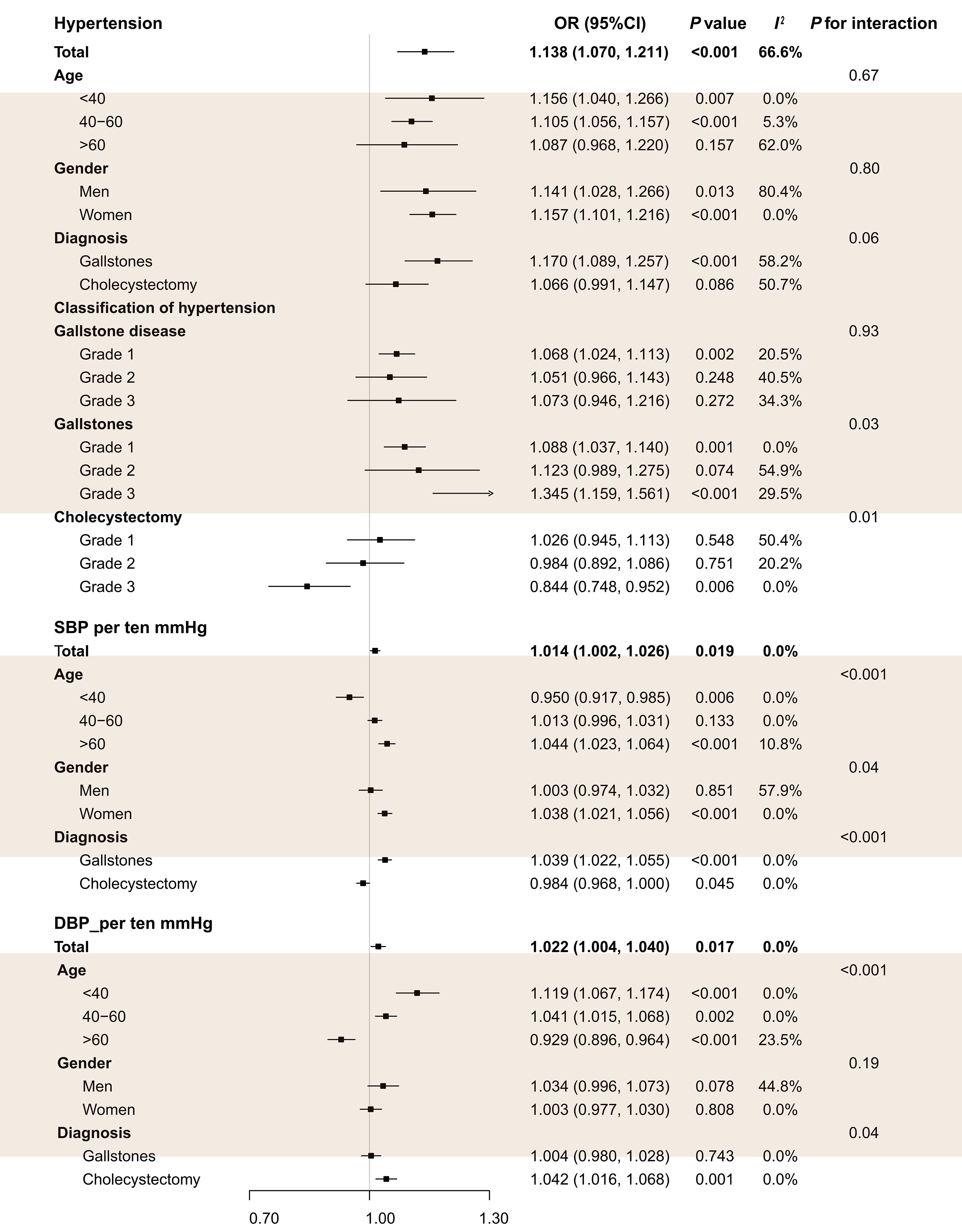
Multivariable regression analysis found that hypertension was significantly associated with the risk of GSD in each of the four hospitals (Table 1). As shown in Figure 1, pooled analyses showed that hypertension increased the risk of GSD. Subgroup analysis suggested hypertension was positively correlated with GSD risk in the young (age <40 years) and middle-aged (age: 40-60 years) population but not in the older (age >60 years). In addition, hypertension significantly increases the risk of GSD, and the higher grade of hypertension, the stronger association with GSD risk. Furthermore, we also found that each ten mmHg increase in SBP and DBP was positively associated with GSD risk. Subgroup analyses showed that each ten mmHg increase in SBP was associated with gallstones but not cholecystectomies.
