Abstract
Although human civilization has developed through genomic evolution, including its fingernail-functional cooperation, the daily lives of humans have resulted in a significant amount of carbon dioxide (CO2) being released into the atmosphere since the Industrial Revolution, which started almost 200 years ago. Recently, climate change has been documented to have spread globally. For example, terrestrial rains have caused severe floods in Europe, and one of the largest tornados in history occurred in the Kentucky region of the United States of America in 2021. Furthermore, glaciers are melting rapidly. This change is clearly due to the accumulation of CO2 in the atmosphere. Therefore, this accumulated CO2 must be eliminated as soon as possible to prevent further worsening of the climate crisis. However, CO2 reduction cannot be achieved simply by the use of solar or wind power. The concept of a carbon-neutral society by 2050 seems too late. Contrarily, CO2 can be captured experimentally from the air or from the exhaust gases through various techniques, including absorption, adsorption, and membrane separation. Adsorption with amines is currently the dominant technology, but it is not largely used because it involves the use of toxic organic solvents. Therefore, this study reports the development of an innovative method for CO2 fixation and storage. Using this method, CO2 is converted to CaCO3, limestone, or corral, a harmless natural compound, using NaOH and CaCl2. This novel method can be used to convert fossil fuels, such as coal, oil, and natural gas, into Earth components using a simple and inexpensive system without environmental concerns.
Keywords
CO2 fixation, Storage, Climate change, Industrial Revolution, Human, Earth, Fossil Fuel, Coal, Oil, Natural gas, Environment, Evolution
Introduction
Charles Darwin made a statement based on his natural selection theory approximately half a century ago “It is not the strongest of the species that survives, nor the most intelligent that survives. Rather, the one that is most adaptable to changes.” However, some people believe in “the law of the jungle” and that human beings are ranked at the top among all the organisms on Earth. Climate change due to atmospheric CO2 began with the Industrial Revolution and has progressed along with civilization. The Industrial Revolution drastically changed our daily lives, making it more luxurious and easy through the use of fossil fuels, such as coal and oil. Our daily lifestyle results in the continuous emission of CO2 into the atmosphere and the accumulation of atmospheric CO2, which has inevitably led to the current climate crisis.
Although there have been several significant environmental changes over time, the Earth’s environment has been unusually stable for the past 10,000 years [1]. In all these years, various natural systems have regulated the Earth’s climate and maintained conditions to facilitate human development. However, these regulatory systems have been significantly disturbed in the recent years, and we may be approaching a threshold beyond which unpredictable environmental changes may occur, such as an increase in the mean global temperature [2]. In this review, the evolution of the human race and Earth and its associations with CO2 are described.
Biological Evolution
The measurement of morphological changes in organisms or geological fossil records has been extensively adopted to evaluate the degree of evolution. The microbial fossils have been discovered in 3.5-billion-year-old rocks [3-6], indicating that primitive organisms existed on Earth. Polynucleotides; deoxyribonucleic acids (DNA), including adenine (A), guanine (G), thymine (T), and cytosine (C); and ribonucleic acids (RNA), including adenine (A), guanine (G), uracil (U), and cytosine (C), comprise ribonucleotides that contain the characteristics of all organisms. These characteristics are transferred to the descendants through polynucleotide sequences. The genomic DNA sequences are replicated to transfer their characteristics to their descendants. However, a part of the DNA is transcripted onto the RNA where the message is translated to produce a protein. Changes in DNA occur spontaneously during these processes because of endogenous or exogenous factors, resulting in mutations. The DNA changes result in biological evolution.
