Abstract
The liver emerges as an ultimate metabolic nexus—a polyfunctional organ that simultaneously performs biochemical detoxification, macromolecular processing, and systemic nutrient governance. While hepatic stellate cells (HSCs) were historically confined to their fibrogenic identity, their broader homeostatic orchestration remained enigmatic. RSPO3 as a linchpin in hepatocyte dynamics, with targeted pharmacological modulation enhancing hepatic lobular repatterning, regenerative capacity, and metabolic optimization. This mechanistically aligns with Wnt/β-catenin signaling’s indispensable role in spatial-metabolic zonation. Although earlier paradigms failed to reconcile the pathological dichotomy of HSCs, emerging evidence unveils their symbiotic crosstalk with hepatocytes through regulation of the Wnt pathway mediated by RSPO3. This works discussed HSCs as biochemical coordinators, modulating hepatocyte transcriptomic landscapes and regenerative programming via paracrine signaling. Recognition of synaptic-like HSCs extensions as architectural interfaces in hepatic microecology demands a paradigm shift—no longer mere fibrotic antagonists, but participate in the regulation of liver function. Within the 15 microns cellular sentinels lies a cryptographic key to deciphering liver regeneration, potentially unlocking therapies to reverse cirrhotic degeneration and restore functional hepatostasis.
Keywords
Hepatic stellate cells, RSPO3, Wnt/β-catenin, Spatial-metabolim, Fibrotic, Hepatocyte, Regeneration
Commentary
In a recently published study in Nature, Robert F. Schwabe's research team deciphered the molecular circuitry governing hepatic stellate cells (HSCs), fundamentally redefining their biological identity. Through systematic molecular profiling, the investigators demonstrated the dual function of HSCs as pathological instigators of fibrotic cascades and master regulators orchestrating hepatic homeostasis. This mechanistic reclassification transcends conventional pathophysiological frameworks, revealing HSCs as dynamic sentinels at the crossroads of liver injury. The elucidation of this dichotomous regulatory effect opens new avenues for targeted interventions in hepatic disorders [1].
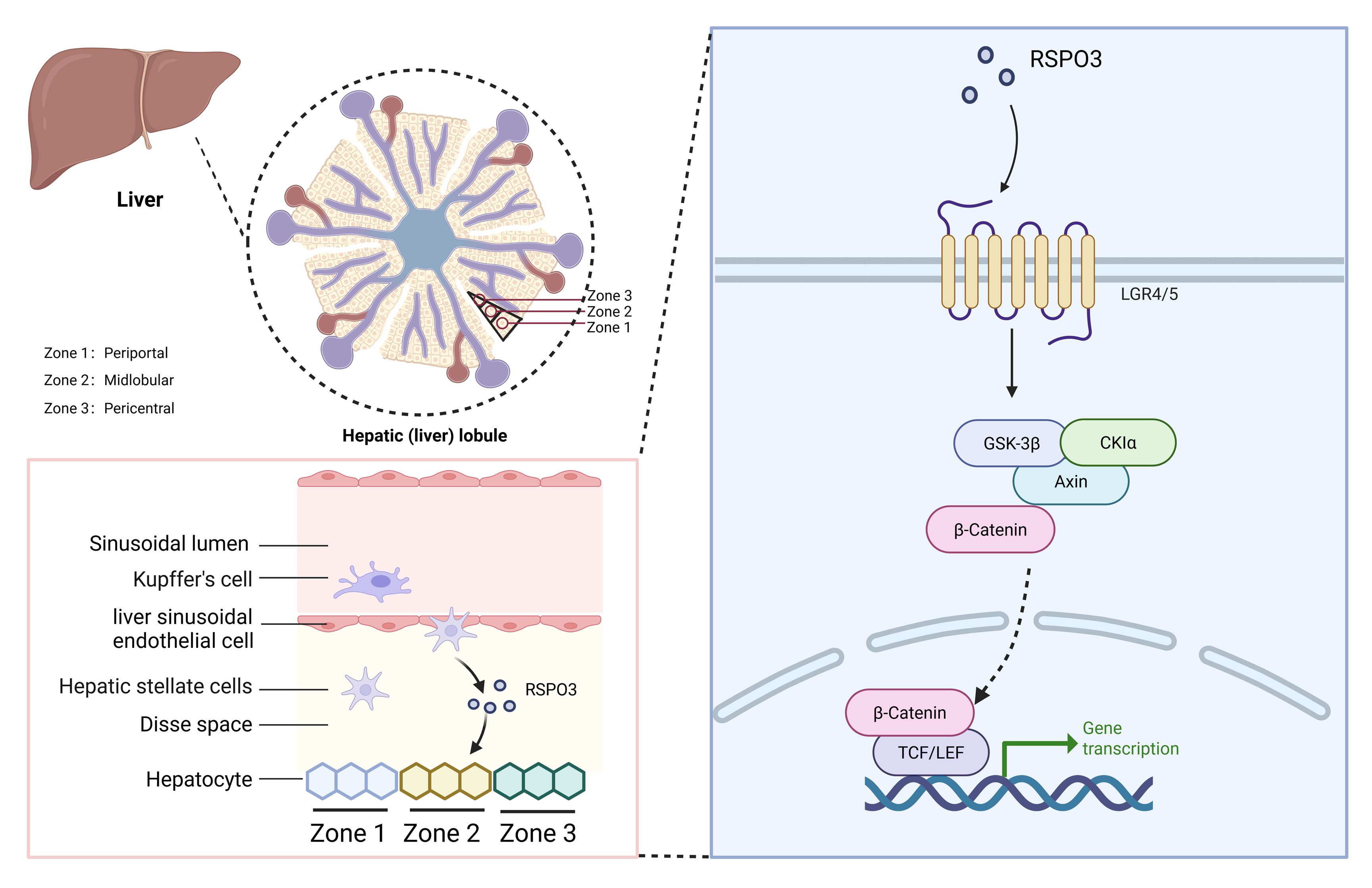
Liver serves as the central regulatory nexus for carbohydrate, glucose, and protein metabolism, orchestrating both xenobiotic detoxification and endogenous metabolic clearance while exhibiting remarkable regenerative plasticity. This metabolic command center operates through spatially orchestrated hepatocyte zonation: pericentral regions specialize in cytochrome P450 mediated detoxification, periportal zones govern gluconeogenesis and urea cycle regulation, while midlobular territories demonstrate metabolic repair capabilities. [2] Disruption of this architectural zonation predisposes to pathophysiological cascades, including oncogenic