|
First affiliated Hospital of Chongqing Medical University |
The People’s Hospital of Kaizhou District of Chongqing |
Beijing Xiaotangshan Hospital |
Tianjin Medical University Cancer Institute and Hospital |
|||||
|
OR (95%CI) |
P |
OR (95%CI) |
P |
OR (95%CI) |
P |
OR (95%CI) |
P |
|
|
Gallstone Disease |
||||||||
|
SBP_per 10 units |
1.007 (0.988, 1.026) |
0.478 |
1.015 (0.997, 1.034) |
0.11 |
1.019 (0.991, 1.048) |
0.185 |
1.063 (0.995, 1.135) |
0.072 |
|
DBP_per 10 units |
1.042 (1.012, 1.072) |
0.005 |
1.011 (0.984, 1.039) |
0.428 |
1.004 (0.960, 1.051) |
0.861 |
1.018 (0.917, 1.131) |
0.732 |
|
Hypertension |
1.153 (1.099, 1.210) |
7.11×10-9 |
1.065 (1.014, 1.118) |
0.012 |
1.176 (1.089, 1.270) |
3.28×10-5 |
1.304 (1.055, 1.613) |
0.014 |
|
Grade 1 |
1.058 (1.001, 1.117) |
0.046 |
1.078 (1.020, 1.138) |
0.007 |
1.032 (0.945, 1.126) |
0.482 |
1.308 (1.038, 1.647) |
0.023 |
|
Grade 2 |
1.072 (0.975, 1.179) |
0.153 |
1.048 (0.964, 1.139) |
0.273 |
0.926 (0.785, 1.093) |
0.365 |
1.352 (0.997, 1.834) |
0.052 |
|
Grade 3 |
1.177 (1.054, 1.315) |
0.004 |
0.996 (0.865, 1.147) |
0.956 |
0.866 (0.569, 1.318) |
0.502 |
1.105 (0.659, 1.854) |
0.705 |
|
Subgroup analysis by age |
||||||||
|
<40 years |
||||||||
|
SBP_per 10 units |
0.962 (0.914, 1.013) |
0.138 |
0.915 (0.861, 0.973) |
0.005 |
0.995 (0.901, 1.098) |
0.918 |
1.001 (0.776, 1.290) |
0.994 |
|
DBP_per 10 units |
1.136 (1.060, 1.217) |
3.15×10-4 |
1.106 (1.025, 1.195) |
0.01 |
1.082 (0.940, 1.246) |
0.273 |
1.176 (0.842, 1.643) |
0.34 |
|
Hypertension |
1.214 (1.053, 1.399) |
0.007 |
1.059 (0.896, 1.252) |
0.502 |
1.156 (0.524, 2.548) |
0.72 |
1.582 (0.824, 3.038) |
0.168 |
|
40 - 60 years |
||||||||
|
SBP_per 10 units |
1.013 (0.983, 1.043) |
0.397 |
1.010 (0.985, 1.035) |
0.452 |
1.019 (0.975, 1.065) |
0.406 |
1.047 (0.940, 1.167) |
0.402 |
|
DBP_per 10 units |
1.039 (0.994, 1.085) |
0.088 |
1.039 (1.002, 1.078) |
0.04 |
1.058 (0.987, 1.134) |
0.11 |
1.027 (0.869, 1.214) |
0.756 |
|
Hypertension |
1.155 (1.083, 1.233) |
1.37×10-5 |
1.068 (1.005, 1.135) |
0.034 |
1.060 (0.872, 1.287) |
0.56 |
1.104 (0.825, 1.476) |
0.507 |
|
>60 years |
||||||||
|
SBP_per 10 units |
1.045 (1.016, 1.075) |
0.002 |
1.058 (1.026, 1.091) |
3.23×10-4 |
1.013 (0.974, 1.054) |
0.518 |
1.075 (0.981, 1.177) |
0.121 |
|
DBP_per 10 units |
0.962 (0.917, 1.010) |
0.122 |
0.904 (0.859, 0.950) |
8.55×10-5 |
0.906 (0.845, 0.971) |
0.005 |
0.970 (0.829, 1.135) |
0.705 |
|
Hypertension |
1.148 (1.056, 1.248) |
0.001 |
1.056 (0.961, 1.160) |
0.259 |
0.902 (0.736, 1.107) |
0.324 |
1.483 (1.027, 2.140) |
0.035 |
|
Subgroup analysis by gender |
||||||||
|
Male |
||||||||
|
SBP_per 10 units |
0.979 (0.952, 1.007) |
0.134 |
0.993 (0.966, 1.020) |
0.603 |
1.017 (0.981, 1.054) |
0.363 |
1.103 (1.002, 1.214) |
0.044 |
|
DBP_per 10 units |
1.076 (1.033, 1.120) |
4.39×10-4 |
1.016 (0.978, 1.056) |
0.416 |
1.006 (0.949, 1.066) |
0.835 |
1.012 (0.869, 1.177) |
0.881 |
|
Hypertension |
1.133 (1.065, 1.205) |
7.38×10-5 |
1.011 (0.948, 1.078) |
0.742 |
1.206 (1.097, 1.325) |
1.05×10-4 |
1.495 (1.112, 2.010) |
0.008 |
|
Female |
||||||||
|
SBP_per 10 units |
1.047 (1.020, 1.075) |
0.001 |
1.038 (1.012, 1.065) |
0.004 |
1.018 (0.973, 1.065) |
0.438 |
1.026 (0.935, 1.125) |
0.594 |
|
DBP_per 10 units |
