Abstract
This commentary discusses our recent findings that, contrary to the β-cell rest hypothesis, early short-term insulin administration in a mouse model of marginal β-cell deficiency paradoxically worsened glycemic control and induced adverse α-cell-mediated islet remodeling. Early insulin intervention is hypothesized to preserve β-cell function; however, its utility in marginal β-cell deficiency is not well-established. Our recent study investigated the effects of early insulin administration versus syngeneic islet transplantation in a 50% pancreatectomy mouse model, which mimics a state of marginal β-cell deficiency while maintaining normoglycemia. Contrary to our expectation, short-term exogenous insulin treatment resulted in a paradoxical worsening of glycemic control and induced adverse islet remodeling. Histological analyses revealed this was driven by a significant expansion of α-cells, attributable to both increased proliferation and neogenesis. In contrast, syngeneic islet transplantation achieved superior glycemic control and preserved near-normal islet architecture. These findings suggest that prophylactic insulin use in early dysglycemia may not confer a protective effect and could inadvertently accelerate islet dysfunction. Rather, islet transplantation remains a more effective strategy for maintaining metabolic stability and islet structure in insulin-deficient states. This work calls for caution in the early initiation of insulin treatment in diabetic patients and highlights the need for further investigation into the effects of exogenous insulin on α-cell biology.
Keywords
50% Pancreatectomy, Islet transplantation, Early insulin treatment, Islet morphology
Commentary
Diabetes mellitus has reached a global pandemic level. Approximately 3.4 million adults aged 20-79 years are estimated to have died because of diabetes or its complications in 2024. This corresponds to 9.3% of global deaths from all causes in this age group. Diabetes led to nearly one in ten (9.3%) of all deaths in this age group [1]. Although type 1 and type 2 diabetes have distinct etiologies, both conditions feature a deficiency in β-cell mass and/or defective functionality [2,3]. The treatment goal of diabetes is to achieve durable normoglycemia while preventing long-term complications. Currently, the most promising approach is pancreas or islet transplantation, which restores physiological insulin secretion and maintains the delicate balance between insulin and glucagon [4] that cannot be fully replicated by exogenous insulin administration alone.
Although numerous strategies have been explored over the past several decades to delay or prevent β-cell destruction, no effective treatment exists yet. Early insulin intervention in overt diabetes has been reported to partially restore endogenous β-cell function by reducing β-cell workload, preserving residual function, and potentially promoting regeneration and inducing a transient improvement in glycemic control, like the honeymoon period in Type 1 diabetes [5,6]. However, it is unclear whether insulin therapy, like islet transplantation, can similarly preserve residual β-cell mass in cases of borderline β-cell deficiency and delay or prevent the onset of diabetes in prediabetic stage.
In our recent publication [7], we investigated this hypothesis using a β-cell deficiency model in mice created through 50% pancreatectomy. This model results in approximately a 50% reduction in β-cell mass while maintaining normoglycemia, thus mimicking the β-cell deficiency, although it has such limitation as pure β-cell mass reduction without insulin resistance, typical of human prediabetes or type 2 diabetes. We treated animals via two approaches: exogenous insulin delivery or islet transplantation. Our initial expectation was that both methods might allow β-cells to rest and regenerate, akin to the honeymoon phase in type 1 diabetes. Surprisingly, we observed that exogenous insulin administration for seven days post-50% pancreatectomy worsened glycemia, with detrimental effects persisting for up to six months. Histological analyses revealed a consistent expansion of α-cells throughout the islets, with an unusual distribution compared to normal pancreas. In contrast, islet transplantation achieved superior glycemic control and preserved near-normal islet morphology. These results reaffirm that islet transplantation provides the most favorable metabolic and structural outcomes, even in marginal insulin-deficient states.