transformation and fibrotic remodeling. Hepatocyte survival within their 3D microniches (The 3D spatial niche of cell survival, including spatial geometry, composition, and spatial distribution of cells and ECMs.) requires synergistic interactions with sinusoidal endothelia (mediating metabolite exchange), cholangiocytes, and Kupffer cells [3]. HSCs, while classically recognized for retinoid storage and fibrogenic transformation, now emerge as enigmatic metabolic sentinels. These cells are located at the sinusoidal junction and undergo myofibroblast activation after injury, driving collagen deposition and progressing from reparative scarring to liver cirrhosis pathogenesis.
Current research is facing a fundamental knowledge gap regarding the stable governance of HSCs. Genetic or pharmacological HSC ablation studies have yielded contradictory results in regeneration and injury response, revealing non-standard functions beyond fibrosis. Emerging evidence suggests that HSCs may regulate liver metabolism through paracrine proximal cross action, possibly affecting zoning patterns through Wnt/RSPO3 signaling gradients. The dual function of cells—quiescent metabolic regulators and activated fibrosis effectors—makes them key arbitrators of liver fate. Deciphering their baseline regulatory circuitry may hold the key to unlocking regenerative reprogramming strategies capable of reversing end-stage hepatic degeneration.
Liver regeneration is orchestrated through hepatocyte hyperplasia and clonal expansion of neighboring parenchymal cells. Crucially, hepatic stellate cells (HSCs) function as metabolic gatekeepers, preserving hepatic equilibrium via paracrine secretion of growth modulators and niche-specific signaling molecules [4,5]. Schwabe et al. now redefine HSCs as cellular conductors of regenerative processes. Genetic ablation of HSCs induces profound regenerative deficits, marked by impaired hepatic mass restoration and suppression of proliferation biomarkers. Strikingly, toxin susceptibility exhibits zonal specificity: midlobular hepatotoxins demonstrate attenuated cytotoxicity, while periportal agents paradoxically exacerbate injury. This toxicodynamic dichotomy probably stems from compartmentalized detoxification machinery-midlobular zones rely on enzymatic biotransformation, while periportal vulnerability arises from lobular restructuring and expansion of toxin-sensitive territories. Collectively, these findings position HSCs as spatiotemporal regulators governing dual hepatic fate trajectories: orchestrating regenerative competence while modulating injury thresholds. Mechanistically, HSCs may exert zonation-selective control over hepatocyte behavior through dynamically secreted morphogens, creating biochemical gradients that shape regional repair responses. Such insights illuminate HSCs as master regulators at the nexus of hepatic degeneration and regeneration.