0.996 (0.956, 1.039) |
0.868 |
1.007 (0.969, 1.047) |
0.714 |
1.008 (0.937, 1.084) |
0.838 |
1.020 (0.881, 1.182) |
0.787 |
|
Hypertension |
1.187 (1.098, 1.283) |
1.70×10-5 |
1.146 (1.062, 1.237) |
4.36×10-4 |
1.117 (0.980, 1.274) |
0.098 |
1.111 (0.814, 1.515) |
0.508 |
|
Subgroup analysis by diagnosis |
||||||||
|
Gallstones |
||||||||
|
SBP_per 10 units |
1.030 (1.004, 1.057) |
0.025 |
1.048 (1.021, 1.076) |
4.32×10-4 |
1.029 (0.996, 1.063) |
0.085 |
1.091 (1.009, 1.179) |
0.028 |
|
DBP_per 10 units |
1.024 (0.985, 1.065) |
0.233 |
0.982 (0.945, 1.021) |
0.367 |
1.011 (0.959, 1.066) |
0.679 |
0.995 (0.880, 1.125) |
0.934 |
|
Hypertension |
1.141 (1.067, 1.221) |
1.13×10-4 |
1.096 (1.022, 1.175) |
0.011 |
1.251 (1.144, 1.367) |
7.75×10-7 |
1.370 (1.067, 1.758) |
0.013 |
|
Grade 1 |
1.067 (0.988, 1.152) |
0.098 |
1.087 (1.005, 1.176) |
0.038 |
1.101 (0.996, 1.217) |
0.059 |
1.276 (0.969, 1.681) |
0.083 |
|
Grade 2 |
1.153 (1.013, 1.314) |
0.031 |
1.054 (0.935, 1.189) |
0.387 |
1.007 (0.835, 1.216) |
0.938 |
1.623 (1.147, 2.298) |
0.006 |
|
Grade 3 |
1.473 (1.277, 1.698) |
9.75×10-8 |
1.307 (1.090, 1.568) |
0.004 |
0.892 (0.549, 1.448) |
0.643 |
1.397 (0.783, 2.491) |
0.257 |
|
Cholecystectomy |
||||||||
|
SBP_per 10 units |
0.981 (0.957, 1.006) |
0.144 |
0.984 (0.961, 1.008) |
0.182 |
0.990 (0.942, 1.041) |
0.708 |
0.996 (0.887, 1.119) |
0.947 |
|
DBP_per 10 units |
1.058 (1.017, 1.100) |
0.005 |
1.038 (1.002, 1.076) |
0.038 |
0.989 (0.911, 1.074) |
0.793 |
1.064 (0.883, 1.283) |
0.512 |
|
Hypertension |
1.138 (1.067, 1.214) |
8.46×10-5 |
1.032 (0.968, 1.100) |
0.336 |
0.986 (0.858, 1.134) |
0.846 |
1.122 (0.770, 1.637) |
0.548 |
|
Grade 1 |
1.040 (0.967, 1.118) |
0.295 |
1.060 (0.987, 1.138) |
0.108 |
0.871 (0.739, 1.026) |
0.097 |
1.310 (0.881, 1.947) |
0.182 |
|
Grade 2 |
0.990 (0.873, 1.124) |
0.882 |
1.037 (0.931, 1.154) |
0.511 |
0.757 (0.550, 1.042) |
0.088 |
0.831 (0.466, 1.482) |
0.531 |
|
Grade 3 |
0.903 (0.774, 1.053) |
0.193 |
0.761 (0.621, 0.932) |
0.008 |
0.821 (0.383, 1.757) |
0.611 |
0.611 (0.214, 1.747) |
0.358 |
|
DBP: Diastolic Blood Pressure; SBP: Systolic Blood Pressure |
||||||||
Figure 1: Pooled analysis of the association between blood pressure and gallstone diseases risk in the multi-center study.
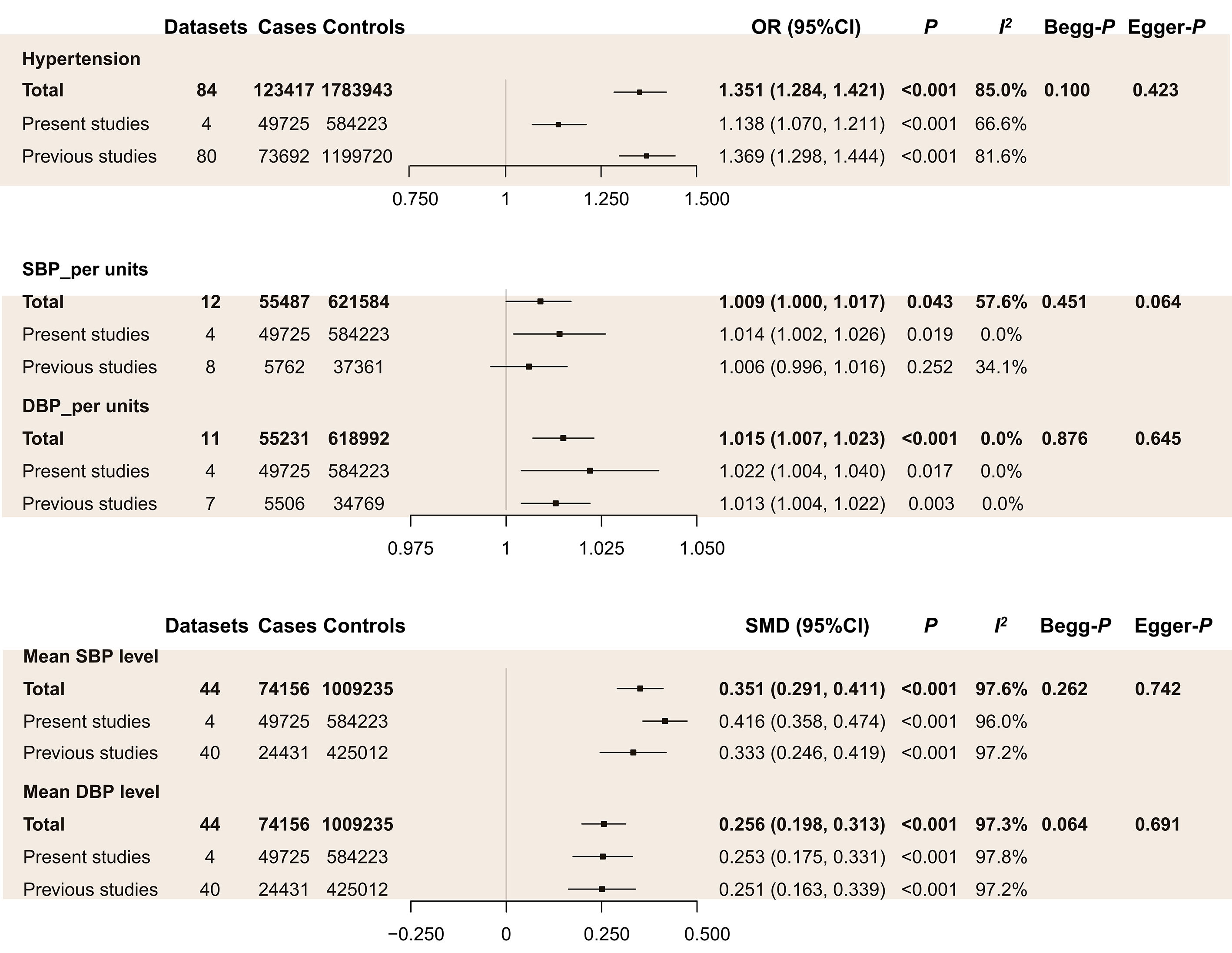
As shown in Figure 2, our systematic review and meta-analysis showed hypertension was positively associated with the risk of GSD in previous studies. Similarly, after adding the results of our multi-center study, hypertension increased GSD risk by about 35%. The funnel plot showed no apparent asymmetry (Supplementary Figure 1). Moreover, Begg’s (p = 0.100) and Egger’s test (p = 0.423) indicated no signi?cant difference in the publication bias (Figure 2). Subgroup analyses by geographic location indicated that hypertension positively related to GSD in Asia and American countries but not in Europe (Table 2). Subgroup analysis by diagnosis showed hypertension was positively associated with gallstone subtypes but not with cholecystectomy (Table 2). Results showed the Z line both crossed the futility boundary and TSA monitoring boundary and achieved the RIS boundary, which suggested the association was conclusive (Supplementary Figure 2).