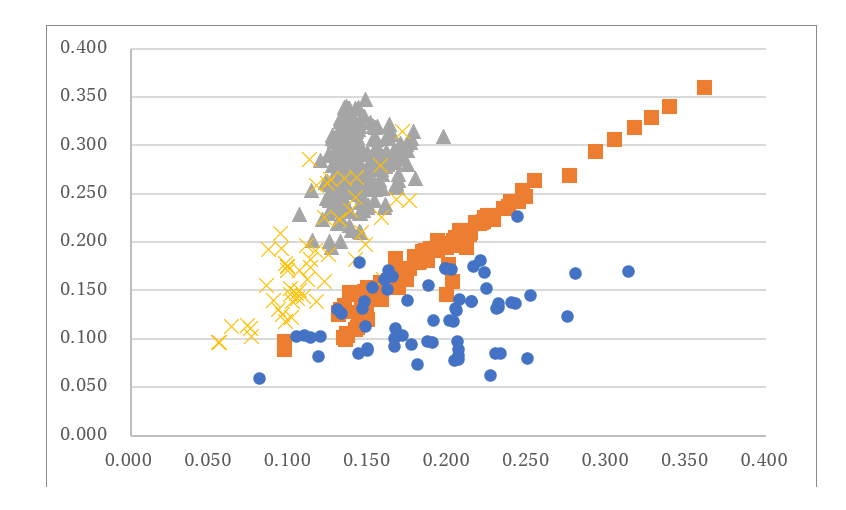
Chargaff reported the first experimental study on cellular DNA content and proposed the first parity rule stating that A = T and G = C in cellular nuclei [7]. This phenomenon may have influenced the discovery of Watson and Crick’s double-stranded DNA structure [8]. In addition, this DNA structure can explain the genetic phenomena. On normalizing the cellular DNA, A + G + C + T = 1, it was revealed that this method represents the entire organism characteristics [9], i.e., the content of each nucleotide in the cells is represented by that of another nucleotide based on Chargaff’s first parity rule [10,11]. For example, by plotting the content of C against that of G in various cellular organelles, linear regression lines were obtained in the chromosomes, chloroplasts, and plant mitochondria. Moreover, the regression lines for animal mitochondria, which were separated into two groups based on the content of C/G, as high or low C/G, intersected at a single point (Figure 1). Therefore, it was concluded that all organisms had a single origin of life [11,12]. In this plot, the content of C in the mitochondria of Monosiga brevicollis is the lowest among the numerous cellular organelles, including prokaryotes and eukaryotes, indicating that this mitochondrion is the most primitive extant organelle [13]. Another research group reported that the mitochondria of M. brevicollis were derived from Reclinomonas americana [14]. However, as per the results of complete genome analysis, King et al. reported that M. brevicollis is the origin of all animal species [15].
Figure 1: Plotting C content against G content. Gray triangles, vertebrate mitochondria from 69 mammals, 42 birds, 63 fishes, 16 Chondrocytes, 32 reptiles, and 22 amphibians; yellow crosses, 54 high C/G content invertebrates; blue circles, 60 low C/G content invertebrate mitochondria; and orange squares, 42 plant mitochondria, 29 chloroplasts, 20 prokaryote chromosomes, 9 archaea chromosomes, and 14 eukaryote chromosomes. This figure was adapted from Sorimachi [53].
The normalized C content of mitochondrial DNA represents biological evolutionary divergence (Figure 1). The mitochondrial C content (0.347) in the pileated woodpecker Dryocopus pileatus was the highest from among the various organisms that were examined, including Homo sapiens (humans; 0.313), Pongo abelii (orangutan; 0.327), and Pan troglodytes (chimpanzee; 0.307). Moreover, Cuvier’s dwarf caiman, Paleosuchus palpebrous (0.340), had the highest mitochondrial C content among all reptiles. Furthermore, hemichordate, including the acorn worm Balanoglossus carnosus (0.314), had high mitochondrial C content. However, the mitochondrial C content of the European hedgehog Erinaceus europaeus (0.201), which was the lowest among all examined mammalian mitochondria, was much lower than those of the hemichordates. These results indicate a discrepancy between genomic and apparent biological evolutions.