To elucidate these regulatory mechanisms at a systemic level, the investigators conducted a multi-omic integration of HSC-hepatocyte crosstalk, revealing profound metabolic-reparative rewiring in HSC-depleted livers. Transcriptomic landscape profiling demonstrated dysregulation of core metabolic modules and regenerative transcriptional programs in hepatocytes. Single-cell resolution coupled with spatial transcriptomic cartography exposed zonation boundary collapse: midlobular cytochrome P450 isoforms exhibited spatial contraction, while periportal markers displayed aberrant territorial expansion. Crucially, Wnt/β-catenin axis-a master regulator of hepatic spatiotemporal regulation governing proliferation, differentiation, and metabolic zonation was systemically suppressed [6]. Notably, RSPO3 emerged as the paracrine signaling axis bridging HSCs and hepatocytes. Mechanistic dissection revealed that RSPO3 engages its cognate receptors LGR4/5 to amplify Wnt signal transduction, forming a ligand-receptor dyad that potentiates hepatocyte proliferative capacity and metabolic competence. This molecular dialogue directly enhances hepatic organoid regeneration fidelity, positioning RSPO3-LGR signaling as a therapeutic rheostat for liver repair [7].
To delineate RSPO3’s role in hepatic morphometric regulation and zonation patterning, the researchers engineered a murine model with HSC-specific RSPO3 ablation. Strikingly, this genetic perturbation recapitulated cardinal features of HSC-depleted systems: diminished hepatosomatic indices, suppression of WNT/β-catenin transcriptional activity, and metabolic zonation disarray [8]. Spatial mapping revealed that HSC-derived RSPO3 establishes a paracrine morphogen gradient spanning midlobular to periportal domains, critically maintaining compartment-specific marker patterning through LGR4/5 receptor agonism. Conditional Rspo3 knockout triggered profound lobular restructuring-Zone 3 exhibited progressive atrophy, while Zone 1 underwent pathological territorial expansion, culminating in global metabolic zonation collapse. The role of RSPO3 as a molecular axis involves the involvement of liver cell LGR4 and β-catenin cascade activation, synchronizing liver size, spatiotemporal regulation, and functional specialization. Translational validation in clinical cohorts demonstrated dynamic RSPO3-LGR4/5 axis modulation across fibrotic pathologies. In early-stage MASLD/ALD, HSCs exhibited compensatory RSPO3 overexpression, correlated with preserved hepatic functionality and active WNT signaling. Conversely, advanced fibrosis exhibited maladaptive RSPO3 hyperexpression—a paradoxical "exhaustion phenotype" marked by metabolic decompensation and regenerative failure. These findings position RSPO3 as an evolutionarily conserved regulatory paradigm and candidate stratification biomarker for hepatic decline trajectories. As a secreted protein, RSPO3 can induce the expression of various pro-fibrotic chemokines and cytokines such as IP-10, MIP-1α, and TNF-α in Kupffer cells and hepatocytes by enhancing the activity of Wnt/β-catenin signaling pathway, which affects the process of liver fibrosis. Wnt signaling plays a conserved evolutionary role in regulating embryonic development, tissue homeostasis, and cell fate determination. Extracellular Wnt ligands and receptors are good targets for specific antibodies. In most cases, mutations in Wnt signaling occur mainly downstream of Wnt ligands, which leads to therapeutic strategies targeting Wnt ligands (including SFRP, DKK, etc.) that may have limited effect, and are relatively easy to use as cell surface receptors (R-spondin).The function of RSPO3 in the Wnt pathway is highly dependent on its specific binding to LGR receptors and its regulation of RNF43/ZNRF3, which is inseparable from the tissue specificity, signal amplification and unique resistance mechanism of RSPO3.