Furthermore, we investigated the differences in the DBP and SBP levels between GSD and the control group. The mean SBP and DBP level was higher in the GSD group than in the control group (Figure 2). And every one mmHg in SBP and DBP was significantly associated with increased GSD risk (Figure 2). Subgroup analyses indicated every one mmHg in SBP and DBP associated with GSD risk in a cross-sectional study and Asia population (Table 2).
Figure 2: Meta-analysis of the association between blood pressure and GSD risk.
|
Effect size of blood pressure on GSD risk |
Mean difference of SBP and DBP |
||||||||||||||
|
Datasets |
OR (95%CI) |
P |
I2 |
P-between groups |
Datasets |
SMD (95%CI) |
P |
I2 |
|||||||
|
SBP |
|
||||||||||||||
|
SBP_per units |
12 |
1.009 (1.000, 1.017) |
0.043 |
57.6% |
44 |
0.351 (0.291, 0.411) |
<0.001 |
97.6% |
|||||||
|
Study_design |
0.34 |
||||||||||||||
|
Cross-sectional study |
7 |
1.010 (1.000, 1.020) |
0.047 |
61.1% |
31 |
0.317 (0.269, 0.366) |
<0.001 |
94.4% |
|||||||
|
Case-control study |
2 |
1.068 (0.995, 1.146) |
0.070 |
1.7% |
9 |
0.549 (0.164, 0.934) |
0.005 |
99.1% |
|||||||
|
Cohort study |
3 |
1.001 (0.952, 1.052) |
0.981 |
26.3% |
4 |
0.183 (0.100, 0.266) |
<0.001 |
80.0% |
|||||||
|
Geographic location |
0.67 |
||||||||||||||
|
Asia |
9 |
1.012 (1.001, 1.022) |
0.030 |
58.1% |
34 |
0.387 (0.324, 0.45) |
<0.001 |
97.8% |
|||||||
|
Europe |
3 |
1.001 (0.952, 1.052) |
0.981 |
26.3% |
6 |
0.175 (-0.011, 0.361) |
0.916 |
95.7% |
|||||||
|
America |
3 |
-0.034 (-0.667, 0.599) |
0.066 |
93.1% |
|||||||||||
|
Africa |
1 |
0.815 (0.450, 1.179) |
<0.001 |
NA |
|||||||||||
|
Gender |
0.12 |
||||||||||||||
|
Men |
6 |
1.000 (0.983, 1.018) |
0.996 |
45.2% |
1 |
0.115 (0.082, 0.148) |
<0.001 |
NA |
|||||||
|
Women |
6 |
1.026 (0.998, 1.054) |
0.071 |
75.4% |
6 |
0.085 (-0.050, 0.219) |
0.218 |
48.2% |
|||||||
|
Subtype diseases |
<0.001 |
||||||||||||||
|
Gallstones |
7 |
1.032 (1.020, 1.044) |
<0.001 |
12.2% |
11 |
0.360 (0.093, 0.626) |
0.008 |
98.9% |
|||||||
|
Cholecystectomy |
4 |
0.984 (0.968, 1.000) |
0.045 |
0.0% |
7 |
0.291 (0.143, 0.439) |
<0.001 |
87.0% |
|||||||
|
Language |
0.06 |
||||||||||||||
|
English |
5 |
1.000 (0.995, 1.004) |
0.969 |
4.7% |
26 |
0.272 (0.190, 0.354) |
<0.001 |
94.6% |
|||||||
|
Chinese |
3 |
1.031 (1.000, 1.063) |
0.053 |
16.3% |
14 |
0.447 (0.235, 0.658) |
<0.001 |
98.5% |
|||||||
|
DBP |
|
||||||||||||||
|
DBP_per units |
11 |
1.015 (1.007, 1.023) |
<0.001 |
0.0% |
44 |
0.256 (0.198, 0.313) |
<0.001 |
97.3% |
|||||||
|
Study_design |
0.57 |
||||||||||||||
|
Cross-sectional study |
7 |
1.015 (1.007, 1.023) |
<0.001 |
0.0% |
31 |
0.192 (0.146, 0.237) |
<0.001 |
93.5% |
|||||||
|
Case-control study |
2 |
1.018 (0.942, 1.099) |
0.654 |
0.0% |
9 |
0.474 (0.048, 0.901) |
0.029 |
99.3% |
|||||||
|
Cohort study |
2 |
0.925 (0.765, 1.119) |
0.424 |
19.0% |
4 |
0.176 (0.132, 0.221) |
<0.001 |
44.3% |
|||||||
|
Geographic location |
0.29 |
||||||||||||||
|
Asia |
9 |
1.015 (1.007, 1.023) |
<0.001 |
0.0% |
34 |
0.293 (0.230, 0.356) |
<0.001 |
97.8% |
|||||||
|
Europe |
2 |
0.925 (0.765, 1.119) |
0.424 |
19.0% |
6 |
-0.077 (-0.365, 0.212) |
0.602 |
77.8% |
|||||||
|
America |
3 |
0.059 (-0.004, 0.122) |
0.066 |
40.8% |
|||||||||||
|
Other |
1 |
0.940 (0.570, 1.309) |
