Commentary
Intrahepatic cholangiocarcinoma (ICC), the second most frequent primary liver tumor, is a highly metastatic malignancy and often leads to disaster outcome in majority of patients, bringing significant challenges in the medical field [1]. Most ICC patients are diagnosed at an advanced stage with metastatic lesions. Of note, less than 30% of patients are eligible for curative resection when diagnosed due to a proclivity for early metastasis [1]. Metastasis is also the deadliest stage in ICC progression. To this end, it is important to reveal the molecular mechanisms driving ICC metastasis to boost the discovery of new therapeutic strategies.
Tumor-repopulating cells (TRCs) are a tumorigenic sub-population of cancer cells with stemness features [2]. TRCs have been implicated in various hallmarks of cancer, including death resistance, metabolic reprogramming, immune evasion, epigenetic reprogramming, and senescence-associated secretory phenotype, etc. [3-7]. TRCs are also one of the most critical factors for promoting the metastasis of cancers. As high metastatic tumor cell sub-populations, even as few as about ten TRCs are sufficient to form lung metastasis [8]. Previous studies showed that low level of SOX2 is required for the metastasis of melanoma via regulating the dormancy propensity of TRCs [9]. Tumor cell-released microparticles reprogram the lung microenvironment and promote the metastasis of TRCs to lung [10]. However, no studies focused on the role of TRCs in ICC metastasis, so an in-depth understanding warrants further investigation.
In a recent research article published in Cellular Signalling [11], Du et al. revealed the function of Hexokinase 2 (HK2) in modulating metastasis of ICC. The researchers calculated a single sample geneset enrichment analysis (ssGSEA) based stemness index in ICC and found its significant association with ICC’s prognosis. They also identified 54 stemness-related anoikis genes (SRAGs) and established a novel anoikis-related classification for ICC. They found anoikis had a close implication in the stemness and metastasis in ICC via bioinformatics analyses. Then, several machine learning algorithms were employed to explore the key regulated SRAGs, HK2. HK2 was up-regulated in ICC-TRCs that possessed enhanced migration and invasion ability, as well as anoikis resistance, which may be due to the activated MTORC1 signaling. Additionally, inhibiting MTORC1 signaling pathway decreased the expression of HK2, and, suppressing HK2 promoted anoikis and thus weakened the migration and invasion ability in ICC-TRCs. Importantly, MTORC1 signaling pathway promoted the expression of HK2, empowering ICC-TRCs with the ability to resist anoikis, which further promote the metastasis of ICC.