The paradoxical worsening of glycemia and α-cell expansion with insulin treatment is an intriguing feature. While exogenous insulin is known to suppress endogenous insulin secretion through feedback inhibition transiently [8], this effect typically resolves within a short period and cannot alone explain the morphological changes observed. Prior work by Liu et al. suggested that insulin and glucagon reciprocally regulate each other’s cell proliferation, raising the possibility that exogenous insulin directly stimulates α-cell proliferation [9]. A crucial question is the origin of the increased alpha cells in response to insulin therapy, and we considered proliferation of pre-existing α-cells, neogenesis from pancreatic progenitors, and trans-differentiation from β-cells. We employed Ki-67 and glucagon immunostaining to assess proliferation and used PDX-1 and glucagon double staining to evaluate neogenesis or trans-differentiation; both processes were confirmed to occur in vivo (Figure 1). The current histological findings are consistent with such a mechanism, although more definitive causal pathways require further investigation through targeted lineage-tracing and molecular studies.
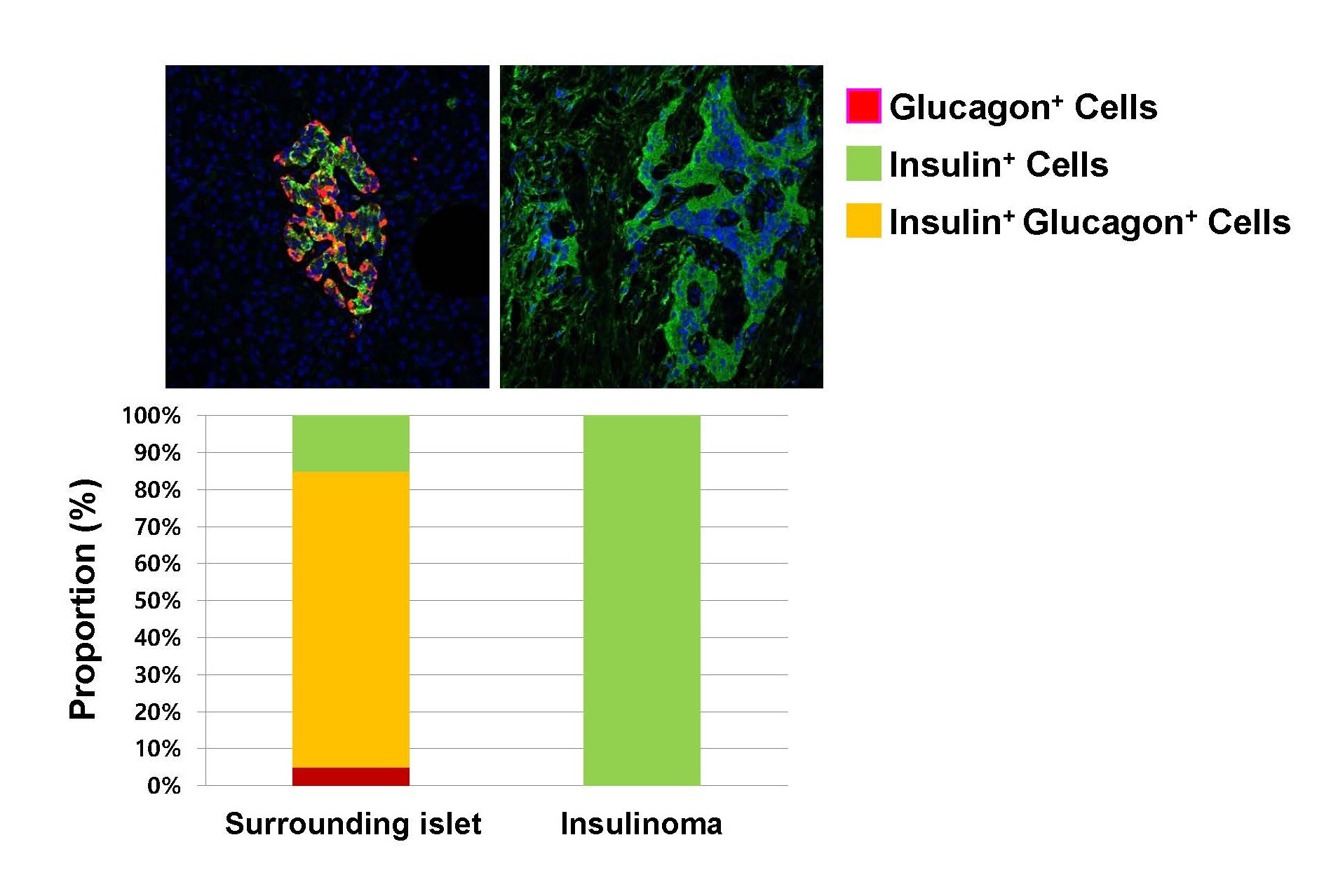
The relevance of these mechanisms to humans remains unclear. Recently, we examined pancreatic histology from a patient with insulinoma, revealing that the insulinoma region was composed exclusively of β-cells, while the surrounding normal islets exhibited a high proportion of insulin- and glucagon-double-positive cells (Figure 2). These double-positive cells could represent trans-differentiation from β- to α-cells or an intermediate phenotype during differentiation of pancreatic progenitors. Recent research indicates that maturation of β- and α-cells derived from human pluripotent stem cells (e.g., iPSCs or embryonic stem cells) remains