Abstract
Introduction: Stroke is among the leading causes of disability and mortality in many countries of the world. The pathogenic mechanisms of ischemic stroke are very complex and have not been completely understood.
Objective: The aim of this study was to estimate the amino acid (AA) pool in the brain hemisphere cortex of rats during Subtotal Cerebral Ischemia (SCI).
Methods: The changes in the pool of AAs and their derivatives in the frontal cortex of the cerebral hemispheres after 2-hour bilateral filament occlusion were studied in 18 rats. The analyses of the levels of AAs and their derivatives were carried out in the supernatant of protein-free tissue homogenates by reversed-phase liquid chromatography using an Agilent 1100 chromatograph.
Results: The results showed that the concentration of several AAs was altered after 2 hours of SCI. There were some specific features of AA imbalance in the left and right hemispheres. In particular, in the left hemisphere, we detected a reduction in the levels of glutamate, threonine, taurine, tyrosine, trypthophan and α-aminoadipine acid, as well as an increase in the level of ornithine. In the right hemisphere, a decrease in the levels of asparagine, serine and phenylalanine was found.
Conclusion: The results of the present study indicate that SCI induced an imbalance in the AA pool in the brain hemisphere cortex of rats. The specific features of the changes in the pool of AAs in the left and right hemispheres bear witness to asymmetry of the AA imbalance during SCI.
Keywords
Amino acids, Brain hemispheres, Subtotal ischemia
Introduction
Stroke is a leading cause of disability and mortality in many countries of the world [1-4]. The pathogenetic mechanisms of ischemic stroke are complex and have not been fully understood. The so-called stages of biochemical cascade are distinguished in the pathogenesis of ischemic stroke: energy deficiency, aspartate and glutamate excitotoxicity, oxidative stress, inflammation and apoptosis [5-8].
The present views of the pathogenesis of ischemic brain damage are far from complete and do not allow to carry out effective treatment. One of the trends in detailed studies on the mechanisms of development of brain damage in ischemia is research into changes in the brain amino acid pool [9,10].
As is known, amino acids and their derivatives (in particular, biogenic amines) play an important role in brain functions both in the normal state and pathology, participating in biosynthesis of membrane proteins and signal molecules, hormones and regulatory peptides [7]. They also act as a source of energy through the tricarboxylic acid cycle and are involved in formation of carbohydrates by gluconeogenesis in their excess [11].
Research into functional asymmetry in various pathological processes is a topical task since it promotes better understanding of the mechanisms of the impairments and allows one to elucidate specific features improving their diagnostics. The aim of the present work was to study the free amino acid pool in the left and right regions of the cortex of the rat brain large hemispheres in subtotal cerebral ischemia.
Materials and Methods
The experiments were carried out on 12 mongrel female albino rats (6 animals in both the control and experimental groups) weighing 180-220 g. The temperature, light and noise regimes were monitored. In our work, we were guided by principles of humane treatment of animals and the experiments complied with requirements of Directive 2010/63/EU of the European Parliament and of the Council on the protection of animals used for scientific purposes of 22 September 2010 [12].
Subtotal ischemia was simulated in experimental group rats by bilateral filament occlusion for two hours [13]. Sham operated animals were in the control group. All the operative manipulations were performed under conditions of intravenous thiopental anesthesia (60 mg/kg). Brains were excised and fragments of the right and left regions of the large hemisphere frontal lobe cortex were taken and frozen in liquid nitrogen.
The range of the compounds assayed included: proteinogenic amino acids, ornithine and citrulline as well as some related compounds (taurine, hydroxyproline, α-aminobutyrate and ethanolamine). The assay of amino acids and their derivatives was carried out using an Agilent 110 chromatograph by reversed-phase chromatography with precolumn derivatization by o-phthalaldehyde and 3-mercaptopropionic acid in sodium borate buffer [14].