<0.001 |
NA |
|||||||||||
|
Gender |
0.52 |
||||||||||||||
|
Men |
6 |
1.021 (0.991, 1.052) |
0.168 |
56.6% |
1 |
0.161 (0.128, 0.194) |
<0.001 |
NA |
|||||||
|
Women |
6 |
1.010 (0.996, 1.024) |
0.160 |
0.0% |
6 |
0.037 (-0.144, 0.217) |
0.69 |
69.5% |
|||||||
|
Subtype diseases |
0.08 |
||||||||||||||
|
Gallstones |
7 |
1.016 (1.003, 1.028) |
0.012 |
0.0% |
11 |
0.434 (0.136, 0.732) |
0.007 |
78.4% |
|||||||
|
Cholecystectomy |
4 |
1.042 (1.016, 1.068) |
0.001 |
0.0% |
7 |
0.106 (-0.010, 0.221) |
0.073 |
99.1% |
|||||||
|
Language |
0.24 |
||||||||||||||
|
English |
4 |
1.009 (0.998, 1.021) |
0.104 |
0.0% |
26 |
0.179 (0.126, 0.231) |
<0.001 |
85.4% |
|||||||
|
Chinese |
3 |
1.02 (1.006, 1.035) |
0.006 |
0.0% |
14 |
0.390 (0.141, 0.640) |
0.002 |
98.9% |
|||||||
|
Hypertension |
|
||||||||||||||
|
Total |
84 |
1.351 (1.284, 1.421) |
<0.001 |
85.0% |
|||||||||||
|
Study_design |
0.01 |
||||||||||||||
|
Cross-sectional study |
53 |
1.395 (1.311, 1.485) |
<0.001 |
85.7% |
|||||||||||
|
Case-control study |
19 |
1.403 (1.235, 1.594) |
<0.001 |
86.0% |
|||||||||||
|
Cohort study |
12 |
1.184 (1.066, 1.314) |
0.002 |
56.3% |
|||||||||||
|
Geographic location |
<0.001 |
||||||||||||||
|
Asia |
70 |
1.356 (1.285, 1.430) |
<0.001 |
84.8% |
|||||||||||
|
Europe |
4 |
1.086 (0.779, 1.514) |
0.626 |
91.1% |
|||||||||||
|
America |
8 |
1.649 (1.232, 2.209) |
0.001 |
80.0% |
|||||||||||
|
Africa |
1 |
1.618 (0.591, 4.430) |
0.349 |
NA |
|||||||||||
|
Other |
1 |
0.509 (0.223, 1.160) |
0.108 |
NA |
|||||||||||
|
Gender |
0.510 |
||||||||||||||
|
Men |
13 |
1.214 (1.100, 1.339) |
<0.001 |
79.8% |
|||||||||||
|
Women |
15 |
1.238 (1.119, 1.370) |
<0.001 |
71.6% |
|||||||||||
|
Subtype diseases |
0.26 |
||||||||||||||
|
Gallstones |
25 |
1.246 (1.159, 1.339) |
<0.001 |
76.0% |
|||||||||||
|
Cholecystectomy |
5 |
1.133 (0.977, 1.314) |
0.099 |
84.8% |
|||||||||||
|
Language |
0.16 |
||||||||||||||
|
English |
39 |
1.395 (1.287, 1.513) |
<0.001 |
80.1% |
|||||||||||
|
Chinese |
41 |
1.354 (1.255, 1.461) |
<0.001 |
81.9% |
|||||||||||
Discussion
This study explores the relationship between hypertension and the risk of GSD based on our published research and some current study findings. In both studies, hypertension was found to be positively correlated with the risk of GSD, and the higher grade of hypertension, the stronger association with gallstone formation.
Consistent with the results of this study, a cross-sectional survey in the Shanghai population [4] also found that hypertension significantly increased the risk of GSD after multi-factor adjustment. A cohort study in the Korean population suggested that hypertension was an independent risk factor for GSD in both genders [16]. However, some studies have shown the opposite conclusion. A case-control study conducted by Gonzalez-Perez and colleagues in the UK population concluded that hypertension is not significantly associated with GSD [20]. A cohort study conducted in the USA population suggested that 0hypertension was not a risk factor for GSD in both genders [25]. These data indicated that the paradoxical results might be due to differences in experimental design, study population, sample size, diagnostic criteria for GSD, or interference from other factors.