challenging in vitro, often requiring in vivo cues that are not yet fully understood and neither recapitulated in vitro [10]. If these double-positive cells indeed originate from differentiating precursor cells, it suggests that full maturation may require additional time or unidentified factors in vivo. These results raise important clinical considerations regarding the optimal timing of insulin initiation. In overt hyperglycemic diabetes, insulin replacement therapy is an essential treatment with proven efficacy. However, in prediabetes, the potential protective effects of insulin can be offset by the worsening insulin resistance, β-cell stress, and systemic metabolic dysfunction caused by persistent hyperinsulinemia [11]. Further research is needed to determine whether early insulin administration at this stage inadvertently accelerates islet dysfunction.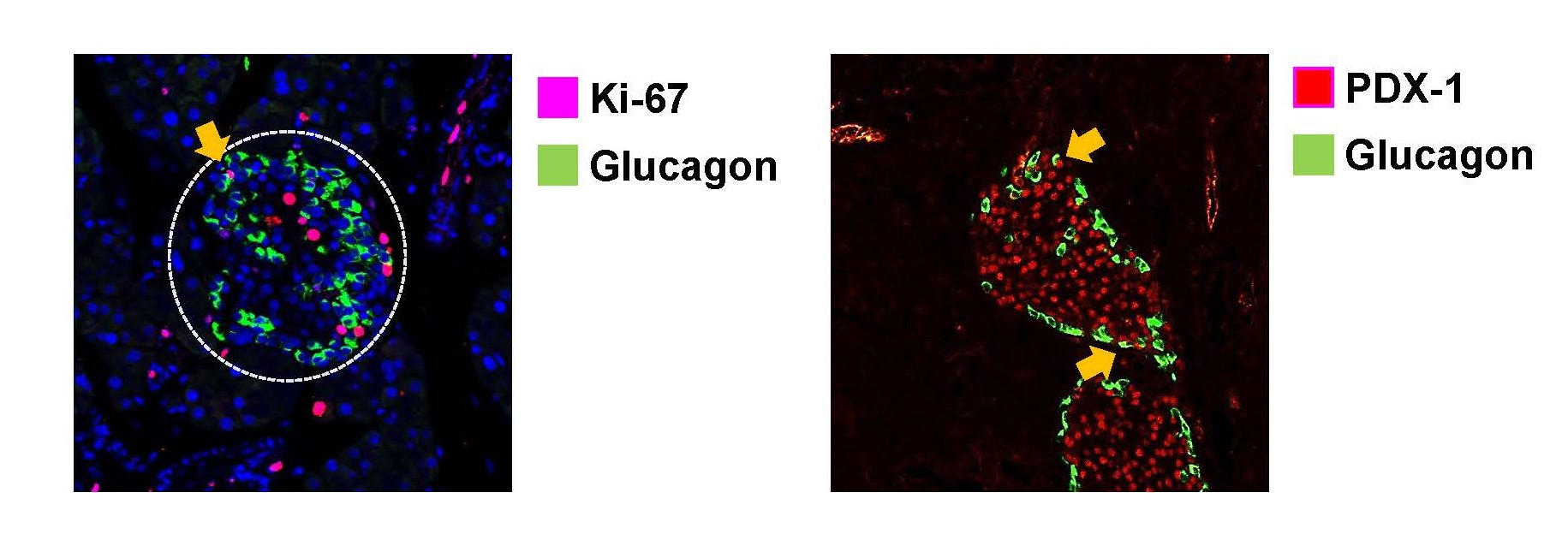
Conclusion
This study highlights that early insulin administration in marginal β-cell deficiency does not provide the expected protective effect and may instead induce adverse islet remodeling and impaired glycemic control. In contrast, islet transplantation like the recently FDA-approved Lantidra, achieved both metabolic stability and preservation of islet structure. Therefore, while insulin remains a life-saving therapy for overt diabetes, our findings sound a note of caution against its prophylactic use in early dysglycemia. They underscore that not all forms of insulin replacement are equal, and they strongly advocate for continued research into strategies that restore the delicate paracrine balance within the islet, such as islet transplantation.
Acknowledgments
This work was supported by the Gachon University research fund of 2023(GCU- 202308920001) and the research fund of 2024 (GCU- 202409220001).
Conflicts of Interest
The authors state that there is no conflict of interest.