Data were processed using the StatSoft Statistica 10.0 software. Methods of descriptive statistics, the 2-way ANOVA of linked samples and the Boruta method of the R software were applied for finding of the most significant parameters.
Results and Discussion
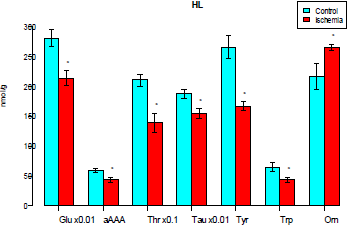
The control animals did not show significant differences in the AA concentrations in the left and right large hemisphere cortex. The subtotal cerebral ischemia was not accompanied by changes in the AA pool, with the pattern in the left and right hemisphere cortex being different. The cortex of the left frontal lobe of the large hemispheres demonstrated some changes in the amino acid pool (Figure 1). In particular, the levels of glutamate, threonine, taurine, tyrosine, tryptophan and α-aminoadipine acid (αAAA) were decreased and that of ornithine was raised. As a consequence, the decreased levels of aromatic amino acids (AAAs) caused an elevation in the ratio of branched chain amino acids (BCAAs) to AAAs (Table 1).
Figure 1: Levels of free amino acids and their derivatives in the cortex of the left frontal lobe of the rat brain large hemispheres (μmol/g) (only the parameters with significantly changed levels are shown here and in Figure 2). Glu-glutamate, αAAA-α-aminoadipine acid, Thr-threonine, Tau-taurine, Phe-phenylalanine, Orn-ornithine. The cortex of the right frontal lobe of the large hemispheres demonstrated reduced levels of glutamate, asparagine, serine, α-aminoadipinic acid, threnonine, taurine, and phenylalanine as well as increased levels of ornithine (Fig.2).
Figure 2: Levels of free amino acids and their derivatives in the cortex of the right frontal lobe of the rat brain large hemispheres (μmol/g). Glu-glutamate, Asn-asparagine, Ser- serine, αAAA-aminoadpine acid, Thr-threonine, Tau-taurine, Phe phenylalanine, Orn-ornithine.
Both the lobes showed depletion of the AA total pool (i.e., of all the AAs assayed, both essential and nonessential) with the pool of ketogenic AA remaining unchanged (Table 1). On the whole the changes in the integral parameters in the large hemisphere cortex coincided and the differences were only noticed in the AAA pools: no increase was recorded in the BCAAs to AAas ratio.
The impairments in AA pool of the large hemisphere cortex also involved the concentrations of neuroactive AAs: both the lobes showed decreased concentrations of excitatory (aspartate, glutamate) and inhibitory (taurine, glycine) neurotransmitter AAs. However, their ratio was unchanged (Table 1).
|
Indices |
Left hemisphere |
Right hemisphere |
||
|
Control |
Ischemia |
Control |
Ischemia |
|
|
BCAA |
491 ± 30,5 |
342 ± 15,2* |
488 ± 37,6 |
415 ± 30,7 |
|
AAA |
821 ± 56,9 |
839 ± 43,6 |
814 ± 69,2 |
853 ± 54,1 |
|