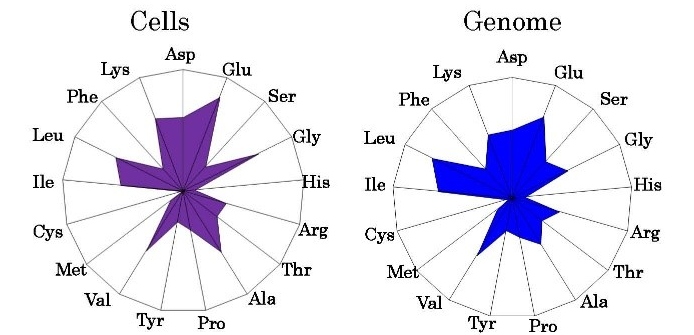
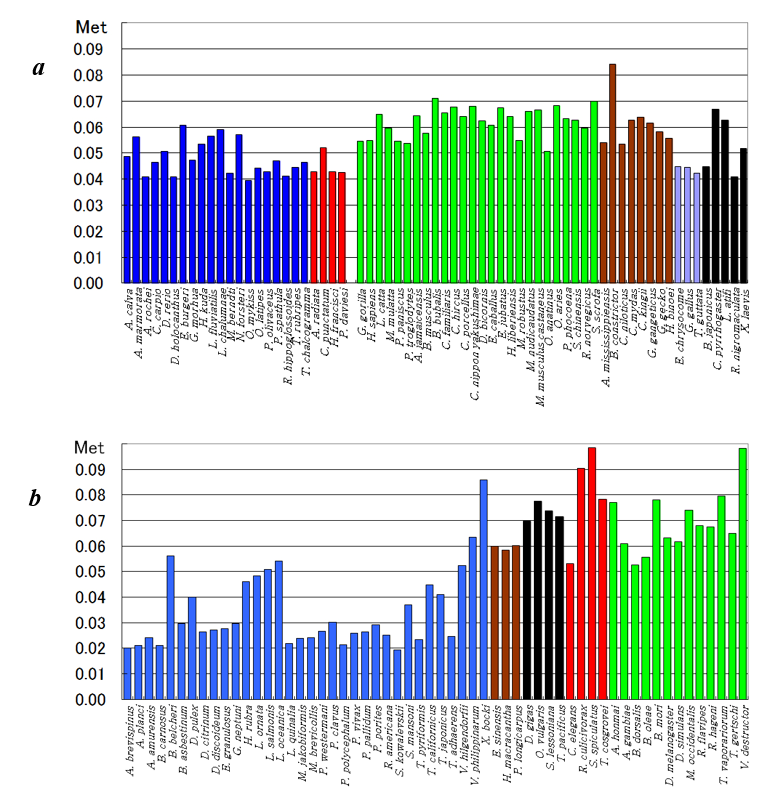
The composition of cellular amino acids in bacterial cells has been analyzed [16], whereas those of other mammalian-cultured cells have been independently examined and expressed as the normalized values using radar charts [17]. The radar chart, based on 20 cellular amino acid compositions, signifies the organism characteristics (Figure 2); the radar charts of various organisms indicate their biological divergence [17]. Furthermore, phylogenetic trees were constructed using the normalized amino acid composition [18,19], and the normalized mitochondrial amino acid composition’s methionine content indicated the evolution of organisms from the ocean to land (Figure 3). Further, eubacteria, including 11 gram-positive and 12 gram-negative bacteria, were categorized into two groups, S-type represented by Staphylococcus aureus and E-type represented by Escherichia coli, according to the patterns of their amino acid compositions presumed from the complete genome. These two groups were characterized by their concentrations of Arg, Ala, and Lys [20]. Moreover, organisms comprising 112 eubacteria, 15 archaea, and 18 eukaryotes were categorized into two major groups as AT-type and GC-type based on cluster analysis using GC contents at the three-codon position [21]. The mitochondrial methionine content increased with the increased adaption to land, and the cellular methionine content of prokaryotes was approximately 0.02%, almost equivalent to that of many aquatic organisms.
Figure 2: Radar charts of cellular and genomic amino acid compositions. Values are expressed as the percentages of total amino acids. Pyrococcus horikoshii was examined. The cellular amino acid composition was obtained from three independent analyses. In genomic calculations, Gln and Asn were also incorporated into Glu and Asp, respectively, to compare with the data based on amino acid analysis. This figure was adapted from Sorimachi [52].
Figure 3: Bar graphs of methionine content in vertebrates (a) and invertebrates (b). This figure was adapted from Sorimachi [11].
The amino acid sequence of proteins [22] and the nucleotide sequence of genes [23] were analyzed to understand biological evolution. Combined with computer technologies, these methodologies could be initially used to determine the complete genome of Haemophilus influenzae [24] and, subsequently, the complete human genome with advancement in technology [25,26]. Surprisingly, these studies revealed that the number of protein genes was not significantly different between the purple sea urchin Strongylocentrotus purpuratus (27,447) [27] and Homo sapiens (19,618) [25,26]. However, the number of total nucleotides were different (921,855,793 and 3,099,441,038, respectively). Figure 2 shows the radar chart of the amino acid composition of the whole-cell homogenates, which is similar to that predicted using the complete genome [28]. This coincidence was because of the homogenous genome structure wherein similar units were repeated [29]. When amino acid compositions were presumed from complete genomes by assuming that each gene is equally expressed in a whole cell, gene assemblies encoding approximately 3,000–7,000 amino residues represented the amino acid compositions presumed from the complete genomes [29].