The mechanisms of hypertension in GSD remain unclear so far. Evidence has shown that there are common risk factors or pathogenic factors between cholelithiasis and hypertension, such as lipid metabolism [26,27] and insulin resistance [28-31] lipid metabolism has long been a research focus in GSD etiology, which is also associated with hypertension [26]. High levels of HDL-C facilitate hepatic bile acid synthesis [32] and decrease the cholesterol saturation index [33,34], which increases cholesterol solubility [35] and thus protects against the formation of gallstones. In addition, insulin resistance is associated with both hypertension and GSD. Studies have reported that insulin resistance may stimulate hepatic cholesterol secretion, biliary cholesterol supersaturated, and gallbladder movement disorder, thus promoting the formation of gallstones [30,36].
Furthermore, studies have shown that Leptin may be related to increased GSD prevalence caused by hypertension [37,38]. Leptin, an adipocyte-derived hormone, regulates blood lipids and glucose. Experimental research and population surveys indicated Leptin is closely related to hypertension [39] and cholelithiasis [38,40,41]. In patients with essential hypertension, serum leptin levels increase significantly with the severity of hypertension [37,42,43]. The increased concentration of Leptin can fortify hydrophilic bile salts, reduce hydrophobic bile salts and the circulating bile acid salt pool, making the cholesterol saturation of bile, which eventually leads to the formation of gallstones [44-46]. Furthermore, a high level of Leptin may cause leptin resistance, which will lead to the increased expression of sterol regulatory element-binding protein (SREBP) and a high level of LDL-C, thus accelerating the formation of gallstones [44,45]. The exact mechanism of the increased risk of GSD caused by hypertension needs to be further explored.
Our studies show that the relationship between hypertension and cholelithiasis is inconsistent among genders. Previous studies have found that the prevalence of GSD in women is higher than in men [47]. For this possible reason, female endogenous estrogen may participate in the liver cholesterol anabolism and promote crystallization nucleation, leading to gallstone formation [47]. In our study, subgroup analysis by gender found that hypertension is positively related to GSD in both men and women. Still, the effect size is relatively more prominent in women than in men, consistent with a cohort study conducted in South Korea [16]. On the contrary, a cross-sectional study found that hypertension was positively associated with GSD risk in females but not in males [48]. Further studies are needed to investigate the influence of gender on the association between hypertension and cholelithiasis.
Moreover, ATP-binding cassette transporters (including ABCA1, ABCG5, and ABCG8) function as efflux facilitators of phospholipids and hepatobiliary cholesterol, playing an essential role in developing GSD [49-51]. Claudia Huesca-Gómez et al. also showed an inverse correlation between ABCA1/ABCG1 and essential arterial hypertension in patients with cIMT [52]. Besides, a single nucleotide polymorphism (SNP) rs11191548 near cytochrome P450 family 17 subfamilies A member 1 (CYP17A1) gene [53,54] is significantly associated with hypertension and hypercholesterolemia [55]. At the same time, variants in this gene are also related to biliary stones among overweight and diabetic subjects [56], suggesting a role of genetic factors in the association of hypertension with GSD.
Limitation
There are some limitations. Firstly, our study is a summary of cross-sectional research results, and thus the association between hypertension and GSD risk might be affected by potential confounders and reverse causality. Secondly, the heterogeneities among the studies were relatively high, which may partly affect the results. Thus, the results should be explained with caution.
Conclusion
The results of this mini-review support the association between hypertension and GSD. The higher the hypertension grade, the greater the risk of GSD. However, the role and mechanism of hypertension in cholelithiasis are not fully understood. More research is needed to explore the pathogenesis of this association.
Funding
National Natural Science Foundation of China (81903398,81902856); Basic Research Foundation of Central Universities, No.YJ2021112; Medical Research Youth Innovation Project of Sichuan Medical Association, No.Q21016; Sichuan Tianfu Emei Youth Talent Project; Natural Science Foundation for Outstanding Youth of Sichuan Province (23NSFJQ0076).
References
2. Portincasa P, Moschetta A, Palasciano G. Cholesterol gallstone disease. The Lancet. 2006 Jul 15;368(9531):230-9.
3. Aerts R, Penninckx F. The burden of gallstone disease in Europe. Alimentary Pharmacology & Therapeutics. 2003 Nov;18:49-53.
4. Song ST, Shi J, Wang XH, Guo YB, Hu PF, Zhu F, et al. Prevalence and risk factors for gallstone disease: A population‐based cross‐sectional study. Journal of Digestive Diseases. 2020 Apr;21(4):237-45.
5. Wang F, Wang J, Li Y, Yuan J, Yao P, Wei S, et al. Gallstone disease and type 2 diabetes risk: a Mendelian randomization study. Hepatology. 2019 Aug;70(2):610-20.
6. Portincasa P, Di Ciaula A, De Bari O, Garruti G, Palmieri VO, Wang DH. Management of gallstones and its related complications. Expert Review of Gastroenterology & Hepatology. 2016 Jan 2;10(1):93-112.
7. Pang Y, Lv J, Kartsonaki C, Guo Y, Yu C, Chen Y, et al. Causal effects of gallstone disease on risk of gastrointestinal cancer in Chinese. British Journal of Cancer. 2021 May;124(11):1864-72.
8. Shabanzadeh DM, Skaaby T, Sørensen LT, Jørgensen T. Screen-detected gallstone disease and cardiovascular disease. European Journal of Epidemiology. 2017 Jun;32(6):501-10.
9. Shabanzadeh DM, Sørensen LT, Jørgensen T. Gallstone disease and mortality: a cohort study. International Journal of Public Health. 2017 Apr;62(3):353-60.
10. Shabanzadeh DM. Incidence of gallstone disease and complications. Current Opinion in Gastroenterology. 2018 Mar 1;34(2):81-9.
11. Nealon WH, Urrutia F, Fleming D, Thompson JC. The economic burden of gallstone lithotripsy. Will cost determine its fate?. Annals of Surgery. 1991 Jun;213(6):645.