Collectively, this investigation delineates the central mechanistic paradigm by which HSCs govern hepatic zonation, regenerative plasticity, and metabolic coordination via the RSPO3-WNT/β-catenin axis-fundamentally recontextualizing HSC pathophysiology while advancing systemic comprehension of hepatic physiology and disease pathogenesis (Figure 1). These insights establish novel therapeutic nodes for hepatic pathologies, yet critical knowledge gaps persist. The RSPO3-dependent signaling lattice remains incompletely mapped, with unresolved questions spanning dose-dependent oncogenic liability from pathway hyperactivation to interspecies translational discrepancies. Similarly, potential interference with conditional knockout models (other cells secreting compensatory factors, etc.), insufficient validation of human disease models, and crosstalk between metabolism and the liver microenvironment should also be considered. The above limitations may serve as an entry point for future research, including the use of organoids or humanized model validation mechanisms, analysis of HSCs heterogeneity in combination with single-cell sequencing, and exploration of the translational potential of R-spondin 3 in the treatment of liver disease. Likewise, abnormally activated WNT signals are the trigger for uncontrolled cell proliferation, which has the potential to promote liver cancer risk and needs to be precisely regulated. Targeted strategy design may be an effective solution to these problems, including the development of RSPO3-specific antibodies or small molecule inhibitors and combination therapy to balance signaling pathway stability, etc. Although small molecule inhibitors targeting their related pathways are actively being developed, targeting HSCs or hepatocytes remains a difficult task, and new nanomaterials or viral vectors may be the breakthrough.
Figure 1. HSCs can exert a protective effect on the liver through RSPO3. Liver lobules can be further divided into 3 zones: periportal (zone 1), midlobular (zone 2) and pericentral (zone 3), which cooperate to maintain liver homeostasis. HCSs play a protective role in the liver by secreting RSPO3 and acting on Wnt/β-catenin. This figure is created by BioRender.
Methodologically, the evolution of high-precision lobular mapping modalities—capable of real-time visualization of metabolic flux-may illuminate zonation dynamics during functional restoration. While preclinical models validate the tripartite guardian role of HSCs, clinical translation demands cross-disciplinary convergence to engineer therapies harmonizing fibrotic resolution, metabolic reprogramming, and regenerative potentiation. Targeting the HSC-RSPO3 axis strategies hold dual promise: not merely arresting fibrogenesis but reinstating metabolic-regenerative equipoise. Such interventions could transcend conventional antifibrotics by orchestrating a therapeutic trifecta—simultaneously dismantling scar matrices, rejuvenating metabolic networks, and rekindling regenerative competence.
Competing Interests
The authors declare that they have no competing interests
Funding
This work was supported by the generous support from the Program of Natural Science Foundation of State (Grant No. 82104733, 81973745).
Authors' Contributions
Qiang Yang: Writing — original draft, Conceptualization Resources, Data Curation. Ying Cai: Resources, Data Curation, Visualization. SiFan Guo: Resources, Data Curation. Zhibo Wang: Resources, Data Curation. Shi Qiu.: Conceptualization, Methodology, Writing – review & editing. Aihua Zhang: Conceptualization, Formal analysis, Methodology, Writing – review & editing, Funding acquisition.
Acknowledgements
We also thank BioRender and MetaboAnalyst for the figure preparation.
Disclosure Instructions
The article was written without the use of AI.
References
2. Santos AA, Delgado TC, Marques V, Ramirez-Moncayo C, Alonso C, Vidal-Puig A, et al. Spatial metabolomics and its application in the liver. Hepatology. 2024 May 1;79(5):1158-79.
3. Guilliams M, Bonnardel J, Haest B, Vanderborght B, Wagner C, Remmerie A, et al. Spatial proteogenomics reveals distinct and evolutionarily conserved hepatic macrophage niches. Cell. 2022 Jan 20;185(2):379-96.e38.
4. Trinh VQ, Lee TF, Lemoinne S, Ray KC, Ybanez MD, Tsuchida T, et al. Hepatic stellate cells maintain liver homeostasis through paracrine neurotrophin-3 signaling that induces hepatocyte proliferation. Sci Signal. 2023 May 30;16(787):eadf6696.
5. Tao J, Wu Z, Liang Y, Wang J, Tang M, Huang S, et al. Lhx2 specifically expressed in HSCs promotes liver regeneration and inhibits liver fibrosis. Hepatology. 2024 Dec 18.
6. Hu S, Liu S, Bian Y, Poddar M, Singh S, Cao C, et al. Single-cell spatial transcriptomics reveals a dynamic control of metabolic zonation and liver regeneration by endothelial cell Wnt2 and Wnt9b. Cell Rep Med. 2022 Oct 18;3(10):100754.
7. Rocha AS, Vidal V, Mertz M, Kendall TJ, Charlet A, Okamoto H, et al. The Angiocrine Factor Rspondin3 Is a Key Determinant of Liver Zonation. Cell Rep. 2015 Dec 1;13(9):1757-64.
8. Planas-Paz L, Orsini V, Boulter L, Calabrese D, Pikiolek M, Nigsch F, et al. The RSPO-LGR4/5-ZNRF3/RNF43 module controls liver zonation and size. Nat Cell Biol. 2016 May;18(5):467-79.