Evolution of the Earth
The solar system is believed to have been was formed approximately 4.6 billion years ago through the collision and assembly of the micro planets, resulting in the formation of the Earth along with seven other planets, including Mercury, Venus, Mars, Jupiter, Saturn, Uranus, and Neptune, and a sub planet, Pluto. However, to the best of our knowledge, only the Earth is inhabitable for living organisms among the several planets in the universe. Even a trace of dead organisms has not been detected on other planets in the universe. This indicates that the Earth is an extremely special and the only inhabitable planet in the universe. The elements present on Earth include iron (32.1%), oxygen (30.1%), silicon (15.1%), magnesium (13.9%), sulfur (2.9%), nickel (1.8%), calcium (1.5%), and aluminum (1.4%), with the remaining 1.2% comprising other elements [30]. Furthermore, fossil fuels, including coal, oil, and natural gas, are also present in the Earth’s crust.
The primitive Earth could have been a hot planet where no organism could survive. However, the land and sea were formed after a certain period, with the land divided into continents according to the plate tectonic theory [31,32]. Biochemical reactions generally occur in the aqueous phase at moderate temperatures on Earth. These temperatures are determined by the distance between the Earth and the Sun; the atmospheric contents, including O2, N2, and the minor amount of CO2; and other elements which control heat radiation. The surface temperature of Venus, which is covered with CO2, is approximately 300°C, where organisms cannot survive. Mars is uninhabitable because of its low temperature and has no atmosphere except for a small amount of dry ice at the polar regions. This makes Earth an extremely valuable planet for living organisms.
Carbon is one of the abundantly available elements in the Earth’s crust. The naturally occurring crystalline forms of carbon are diamond and graphite, and the amorphous form is coal. Additionally, oil and natural gases comprising carbon, hydrogen, oxygen, and minor elements are present in the Earth’s crust as they are formed by organisms that have dead throughout Earth’s long history. Natural phenomena and organisms have produced these carbon compounds. The atmospheric CO2, along with H2O and sunlight, is used by plants to produce carbohydrates. Since ancient times, terrestrial heat has converted these dead plants into coal. CO2 has been converted to corral and stromatolite by coral polyps and cyanobacteria, respectively, forming limestone (CaCO3) in the ocean. These organisms contribute to CO2 fixation, which helps in preserving the beautiful blue planet, Earth (Figure 4).
Figure 4: Earth. This photo was donated by JAXA.
CO2 Characteristics
Carbon is the 15th most abundant element in the Earth’s crust and the fourth most abundant element by mass in the universe, after hydrogen, helium, and oxygen. Carbon compounds, including fossil fuels such as coal, oil, and natural gases, produce energy, and release CO2 in the presence of oxygen. Moreover, modern inventions, such as the steam engine and internal combustion engine, which use fossil fuels, have added CO2 into the atmosphere since the Industrial Revolution that started approximately 200 years ago. Recently, it has been established that CO2, which contributes to the greenhouse effect, induces climate change that increases the global temperature. Several methods to fix CO2, such as absorption, adsorption, penetration, and chemical absorption, have been developed experimentally, including the method using amines; this method is currently used to fix CO2 released from several reactions [33-40]. However, the amines method is not extensively used as amines are toxic organic compounds. Gases are known to penetrate through polymer membranes [41-43], and the penetrability of CO2 is extremely high compared with that of other gases, such as CO2, O2, N2, H2, and CH4 [44]. This high permeability is because of its physicochemical characteristics and not because of the molecular sieve effect. Therefore, membrane separation for CO2 is a useful method because of its simple system [44].
Recently, we developed an innovative method for CO2 fixation and storage [45-47]. In this method, CO2 in the ambient air and artificially prepared high concentration CO2 gas were fixed using low-concentration NaOH and CaCl2 solutions, leading to the formation of CaCO3 and NaCl. These compounds are harmless and stable under normal and natural environmental conditions. Further, as NaOH is produced by NaCl electrolysis, systems associated with generators, such as solar panels, wind power, hydropower, and nuclear reactors, can fix CO2 without the need for external chemical materials using seawater instead of NaCl, without any environmental impact. This innovative method can simultaneously achieve CO2 fixation and storage [44-47]. Furthermore, this combination of simple CO2 fixation and storage and membrane separation can be reproduced to form an artificial forest model [46].