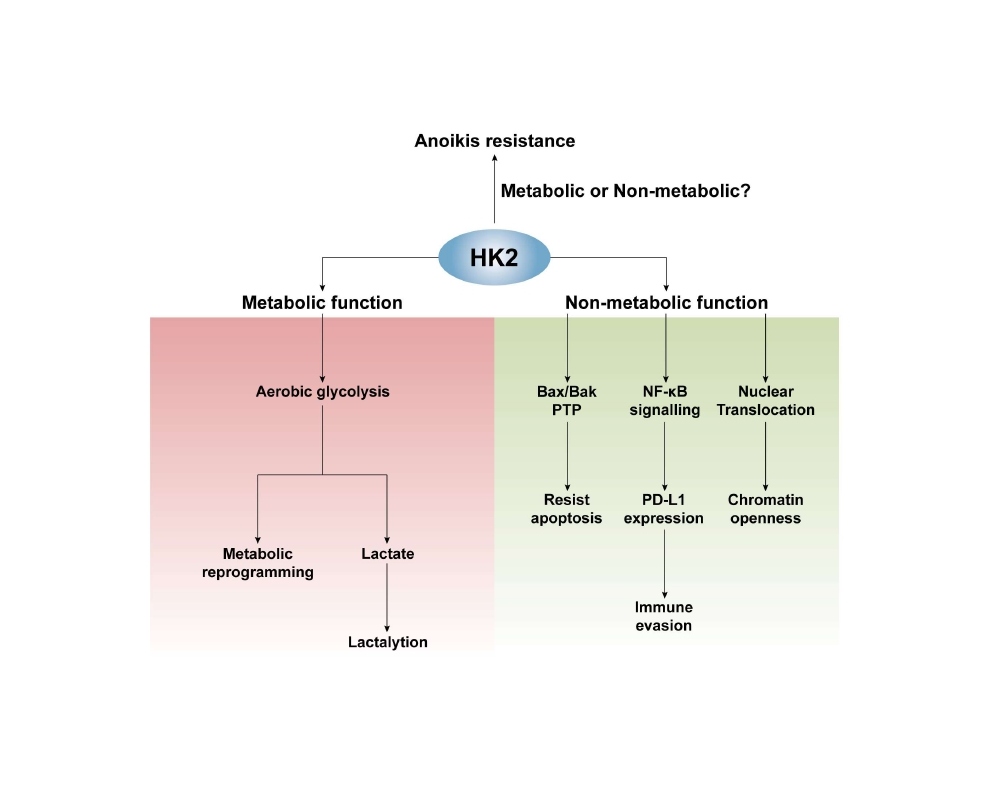
HK2 acts both metabolic and non-metabolic function in cancers. First of all, HK2 can directly combine with mitochondrial VDAC and catalyze the conversion of glucose to glucose-6-phosphate, the first rate-limiting step of aerobic glycolysis, which is the major energy metabolism mode of cancer [12]. Accordingly, HK2 fuels tumor progression and metastasis via regulating metabolic reprogramming. Recent studies reveal novel function of lactate, the main metabolite of aerobic glycolysis, that is lactylation [13]. Both histone and nonhistone proteins can be modified by lactylation, altering the activity, stability, and cellular location of related proteins in cancers [13-15]. These studies closely link aerobic glycolysis and gene regulation as well protein function, greatly enriching HK2’s metabolism function in cancers. Secondly, HK2 can directly antagonize apoptosis stimuli. It suppresses the activity of pro-apoptotic proteins, such as Bax and Bak, yet protects cells from apoptosis also independently of them [12]. Mitochondria HK2 can transduce a permeability transition pore (PTP) closing signal, restricting the release of apoptogenic proteins, and thus resisting cell apoptosis. And then, HK2 can phosphorylation-dependently localize to the nuclear. Nuclear HK2 interacts with nuclear proteins and thus regulates chromatin openness, leading to the increase of chromatin accessibilities at stem cell-positive signature and DNA-repair sites in leukemia, which indicated the directed regulation of HK2 on gene expression [16]. Next, HK2 can act as a kinase to phosphorylate IκBα, resulting in the activation of NF-κB signaling that up-regulates the expression of PD-L1 and further promotes immune evasion [17]. Finally, this study revealed that HK2 can protect ICC-TRCs from anoikis, which expanded a novel function of HK2 in cancer. (Figure 1).
Figure 1: The function of HK2 in cancers.
However, this paper didn’t provide the detailed mechanism under this function of HK2. Further research can focus on two aspects. For one thing, the lactylation of some proteins may block anoikis. For another, the regulation of nuclear HK2 on gene expression may alter the expression of anoikis-related genes. In addition, this paper draws their conclusions only based on cell and database data. More convinced data should be obtained from animal models or clinical samples.
Funding Information
This work was supported by the Shanghai East Hospital Youth Research Cultivation Fund (DFPY2023010).
Declaration of Interest
None.
References
2. Du X, Qi Z, Xu J, Guo M, Zhang X, Yu Z, et al. Loss of GABARAPL1 confers ferroptosis resistance to cancer stem‐like cells in hepatocellular carcinoma. Molecular Oncology. 2022 Oct;16(20):3703-19.