12. Shaffer EA. Epidemiology of gallbladder stone disease. Best practice & Research Clinical Gastroenterology. 2006 Jan 1;20(6):981-96.
13. Lin I, Yang YW, Wu MF, Yeh YH, Liou JC, Lin YL, et al. The association of metabolic syndrome and its factors with gallstone disease. BMC Family Practice. 2014 Dec;15(1):1-6.
14. Hung MC, Chen CF, Tsou MT, Lin HH, Hwang LC, Hsu CP. Relationship Between Gallstone Disease and Cardiometabolic Risk Factors in Elderly People with Non-Alcoholic Fatty Liver Disease. Diabetes, Metabolic Syndrome and Obesity: Targets and Therapy. 2020;13:3579.
15. Almobarak AO, Jervase A, Fadl AA, Garelnabi NI, Al Hakem S, Hussein TM, et al. The prevalence of diabetes and metabolic syndrome and associated risk factors in Sudanese individuals with gallstones: a cross sectional survey. Translational Gastroenterology and Hepatology. 2020;5.
16. Chang Y, Noh YH, Suh BS, Kim Y, Sung E, Jung HS, et al. Bidirectional association between nonalcoholic fatty liver disease and gallstone disease: a cohort study. Journal of Clinical Medicine. 2018 Nov 21;7(11):458.
17. Shabanzadeh DM, Holmboe SA, Sørensen LT, Linneberg A, Andersson AM, Jørgensen T. Are incident gallstones associated to sex‐dependent changes with age? A cohort study. Andrology. 2017 Sep;5(5):931-8.
18. Waniek S, Di Giuseppe R, Esatbeyoglu T, Ratjen I, Enderle J, Jacobs G, et al. Association of circulating vitamin E (α-and γ-tocopherol) levels with Gallstone Disease. Nutrients. 2018 Jan 27;10(2):133.
19. Cha BH, Lee BS, Lee SH, Kang SJ, Park MJ. A study of alcohol consumption and obesity as main risk factor for symptomatic gallbladder stone: a case-control study. Asian Pacific Journal of Cancer Prevention: APJCP. 2017;18(3):715.
20. González‐Pérez BPharm A, Garcia Rodriguez LA. Gallbladder disease in the general population: association with cardiovascular morbidity and therapy. Pharmacoepidemiology and Drug Safety. 2007 May;16(5):524-31.
21. Zhang Y, Sun L, Wang X, Chen Z. The association between hypertension and the risk of gallstone disease: a cross-sectional study. BMC Gastroenterology. 2022 Dec;22(1):1-0.
22. Liu CM, Tung TH, Chou P, Chen VT, Hsu CT, Chien WS, et al. Clinical correlation of gallstone disease in a Chinese population in Taiwan: experience at Cheng Hsin General Hospital. World Journal of Gastroenterology. 2006 Feb 2;12(8):1281.
23. Stroup DF, Berlin JA, Morton SC, Olkin I, Williamson GD, Rennie D, et al. Meta-analysis of observational studies in epidemiology: a proposal for reporting. JAMA. 2000 Apr 19;283(15):2008-12.
24. Brok J, Thorlund K, Gluud C, Wetterslev J. Trial sequential analysis reveals insufficient information size and potentially false positive results in many meta-analyses. Journal of Clinical Epidemiology. 2008 Aug 1;61(8):763-9.
25. Boland LL, Folsom AR, Rosamond WD, Atherosclerosis Risk in Communities (ARIC) Study Investigators. Hyperinsulinemia, dyslipidemia, and obesity as risk factors for hospitalized gallbladder disease: a prospective study. Annals of Epidemiology. 2002 Feb 1;12(2):131-40.
26. Wei W, Li Y, Chen F, Chen C, Sun T, Sun Z, et al. Dyslipidaemia, combined oral contraceptives use and their interaction on the risk of hypertension in Chinese women. Journal of Human Hypertension. 2011 Jun;25(6):364-71.
27. Connolly RJ, Anderson JM, Kambic HE, Greisler H, Merrill EW. Small diameter vascular prostheses. ASAIO Transactions. 1988, 34(4):1043-1046.
28. Han T, Lan L, Qu R, Xu Q, Jiang R, Na L, et al. Temporal relationship between hyperuricemia and insulin resistance and its impact on future risk of hypertension. Hypertension. 2017 Oct;70(4):703-11.
29. Wang F, Han L, Hu D. Fasting insulin, insulin resistance and risk of hypertension in the general population: a meta-analysis. Clinica Chimica Acta. 2017 Jan 1;464:57-63.
30. Tsai CJ, Leitzmann MF, Willett WC, Giovannucci EL. Macronutrients and insulin resistance in cholesterol gallstone disease. Official Journal of the American College of Gastroenterology. 2008 Nov 1;103(11):2932-9.
31. Cortés VA, Barrera F, Nervi F. Pathophysiological connections between gallstone disease, insulin resistance, and obesity. Obesity Reviews. 2020 Apr;21(4):e12983.
32. Janowitz P, Wechsler JG, Kuhn K, Kratzer W, Tudyka J, Swobodnik W, et al. The relationship between serum lipids, nucleation time, and biliary lipids in patients with gallstones. The Clinical Investigator. 1992 May;70(5):430-6.
33. Thornton JR, Heaton KW, Macfarlane DG. A relation between high-density-lipoprotein cholesterol and bile cholesterol saturation. Br Med J (Clin Res Ed). 1981 Nov 21;283(6303):1352-4.
34. Scragg RK, Calvert GD, Oliver JR. Plasma lipids and insulin in gall stone disease: a case-control study. Br Med J (Clin Res Ed). 1984 Sep 1;289(6444):521-5.