Discussion
Primitive organisms may have formed 3.5 billion years ago in the ocean after chemical evolution. The CO2 dissolved in the ocean forms H2CO3, HCO3−, and CO32−, accounting for approximately 50 times as much carbon dissolved in the oceans as in the atmosphere [48]. Generally, 1.4 g/L of CO2 dissolves in water at 100 kPa and 25°C. However, as observed in the following experiment, almost the entire dissolved CO2 content in water existed as CO2 molecules without forming carbonate ions [44]. When a 2-L polyethylene terephthalate (PET) bottle filled with 1 L of CO2 gas and 1 L of water was shaken vigorously by hand, the PET bottle was completely dented with small gas spaces. If the CO2 molecules formed carbonates, white precipitate of CaCO3 should have been produced after adding CaCl2 solution. However, no precipitate appeared in the solution. In contrast, precipitation occurred in the solution after the alkalization of the solution by NaOH [42]. These results indicate that CO2 does not react directly with Ca2+ in the ocean and that organisms such as coral polyps or cyanobacteria contribute to forming CaCO3, which is a component of coral and stromatolite. Ultimately, these organisms contribute to producing limestone, which is an element of the Earth’s crust. Furthermore, CO2 storage through geosequestration, i.e., by injecting CO2 into underground geological formations, such as oil fields, gas fields, and saline formations, has been proposed [49,50]. However, whether these techniques could be used to safely store a huge amount of CO2 without environmental concerns remains unknown as such technologies are yet to be completely established.
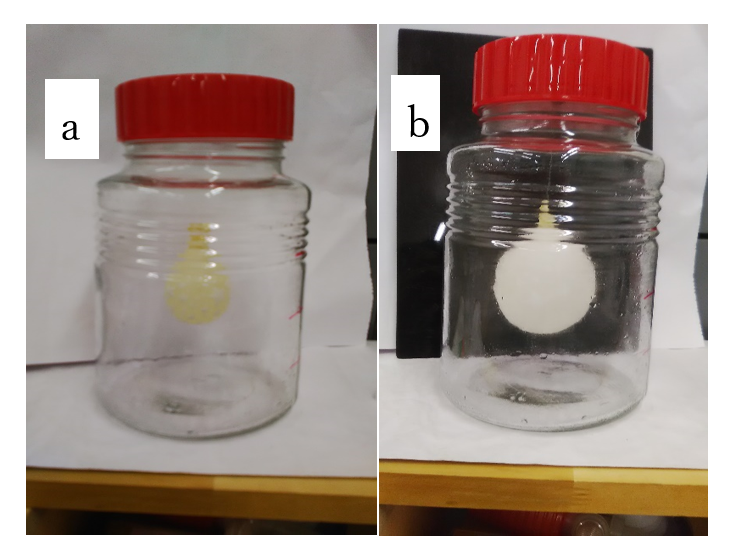
Plants use chlorophyll to fix CO2 and produce carbohydrates through photosynthesis under sunlight. Recently, we demonstrated that CO2 gas easily penetrates the cellulose membranes and visking tubes, whereas N2 and O2 did not [44]. In contrast, H2 and CH4 penetrate the cellulose membranes, whereas their water penetration characteristics are entirely inhibited by water [44]. The inhibitory effect of H2O on H2 or CH4 penetration through the cellulose membranes may be because of their extremely low solubility in water. Furthermore, CO2 and H2O are the final products of the tricarboxylic acid cycle in carbohydrates metabolism, leading to the production of ATP in organisms. Therefore, the characteristic that CO2 penetrates polymer membranes, including cellulose membranes, is extremely crucial in all organisms, including human bodies. The penetration of CO2 occurs smoothly without active transport in the lung tissues as well as in the cellular organelles, including mitochondria, which results in the concentration gradient force pseudo-osmosis in the gas phase [44] (Figure 5). Beginning in early February 2020, extracorporeal membrane oxygenation (ECMO) was used for respiratory support to the patients exhibiting acute viral pneumonia associated with SARS-CoV-2 infection [51]. The principle of the ECMO is based on the high permeability of CO2 through polymer membranes, which separate air and blood. Prokaryotes covered with polysaccharide cell walls can prevent CO2 from entering the cell without a special CO2 excluding system. However, plants can efficiently uptake the atmospheric CO2 from the bodies of organisms through cellulose membranes due to the characteristics of CO2. Therefore, it should be noted that organisms have evolved based on CO2 characteristics.
