Abstract
Neuropsychiatric disorders have been the constant burden impacting healthcare since 1990, and more than 3 billion people worldwide are living with neurological conditions. Mental health conditions with cognitive decline were also observed in people who contracted SARSCoV-2 and later presented the PACS related disease after the resolution of primary infection. Long term dysbiosis, inflammatory bowel disease (IBD), and neurological inflammations signify the establishment of major depressive disorders (MDD), Alzheimer’s disease (AD), Schizophrenia, Parkinson’s disease (PD), lateral sclerosis etc. More than 4% of cases of periodontitis are likely to develop brain related abnormalities. Conversely, cellular and molecular alterations keep restoring Amyloid beta peptides (Aβ), tau protein, and 2-synuclein protein with plaque formation allow the disease associate microglia (DAM TREM) phenotype to transfer the aberrant signals through post synaptic neurons. Brain related inflammations are accompanied by various agonist molecules, Lipopolysaccharides (LPS), increased C-reactive protein, tumor necrosis factor alpha (TNF-α), and mitochondrial dysfunctions. Firmicutes with Lactobacillus spp., Ruminococcus spp., Lachnospira spp. are the known short-chain fatty acids (SCFAs) producers, those meant to involve in regulating the immunological and physiological functions from Gut-Brain-Gut building homeostasis. Aβ stimulation of glial cells produces anti-inflammatory effects, while controlling the ERK/MAPK/JAK/NFkβ pathways-based inflammations. Serotonin, dopamine, gamma aminobutyric acid (GABA), kynurenic acid are the regulatory neurotransmitters that contribute to the prognosis of neurodegenerative diseases and also reduce the stress related corticosterone. Impaired molecular signals disrupt the endothelial layer of gut and blood brain barrier (BBB) impacting the serotonin production in brain. It is imperative to utilize pre, pro, and postbiotics to modulate the depleted microbiota, could reverse the aberrant signals instigating a better disease prognosis.
Keywords
Neurological disorders, Microbial metabolites, Gut-brain axis, Neurotransmitters, Dysregulated signaling, Microbiota
Introduction
One out of 3 people is being affected by neurological illness. The mortality rate has been reported to surpass 80% in middle- and low-income countries [1,2]. Only major depressive disorder (MDD) disease has been discovered to affect ~35 million people worldwide, which is characterized by continuous mood changes with reduction in routine activities [3]. Poor mental health determinants had taken a great lead due to the environments created during COVID-19. In 2020 pandemic, due rise of infections’ rate had restricted people’s mobility by implementation of heavy lockdowns could have resulted 27.6% additional increase of MDD cases along with 76.2% rise in anxiety disorders worldwide [4]. Thereafter, PACS cases came into picture affecting people who have already recovered from the primary infection, also associated with other illnesses, regular antibiotic use, genetic and environmental changes, and poor dietary factors. Microbiomics studies have been in the limelight for more than a couple of decades, implicated to cause the mental disorders, depression and psychosis with involved long-term dysbiosis. That also confers a relationship and communications’ imbalance among Gut-brain-Gut signaling affecting the neurons largely. Current evidence has shown that gastrointestinal (GI) microbiota is to be involved with abnormal immune signals and metabolomic alterations progress neurons convey opposite molecular signaling from GI tract to brain and vice versa [3]. However, it is still unclear how the neuron cells get affected causing the permanent pathology for more severe cases such as Alzheimer’s patients. Therefore, it warrants further investigations to disclose the complex relationships among cellular and molecular pathways to utilize every possible strategy including early interventional studies that could benefit the population. Similar neurological features like fatigue, breathing difficulties, pain, cognitive dysfunction, and with an inability to carry out daily activities observed in COVID-19 patients. Approximately 65 million people have been diagnosed with PACS, typically lasting for 3 to 6 months [5]. One third of non-hospitalized PACS cases were reported with “brain fog” and “cognitive dysfunction” and found often associated with damage of cerebral vessels and intestinal wall. Increased permeability of toxins, harmful molecules to brain and angiotensin-converting enzyme 2 (ACE-2) abundance was the result of intense cytokine storm formation exacerbated the PACS pathogenesis. Indirect neurological damage has been reported in deceased patients who did not recover from the virus. These inflammatory profiles have been sustained in the long term due to elevated levels of TNF-α, Interleukin (IL)-6, IL-4, and monocytes derived macrophages, resulting in persistent aberrant immune responses associated with leukopenia. The continued secretion of leukotrienes has led to increase the chemokine secretion and induction of fatty