Nonessential AA |
52800 ± 1560 |
45500 ± 1130* |
54200 ± 1340 |
44600 ± 1170* |
|
Essential AA |
3900 ± 92,9 |
3250 ± 214* |
4010 ± 110 |
3570 ± 164* |
|
Glucogenic AA |
55500 ± 1630 |
47600 ± 1120* |
56900 ± 1330 |
46900 ± 1210* |
|
Ketogenic AA |
799 ± 29,8 |
915 ± 65,1 |
842 ± 38,2 |
952 ± 93,4 |
|
Neurotransmitter AA |
62500 ± 1820 |
50100 ± 1750* |
62000 ± 2590 |
48200 ± 1070* |
|
Excitatory AA |
34300 ± 1590 |
27700 ± 978* |
35900 ± 1470 |
27200 ± 1100* |
|
Inhibitory AA |
28200 ± 2360 |
22400 ± 1020* |
26100 ± 1870 |
21000 ± 1080* |
|
BCAA/AAA |
1,71 ± 0,136 |
2,48 ± 0,177* |
1,73 ± 0,177 |
2,11 ± 0,205 |
|
Nonessential/Essential AA |
13,6 ± 0,342 |
14,3 ± 1,12 |
13,6 ± 0,522 |
12,6 ± 0,401* |
|
Glucogenic/Ketogenic AA |
70,4 ± 4,34 |
53,3 ± 4,96* |
68,3 ± 2,99 |
50,6 ± 3,58* |
|
Excitory/Inhibitory AA |
1,28 ± 0,139 |
1,24 ± 0,0493 |
1,41 ± 0,0965 |
1,31 ± 0,101 |
|
Total concentration of AA |
83600 ± 2320 |
70100 ± 1550* |
83000 ± 2590 |
68300 ± 1430* |
|
-p <0,05 |
||||
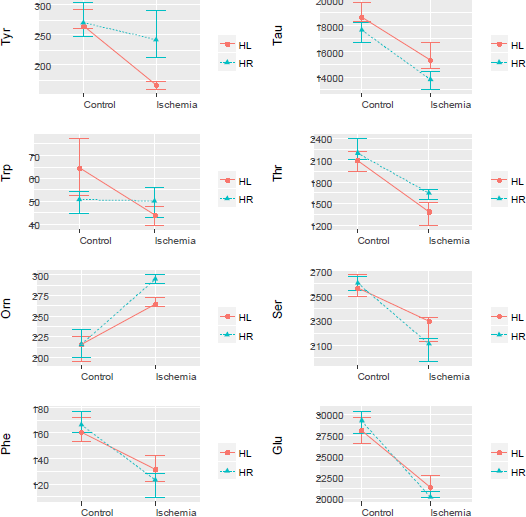
The analysis of the interaction of two factors, the side of the large hemispheres and the presence/ the absence of ischemia, indicates differences in the effects of subtotal ischemia on the levels of tyrosine, tryptophan and ornithine in the left and right lobes of the large hemispheres.
(Figure 3) shows that the changes in the tyrosine and tryptophan levels occurred only in the left lobe. The level of ornithine was raised in the cortex of both the lobes, but the elevation was significantly greater in the right lobe compared to the left one. The phenylalanine, taurine, serine, thereonine and glutamate concentrations diminished unidirectionally in both the lobes.
Figure 3: Analysis of interactions of the factors “left lobe side” and “the presence/the absence of ischemia”. The plots show the average and 95% confidence intervals.
To find the most informative indices, stepwise procedures are traditionally used. However, this approach often depends on the choice of the initial conditions of the search, which can produce ambiguous results. Therefore, to solve this problem, we applied the Boruta procedure from the R statistical software package, with the algorithm being independent of the initial conditions.
The Boruta results (Figure 4) showed that the most significant indices characterizing alterations in the AA pool of the cortex of the large hemisphere left frontal lobe in subtotal cerebral ischemia were threonine, glutamate, ornitine, tyrosine, αAAA and taurine. In the right frontal lobe of the large hemispheres, most significant were threonine, asparagine, glutamate, serine, ornithine, β-alanine, phenylalanine and taurine.
Figure 4: Results of Boruta algorithm selection of the most significant indices.