3. Tang K, Zhu L, Chen J, Wang D, Zeng L, Chen C, et al. Hypoxia promotes breast cancer cell growth by activating a glycogen metabolic program. Cancer Research. 2021 Oct 1;81(19):4949-63.
4. Luo S, Li Y, Ma R, Liu J, Xu P, Zhang H, et al. Downregulation of PCK2 remodels tricarboxylic acid cycle in tumor-repopulating cells of melanoma. Oncogene. 2017 Jun;36(25):3609-17.
5. Liu Y, Liang X, Dong W, Fang Y, Lv J, Zhang T, et al. Tumor-repopulating cells induce PD-1 expression in CD8+ T cells by transferring kynurenine and AhR activation. Cancer Cell. 2018 Mar 12;33(3):480-94.
6. Liu Y, Lv J, Liang X, Yin X, Zhang L, Chen D, et al. Fibrin stiffness mediates dormancy of tumor-repopulating cells via a Cdc42-driven Tet2 epigenetic program. Cancer Research. 2018 Jul 15;78(14):3926-37.
7. Du X, Zhang X, Qi Z, Zeng Z, Xu Y, Yu Z, et al. HELLS modulates the stemness of intrahepatic cholangiocarcinoma through promoting senescence-associated secretory phenotype. Computational and Structural Biotechnology Journal. 2023 Jan 1;21:5174-85.
8. Liu J, Tan Y, Zhang H, Zhang Y, Xu P, Chen J, et al. Soft fibrin gels promote selection and growth of tumorigenic cells. Nature Materials. 2012 Aug;11(8):734-41.
9. Jia Q, Yang F, Huang W, Zhang Y, Bao B, Li K, et al. Low levels of Sox2 are required for melanoma tumor-repopulating cell dormancy. Theranostics. 2019;9(2):424-35.
10. Zhang H, Yu Y, Zhou L, Ma J, Tang K, Xu P, et al. Circulating tumor microparticles promote lung metastasis by reprogramming inflammatory and mechanical niches via a macrophage-dependent pathway. Cancer Immunology Research. 2018 Sep 1;6(9):1046-56.
11. Du X, Qi Z, Jiao Y, Wu W, Huang Q, Sun X, et al. HK2 promotes migration and invasion of intrahepatic cholangiocarcinoma via enhancing cancer stem-like cells' resistance to anoikis. Cellular Signalling. 2024 Jun 1;118:111126.
12. Ciscato F, Ferrone L, Masgras I, Laquatra C, Rasola A. Hexokinase 2 in cancer: a prima donna playing multiple characters. International journal of molecular sciences. 2021 Apr 29;22(9):4716.
13. Zhang D, Tang Z, Huang H, Zhou G, Cui C, Weng Y, et al. Metabolic regulation of gene expression by histone lactylation. Nature. 2019 Oct 24;574(7779):575-80.
14. Meng Q, Sun H, Zhang Y, Yang X, Hao S, Liu B, et al. Lactylation stabilizes DCBLD1 activating the pentose phosphate pathway to promote cervical cancer progression. Journal of Experimental & Clinical Cancer Research. 2024 Jan 31;43(1):36.
15. Yang K, Fan M, Wang X, Xu J, Wang Y, Tu F, et al. Lactate promotes macrophage HMGB1 lactylation, acetylation, and exosomal release in polymicrobial sepsis. Cell Death & Differentiation. 2022 Jan;29(1):133-46.
16. Thomas GE, Egan G, García-Prat L, Botham A, Voisin V, Patel PS, et al. The metabolic enzyme hexokinase 2 localizes to the nucleus in AML and normal haematopoietic stem and progenitor cells to maintain stemness. Nature Cell Biology. 2022 Jun;24(6):872-84.
17. Guo D, Tong Y, Jiang X, Meng Y, Jiang H, Du L, et al. Aerobic glycolysis promotes tumor immune evasion by hexokinase2-mediated phosphorylation of IκBα. Cell Metabolism. 2022 Sep 6;34(9):1312-24.