Figure 5: Pseudo osmosis in the gas phase. (a) The initial sate of a latex balloon, (b) the balloon treated with 80% CO2 in a 4-L glass bottle for 4 h. This partially modified photo was adapted from Sorimachi [44].
Conclusion
Only Homo sapiens have achieved the ultimate evolution due to the cooperation of cerebral and sensitive five-fingernail development. However, their genomic evolution falls behind birds, which are ranked the most evolved organisms, following the complete mitochondrial genomes among the examined organisms [52,53]. Humans continuously produce and release CO2 into the atmosphere to preserve their advanced lifestyle and have been using fossil fuels since the Industrial Revolution. However, it is generally recognized that the increased atmospheric CO2 has induced intense climate changes globally. Human beings are majorly responsible for this crisis. Therefore, it is our moral duty to address the situation through global cooperation to protect our beautiful blue planet not only for the future generations but also for the other organisms who are not responsible for the climate crisis.
Charles Darwin was right in stating that “It is not the strongest of the species that survives, nor the most intelligent that survives. Rather, the one that is most adaptable to changes”.
Acknowledgments
The author thanks Enago (https://www.enago.jp) for editing a draft of this manuscript.
Author Contributions
KS conceived and designed the study and wrote the manuscript.
Competing Financial Interests
The author declares that the present data have been used to support applications to the Japan Patent Office (PCT/JP2019/03400, PTC/JP2019/045389, PCT/JP2019/045390, PCT/2020/026989, PCT/JP2019/048178, PCT/2020/002064, PCT/2020/026990, PCT/JP2020/029505, PCT/JP2020/029504, JP2020/79418, JP2021-090928, JP2021-126892, JP Patent #6783436, 6788170, 6878666, 6788169, 6830564, 6788162, 6739680, 6817485, 6906111, 6864143).
References
2. Rockström J, Steffen W, Noone K, Persson Å, Chapin FS, Lambin EF, et al. A safe operating space for humanity. Nature. 2009 Sep;461(7263):472-5.
3. Schopf JW, Barghoorn ES. Alga-like fossils from the early Precambrian of South Africa. Science. 1967 Apr 28;156(3774):508-12.
4. Nagy B, Zumberge JE, Nagy LA. Abiotic, graphitic microstructures in micaceous metaquartzite about 3760 million years old from southwestern Greenland: implications for early Precambrian microfossils. Proceedings of the National Academy of Sciences. 1975 Mar;72(3):1206-9.
5. Noffke N, Christian D, Wacey D, Hazen RM. Microbially induced sedimentary structures recording an ancient ecosystem in the ca. 3.48 billion-year-old Dresser Formation, Pilbara, Western Australia. Astrobiology. 2013 Dec 1;13(12):1103-24.
6. Schopf JW. Fossil evidence of Archaean life. Philosophical Transactions of the Royal Society B: Biological Sciences. 2006 Jun 29;361(1470):869-85.
7. Chargaff E. Chemical specificity of nucleic acids and mechanism of their enzymatic degradation. Experientia. 1950 Jun;6(6):201-9.
8. Jd W, Fh C. Genetical implications of the structure of deoxyribonucleic acid. Nature. 1953 May 1;171(4361):964-7.
9. Sorimachi K, Okayasu T, Ohhira S. Normalization of Complete Genome Characteristics: Application to Evolution from Primitive Organisms to Homo sapiens. Current Genomics. 2015 Apr 1;16(2):99-106.
10. Sorimachi K, Okayasu T. Universal rules governing genome evolution expressed by linear formulas. The Open Genomics Journal. 2008 Oct 24;1(1):33-43.
11. Sorimachi K. Origin of Life in the Ocean: Direct Derivation of Mitochondria from Primitive Organisms based on Complete Genomes. Current Chemical Biology. 2015 Apr 1;9(1):23-35.
12. Sorimachi K. Genomic data provides simple evidence for a single origin of life. Natural Science. 2010 Jun 1;2(05):519.