35. Hussaini SH, Pereira SP, Murphy GM, Dowling RH. Deoxycholic acid influences cholesterol solubilization and microcrystal nucleation time in gallbladder bile. Hepatology. 1995 Dec 1;22(6):1735-44.
36. Ruhl CE, Everhart JE. Association of diabetes, serum insulin, and C‐peptide with gallbladder disease. Hepatology. 2000 Feb;31(2):299-303.
37. Shankar A, Xiao J. Positive relationship between plasma leptin level and hypertension. Hypertension. 2010 Oct 1;56(4):623-8.
38. Saraç S, Atamer A, Atamer Y, Can AS, Bilici A, Taçyildiz I, et al. Leptin levels and lipoprotein profiles in patients with cholelithiasis. Journal of International Medical Research. 2015 Jun;43(3):385-92.
39. Zhang Y, Chua Jr S. Leptin function and regulation. Comprehensive Physiology. 2011 Jan 17;8(1):351-69.
40. Graewin SJ, Kiely JM, Lu D, Svatek CL, Al-Azzawi HH, Swartz-Basile DA, et al. Leptin regulates gallbladder genes related to gallstone pathogenesis in leptin-deficient mice. Journal of the American College of Surgeons. 2008 Mar 1;206(3):503-10.
41. Wang SN, Yeh YT, Yu ML, Dai CY, Chi WC, Chung WL, et al. Hyperleptinaemia and hypoadiponectinaemia are associated with gallstone disease. European Journal of Clinical Investigation. 2006 Mar;36(3):176-80.
42. Kaze AD, Musani SK, Bidulescu A, Correa A, Bertoni AG, Ahima RS, et al. Plasma leptin and blood pressure progression in Blacks: the Jackson Heart Study. Hypertension. 2021 Apr;77(4):1069-75.
43. Beltowski J. Role of leptin in blood pressure regulation and arterial hypertension. Journal of Hypertension. 2006 May 1;24(5):789-801.
44. Roy S, Hyogo H, Yadav SK, Wu MK, Jelicks LA, Locker JD, et al. A biphasic response of hepatobiliary cholesterol metabolism to dietary fat at the onset of obesity in the mouse. Hepatology. 2005 Apr;41(4):887-95.
45. Hyogo H, Roy S, Paigen B, Cohen DE. Leptin promotes biliary cholesterol elimination during weight loss in ob/ob mice by regulating the enterohepatic circulation of bile salts. Journal of Biological Chemistry. 2002 Sep 13;277(37):34117-24.
46. VanPatten S, Ranginani N, Shefer S, Nguyen LB, Rossetti L, Cohen DE. Impaired biliary lipid secretion in obese Zucker rats: leptin promotes hepatic cholesterol clearance. American Journal of Physiology-Gastrointestinal and Liver Physiology. 2001 Aug 1;281(2):G393-404.
47. Wang HH, Liu M, Clegg DJ, Portincasa P, Wang DQ. New insights into the molecular mechanisms underlying effects of estrogen on cholesterol gallstone formation. Biochimica et Biophysica Acta (BBA)-Molecular and Cell Biology of Lipids. 2009 Nov 1;1791(11):1037-47.
48. Chi Z, Ye B, YuTong W, Ben Z, DongQing G, Xin W. Prevalence of gallstones among university teachers and related risk factors: A multicenter study. Journal of Clinical Hepatobiliary Diseases (Chinese). 2020 Mar 20;36(3):624-30.
49. Grünhage F, Acalovschi M, Tirziu S, Walier M, Wienker TF, Ciocan A, et al. Increased gallstone risk in humans conferred by common variant of hepatic ATP‐binding cassette transporter for cholesterol. Hepatology. 2007 Sep;46(3):793-801.
50. Stender S, Frikke-Schmidt R, Nordestgaard BG, Tybjærg-Hansen A. The ABCG5/8 cholesterol transporter and myocardial infarction versus gallstone disease. Journal of the American College of Cardiology. 2014 May 27;63(20):2121-8.
51. Lee J, Tauscher A, Seo DW, Oram JF, Kuver R. Cultured gallbladder epithelial cells synthesize apolipoproteins AI and E. American Journal of Physiology-Gastrointestinal and Liver Physiology. 2003 Sep;285(3):G630-41.
52. Huesca-Gómez C, Torres-Paz YE, Martínez-Alvarado R, Fuentevilla-Álvarez G, Valle-Mondragón D, Torres-Tamayo M, et al. Association between the transporters ABCA1/G1 and the expression of miR-33a/144 and the carotid intima media thickness in patients with arterial hypertension. Molecular Biology Reports. 2020 Feb;47(2):1321-9.
53. Zhang N, Jia J, Ding Q, Chen H, Ye X, Ding H, et al. Common variant rs11191548 near the CYP17A1 gene is associated with hypertension and the serum 25 (OH) D levels in Han Chinese. Journal of Human Genetics. 2018 Jun;63(6):731-7.
54. Xi B, Shen Y, Reilly KH, Wang X, Mi J. Recapitulation of four hypertension susceptibility genes (CSK, CYP17A1, MTHFR, and FGF5) in East Asians. Metabolism. 2013 Feb 1;62(2):196-203.
55. Zhang N, Chen H, Jia J, Ye X, Ding H, Zhan Y. The CYP17A1 gene polymorphisms are associated with hypercholesterolemia in Han Chinese. The Journal of Gene Medicine. 2019 Aug;21(8):e3102.
56. Hou L, Xu J, Gao YT, Rashid A, Zheng SL, Sakoda LC, et al. CYP17 MspA1 polymorphism and risk of biliary tract cancers and gallstones: A population‐based study in Shanghai, China. International Journal of Cancer. 2006 Jun 1;118(11):2847-53.