Keywords
Cardiac hypertrophy, Fabry disease, α-Gal; pathway, inflammation
Commentary
Cardiac hypertrophy is one of the most common manifestations of Fabry disease (FD) [1] and it can be the only clinical expression, in the so called cardiac variant, as result of specific mutation of alpha-galactosidase-A gene (GLA), as the N215S one [2].
The organic substrate of myocardial hypertrophy in women with FD is attributed to the presence of normal and large cardiomyocytes containing vacuoles of globotriaosylceramide (Gb3) because of deficiency of the lysosomal enzyme alpha-galactosidase A (α-Gal). This “cell mosaic” is motivated by the alternate expression in female Fabry cardiac cells of normal and mutated X-chromosome (process of Lionization).
Specifically, the coexistence of normal and affected cells involves all cardiac cell types including myocytes, smooth muscle cells and endothelial cells of vessels and all the cell components of cardiac conduction tissue [3,4]. The ratio between normal and affected cells would be responsible for the severity of myocardial, coronary and electrical manifestations.
Indeed, in the above-mentioned study [5], it is reported that even unaffected cardiomyocytes although expressing a normal GLA gene and normal level of α-Gal enzyme, concur to myocardial hypertrophy and then to the severity and progression of Fabry disease cardiomyopathy (FDCM). Furthermore, the interstitial release of Gb3 in the context of the “constitutional secretory pathway” may be followed by Gb3 adhesion to plasma membrane of unaffected cells promoting an immune-mediated inflammatory reaction [6] which brings an additional form of damage.
What remains unclear is the molecular pathway involved in the pathogenic contribution of unaffected cardiac cells in the female with FDCM.
It is speculated that metabolites released by affected cells like lyso-Gb3 or the same Gb3 can act as paracrine stimulator of cell hypertrophy and cell inflammation.
A possible therapeutic solution to the environmental abnormality of FDCM is an enhanced uptake of enzyme replacement therapy by both affected and unaffected cardiac cells. Actually, it seems to be limited by a remarkable down regulation of mannose-6-phosphate plasma-membrane receptor [7].
Increasing M6P expression by specific molecular inductors like Growth Hormone or Estradiol [7] could be the solution for the metabolic and immunologic abnormalities associated with FDCM.
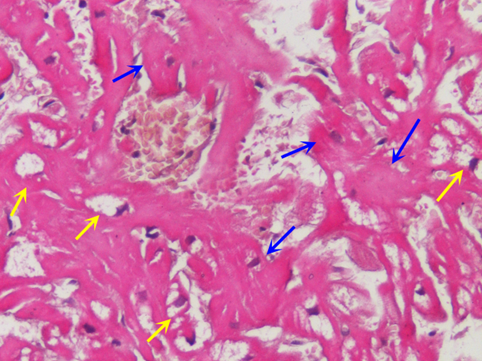
Figure 1. Severe hypertrophy with disarray of unaffected cardiomyocytes (see blue arrow) in a female with Fabry cardiomyopathy. Yellow arrows indicate affected (vacuolated) cells which show equal or less degree of enlargement. (H&E 200 x).
Funding
This study was supported by an Investigator Initiated Research grant from Takeda Pharmaceuticals International AG, a member of the Takeda group of companies (IISR-2018-104317) and partially by the Italian Health Ministry (IRCCS San Raffaele Roma—Ricerca Corrente #2020/1) and Fondazione Roma (MEBIC # 18/6/2019), Mebic San Raffaele Pisana.
Conflicts of Interest
The authors declare no conflicts of interest.
References
2. Scheidt WV, Eng CM, Fitzmaurice TF, Erdmann E, Hübner G, Olsen EG, et al. An atypical variant of Fabry's disease with manifestations confined to the myocardium. New England Journal of Medicine. 1991 Feb 7;324(6):395-9. Chimenti C, Morgante E, Tanzilli G, Mangieri E, Critelli G, Gaudio C, et al. Angina in Fabry disease reflects coronary small vessel disease. Circulation: Heart Failure. 2008 Sep 1;1(3):161-9.
3. Frustaci A, Morgante E, Russo MA, Scopelliti F, Grande C, Verardo R, et al. Pathology and function of conduction tissue in Fabry disease cardiomyopathy. Circulation: Arrhythmia and Electrophysiology. 2015 Aug;8(4):799-805.
4. Chimenti C, Verardo R, Frustaci A. Hypertrophy of unaffected cardiomyocytes correlates with severity of cardiomyopathy in female patients with Fabry disease. Orphanet Journal of Rare Diseases. 2021 Dec;16:1-4.
5. Frustaci A, Verardo R, Grande C, Galea N, Piselli P, Carbone I, et al. Immune‐Mediated myocarditis in Fabry disease cardiomyopathy. Journal of the American Heart Association. 2018 Sep 4;7(17):e009052.
6. Frustaci A, Verardo R, Scialla R, Bagnato G, Verardo M, Alfarano M, Russo MA. Downregulation of mannose-6-phosphate receptors in fabry disease cardiomyopathy: A potential target for enzyme therapy enhancement. Journal of Clinical Medicine. 2022 Sep 16;11(18):5440.