References
2. Donath MY, Halban PA. Decreased beta-cell mass in diabetes: significance, mechanisms and therapeutic implications. Diabetologia. 2004 Mar;47(3):581–9.
3. Matveyenko AV, Butler PC. Relationship between beta-cell mass and diabetes onset. Diabetes Obes Metab. 2008 Nov;10 Suppl 4(0 4):23–31.
4. Wang Q, Huang YX, Liu L, Zhao XH, Sun Y, Mao X, et al. Pancreatic islet transplantation: current advances and challenges. Front Immunol. 2024 Jun 3;15:1391504.
5. Raz I, Mosenzon O. Early insulinization to prevent diabetes progression. Diabetes Care. 2013 Aug;36 Suppl 2(Suppl 2):S190–7.
6. Chon S, Oh S, Kim SW, Kim JW, Kim YS, Woo JT. The effect of early insulin therapy on pancreatic β-cell function and long-term glycemic control in newly diagnosed type 2 diabetic patients. Korean J Intern Med. 2010 Sep;25(3):273–81.
7. Kim BJ, Park H, Shin JS, Kim JK, Kim KW. Islet Transplantation Provides Superior Glycemic Control and Maintenance of Better Islet Architecture Compared to Early Insulin Treatment in 50% Pancreatectomized Mice. Transplant Proc. 2025 Jul-Aug;57(6):1196–200.
8. Liljenquist JE, Horwitz DL, Jennings AS, Chiasson JL, Keller U, Rubenstein AH. Inhibition of insulin secretion by exogenous insulin in normal man as demonstrated by C-peptide assay. Diabetes. 1978 May;27(5):563–70.
9. Liu Z, Kim W, Chen Z, Shin YK, Carlson OD, Fiori JL, et al. Insulin and glucagon regulate pancreatic α-cell proliferation. PLoS One. 2011 Jan 25;6(1):e16096.
10. Sali S, Azzam L, Jaro T, Ali AAG, Mardini A, Al-Dajani O, et al. A perfect islet: reviewing recent protocol developments and proposing strategies for stem cell derived functional pancreatic islets. Stem Cell Res Ther. 2025 Mar 31;16(1):160.
11. Zhang AMY, Wellberg EA, Kopp JL, Johnson JD. Hyperinsulinemia in Obesity, Inflammation, and Cancer. Diabetes Metab J. 2021 May;45(3):285–311.