The development of asymmetry of the free amino acid pool of the large hemisphere frontal lobe cortex was confirmed by the method of multidimensional analysis. The linear discriminant analysis showed an increase on the plane of two main components of the distance between the regions of the large hemisphere right and left frontal lobes in the brain of animals with ischemia ( D2, the square of the Mahalanobis distance, was 8.8) as compared to that of the control group (D2=2.3, Figure 5). The greatest relative contribution to the values of the 1st root of the discriminant function was made by glutamate, threonine and tryptophan while threonine and taurine contributed to the value of the 2nd root (Fig.5). The analysis of the intra-group correlation coefficients of the variable discriminant function coefficients and the standardized canonical discriminant function coefficients demonstrated that the 1st root was closely related to the level of glutamate, whereas the 2nd root was connected with that of tyrosine (Fig.5). We can suggest to interpret the roots as general (independent of the side of the frontal lobe, the 1st root) and specific (the 2nd root) effects of ischemia since it is the shift of the CL ischemia group along the second root that stipulates the asymmetry of the brain cortex amino acid pool in ischemia. Since the first root accounts to more than 83% of the total dispersion (which considerably exceeds the corresponding parameter for the second root-14.3), the asymmetry of the amino acid imbalance is insignificant in comparison with the pronounced AA imbalance.
Figure 5: Location of groups on the plane of the first two roots of the discriminant function. CL is the cortex of the left frontal lobe of the brain large hemispheres. CR is the cortex of the right frontal lobe of the brain large hemispheres.
Conclusion
The subtotal cerebral ischemia was accompanied by a pronounced imbalance of the amino acid pool of the large hemisphere cortex. The differences in the pattern of changes in the AA levels in the left and right regions of the large hemisphere cortex indicate interhemispheric asymmetry of the amino acid imbalance in cerebral ischemia.
References
2. Stakhovskaia LV, Klochikhina OA, Bogatyreva MD, Kovalenko VV. Epidemiology of stroke in the Russian Federation: results of territory's population registry (2009-2010). Zhurnal nevrologii i psikhiatrii imeni SS Korsakova. 2013;113(5):4-10.
3. Razvodovsky YE. Alcohol consumption and stroke mortality in Belarus. Voprosy Narcologii. The Questions of Narcology.2009; 6:82-92.
4. Razvodovsky YE. Alcohol attributable fraction of stroke mortality in Russia. Journal of the Neurological Science 2013; 333(1): 231.
5. Gusev EI, Martynov MJ, Kamchatnov PR. Ischemic stroke. The current state of the problem. Doctor. RU. 2013; 5 (83): 7-12.
6. Zimatkin SM, Bon EI, Maksimovich NOT. The role of neuroglobin in cerebral ischemia / hypoxia and other neuropathology. Journal of Grodno State Medical University. 2018; 16 (6).. (in Russ.].
7. Kulesh SD. Pathogenesis of ischemic stroke: biochemical mechanisms and role of neiroactive amino acids]. Medicinskie Novosci. Medical News. 1998; 1: 21-24.
8. Zaldocka B, Domanska-Janik K. Enhancement of [3~]~-aspartate release during ischemia like conditions in rat hippocampal slices: source of excitatory amino. Acta Neurobiol. Exp.. 1996 Jan 1;56:63-70.
9. Razvodovsky YE, Troyan EI, Doroshenko YM, Smirnov VY, Maksimovich NY. Levels of Free Amino Acids and their Derivatives in the Brain Cortex of Rats During Unilateral Ischemia.
10. Skvortsova VI, Raevskiy KS, Kovalenko AV. Concentration of neurotransmitters amino acids in the spinal cord liquid in patients with acute ischemic stroke. Zhurnal nevrologii i psychiatrii. 1999;2:34-8.
11. Fernstrom JD. Branched-chain amino acids and brain function. The Journal of nutrition. 2005 Jun 1;135(6):1539S-46S.
12. European Convention for the protection of vertebrate animals used for experimental and other scientific purposes. ETS N 123.-Strasbourg, 1986.- P.34-42.
13. Rozvadovsky VD. Trenin SO, Telpuchov VI. Microsurgery model of brain ischemia. Journal patologitchescoy phisiologii I obschey patalogii Journal of pathological physiology and general pathology.1985;2: 87-92.
14. Barcovskij EB. Bocun SB., Borodinskij AN. and other. Contemporary problems of biochemistry. Methods of investigation. Minsk: The highest school, 2013.-491 p.