13. Sorimachi K. The most primitive extant ancestor of organisms and discovery of definitive evolutionary equations based on complete genome structures. Natural Science. 2018 Sep 4;10(9):338-69.
14. Andersson SG, Zomorodipour A, Andersson JO, Sicheritz-Pontén T, Alsmark UC, Podowski RM, et al. The genome sequence of Rickettsia prowazekii and the origin of mitochondria. Nature. 1998 Nov;396(6707):133-40.
15. King N, Westbrook MJ, Young SL, Kuo A, Abedin M, Chapman J, et al. The genome of the choanoflagellate Monosiga brevicollis and the origin of metazoans. Nature. 2008 Feb;451(7180):783-8.
16. Sueoka N. Correlation between base composition of deoxyribonucleic acid and amino acid composition of protein. Proceedings of the National Academy of Sciences. 1961 Aug;47(8):1141-9.
17. Sorimachi K. Evolutionary changes reflected by the cellular amino acid composition. Amino Acids. 1999 Jun;17(2):207-26.
18. Sorimachi K, Okayasu T, Ebara Y, Furuta E, Ohhira S. Phylogenetic position of Xenoturbella bocki and Hemichordates Balanoglossus carnosus and Saccoglossus kowalevskii based on amino acid composition or nucleotide content of complete mitochondrial genomes. International Journal of Biology. 2014 Jan 1;6(1):82.
19. Sorimachi K, Okayasu T. Classification of non-animals and invertebrates based on amino acid composition of complete mitochondrial genomes. International Journal of Biology. 2014 Jan 1;6(1):1.
20. Sorimachi K, Okayasu T. Classification of eubacteria based on their complete genome: where does Mycoplasmataceae belong?. Proceedings of the Royal Society of London. Series B: Biological Sciences. 2004 May 7;271(suppl_4):S127-30.
21. Okayasu T, Sorimachi K. Organisms can essentially be classified according to two codon patterns. Amino Acids. 2009 Feb;36(2):261-71.
22. Sanger F, Coulson AR. A rapid method for determining sequences in DNA by primed synthesis with DNA polymerase. Journal of Molecular Biology. 1975 May 25;94(3):441-8.
23. Maxam AM, Gilbert W. A new method for sequencing DNA. Proceedings of the National Academy of Sciences. 1977 Feb;74(2):560-4.
24. Fleischmann RD, Adams MD, White O, Clayton RA, Kirkness EF, Kerlavage AR, et al. Whole-genome random sequencing and assembly of Haemophilus Influenzae Rd. Science. 1995 Jul 28;269(5223):496-512.
25. Lander ES. Initial sequencing and analysis of the human genome. International Human Genome Sequencing Consortium. Nature. 2001;409:860-921.
26. Venter JC, Adams MD, Myers EW, Li PW, Mural RJ, Sutton GG, et al. The sequence of the human genome. Science. 2001 Feb 16;291(5507):1304-51.
27. Sodergren E, Weinstock GM, Davidson EH, Cameron RA, Gibbs RA, Angerer RC, et al. The genome of the sea urchin Strongylocentrotus purpuratus. Science. 2006 Nov 10;314(5801):941-52.
28. Sorimachi K, Itoh T, Kawarabayasi Y, Okayasu T, Akimoto K, Niwa A. Conservation of the basic pattern of cellular amino acid composition of archaeobacteria during biological evolution and the putative amino acid composition of primitive life forms. Amino Acids. 2001 Dec;21(4):393-9.
29. Sorimachi K, Okayasu T. Gene assembly consisting of small units with similar amino acid composition in the Saccharomyces cerevisiae genome. Mycoscience. 2003 Oct 1;44(5):415-7.
30. Morgan JW, Anders E. Chemical composition of earth, Venus, and Mercury. Proceedings of the National Academy of Sciences. 1980 Dec;77(12):6973-7.
31. Moser DE, Flowers RM, Hart RJ. Birth of the Kaapvaal tectosphere 3.08 billion years ago. Science. 2001 Jan 19;291(5503):465-8.
32. Chowdhury P, Mulder JA, Cawood PA, Bhattacharjee S, Roy S, Wainwright AN, et al. Magmatic thickening of crust in non–plate tectonic settings initiated the subaerial rise of Earth’s first continents 3.3 to 3.2 billion years ago. Proceedings of the National Academy of Sciences. 2021 Nov 16;118(46):e2105746118.
33. Lv B, Guo B, Zhou Z, Jing G. Mechanisms of CO2 capture into monoethanolamine solution with different CO2 loading during the absorption/desorption processes. Environmental Science & Technology. 2015 Sep 1;49(17):10728-35.
34. Choi S, Drese JH, Jones CW. Adsorbent materials for carbon dioxide capture from large anthropogenic point sources. ChemSusChem: Chemistry & Sustainability Energy & Materials. 2009 Sep 21;2(9):796-854.
35. Jones CW. CO2 capture from dilute gases as a component of modern global carbon management. Annual Review of Chemical and Biomolecular Engineering. 2011 Jul 15;2:31-52.
36. Nandi M, Okada K, Dutta A, Bhaumik A, Maruyama J, Derks D, et al. Unprecedented CO2 uptake over highly porous N-doped activated carbon monoliths prepared by physical activation. Chemical Communications. 2012;48(83):10283-5.
37. Hajra S, Biswas A. Efficient chemical fixation and defixation cycle of carbon dioxide under ambient conditions. Scientific Reports. 2020 Sep 25;10(1): 15825.
38. Hiraide S, Sakanaka Y, Kajiro H, Kawaguchi S, Miyahara MT, Tanaka H. High-throughput gas separation by flexible metal–organic frameworks with fast gating and thermal management capabilities. Nature Communications. 2020 Aug 3;11(1):1-5.
39. Modak A, Nandi M, Mondal J, Bhaumik A. Porphyrin based porous organic polymers: novel synthetic strategy and exceptionally high CO 2 adsorption capacity. Chemical Communications. 2012;48(2):248-50.
40. Qiao Z, Zhao S, Wang J, Wang S, Wang Z, Guiver MD. A highly permeable aligned montmorillonite mixed‐matrix membrane for CO2 separation. Angewandte Chemie. 2016 Aug 1;128(32):9467-71.
41. Araújo T, Bernardo G, Mendes A. Cellulose-based carbon molecular sieve membranes for gas separation: a review. Molecules. 2020 Aug 1;25(15):3532.
42. Xu S, Zhou H, Jia H, Xu J, Ma L, Zang Y, et al. Preparation and high performance of cellulose acetate films by grafting with imidazole ionic liquid. ACS Omega. 2021 May 3;6(19):12500-6.
43. Ho NA, Leo CP. A review on the emerging applications of cellulose, cellulose derivatives and nanocellulose in carbon capture. Environmental Research. 2021 Jun 1;197:111100.
44. Sorimachi K. Epoch-Making Discovery for CO2 Characteristics:“Pseudo Osmosis” in the Gas Phase.
45. Sorimachi K. Innovative method for CO2 fixation and storage. Scientific Reports. 2022 Feb 1;12(1):1-9.
46. Sorimachi K. Novel Method for CO2 Fixation and Storage Preventing Climate Crisis: an “Artificial Forest” Model. Petro Chem Indus Intern, 5 (1), 01. 2022;4:1-4.
47. Sorimachi K. Pilot plant model for sequential CO2 fixation and storage. Adv Environ Stud 2022; 6: 479-83.
48. Sorimachi K. Innovative method for CO2 fixation and storage. Scientific Reports. 2022 Feb 1;12(1):1-9.
49. Eccles J, Pratson LF, Chandel MK. Effects of well spacing on geological storage site distribution costs and surface footprint. Environmental Science & Technology. 2012 Apr 17;46(8):4649-56.
50. Carroll SA, Iyer J, Walsh SD. Influence of chemical, mechanical, and transport processes on wellbore leakage from geologic CO2 storage reservoirs. Accounts of Chemical Research. 2017 Aug 15;50(8):1829-37.
51. Umakanthan S, Sahu P, Ranade AV, Bukelo MM, Rao JS, Abrahao-Machado L, Fet al. Origin, transmission, diagnosis and management of coronavirus disease 2019 (COVID-19). Postgraduate Medical Journal. 2020 Dec 1;96(1142):753-8.
52. Sorimachi K. Visible evolution from primitive organisms to Homo sapiens. Cheminformatics and its Applications. 2020 Feb 12:9-30.
53. Sorimachi K. Study on ultimate human evolution: Cooperation of cerebral and five-fingernail development. New Visions in Biological Science Vol. 3. 2021 Sep 18:50-64.