acid synthesis as well [6-10]. Autism is directly related to a tendency of hematopoietic abnormalities linked with epigenetic alterations pertaining to the ancestral immune cells’ behavior. Gut brain abnormalities demonstrate the dysregulations between Treg cells and gut microbiome (Autistic mice model) [11]. Neurodevelopmental disorders can be the result of different patterns in gene expression, including DNA methylation and modifications to histone proteins with changing deacetylase activities. The interconnected endocrine, metabolic, and immune activities among gut and other body organs generally communicate through metabolites and cytokines circulation. Therefore, the negative effects on brain function, cognition and mental health disorders occur for obvious reasons [12]. Systemic communication among brain, gut, and other organs signifies its role as involved influencer of cellular and molecular activities affecting brain including other organs in the severe disease state. The resident normal microflora influences the neurological activities, unless it becomes aberrant during co-morbidities/diseased state refers to involving enteropathy with psychopathy [13]. Therefore, a healthy microbiome is also considered as an organ that regulates the neurological functions to support mental health and wellbeing. This review is based upon the accumulated molecular and cellular pathologies in various neuropsychiatric disorders presenting the dysregulated pattern of neurotransmitters with long term dysbiosis have the capability to alter the functions of microglial cells and neurons to transmit the opposite signals through post synaptic neurons result in disruption of synaptic communications allows the neurons death by massive inflammations. Thorough explanations are provided that could assist to rectify the bidirectional signals using treatment modalities as early interventional therapy, might be a recipe for living healthy life.
Possible Cause of Neurological Diseases
Neuropsychiatric disorders like schizophrenia, autism, spectrum disorder, major depressive disorder, bipolar and anxiety disorders have been on the rise since 1990 [14]. A state of stress in the hypothalamic-pituitary-adrenal glands (HPA) can directly contribute to the manifestation of MDD. The outcomes of depressive state and dysbiosis have also been demonstrated in chronic depression and anxiety-like disorders [13]. These can be further elucidated by various molecular activities, including:
- Alterations in amine receptors in the corticolimbic region of the brain.
- An increased concentration of pro-inflammatory cytokines, which could result from enhanced interactions between resident microbiota and pathogens.
- Elevated antibody levels against lipopolysaccharides (LPS).
- Increased gut permeability, commonly referred to as “leaky gut syndrome,” and bacterial translocation.
- Changes in the Bacteroidetes/Firmicutes ratio during MDD, along with depletion of Blautia, Faecalibacterium, and Coprococcus.
- Periodontal conditions also increase the risk of psychiatric disorders such as schizophrenia [14].
Parkinson’s disease is described by pathology in context with accumulation of α-synuclein responsible for microglial dysfunctions resulting in neurons deficit [15,16]. Amyotrophic lateral Sclerosis (ALS) is associated with microbiota influenced neuroinflammation [16]. Akkemansia muciniphila abundance is reduced in ALS with reduced nicotinamide production that in turn increase the motor neurons dysfunction and brain atrophy. These characteristics could be restored by the supplementation with Akkermansia muciniphila [16,17]. Akkermansia muciniphila reduces the inflammatory response, whereas Ruminococcus torques and Parabacteroides distasonis increase the symptoms of ALS [18]. Huntingtin protein toxicity in huntingtin disease (HD) is associated with N-terminal fragments in claudin neurons, microglia, and astrocytes, that is mainly increased due to aberrations in HTT gene with CAG repeat [19]. PD, AD, multiple sclerosis (MS), and HD are often associated with decreased levels of brain-derived neurotrophic factors (BDNF), which regulates energy metabolism to prevent the depletion of β cells [20,21]. Dysbiosis in diabetes, age-related, and even in obese condition can also be the consequence for regular cognitive decline [13]. The gut microbiota, gliocytes and astrocytes relationship has been explained in detail in context with PD, AD, HD, MS etc., targeting the CNS inflammation [16,22]. Dysbiosis and an irregular immune response causing neuroinflammation promoting the psycopathogenesis have also been reported in schizophrenia patients, often diagnosed with serological markers and proinflammatory cytokines. Furthermore, gene-related disorders can worsen the activities related to brain development, memory, and cognition for example, the major histocompatibility complex (MHC) located with the marker neurogranin gene (NRGN) and transcriptional factor 4 (TCF 4) [13].
Molecular Factors to Influence the Neurological Diseases
Molecular factors increase hypersensitivity of the neuronal cells getting inflamed in AD and AD related neuropathy. Entorhinal cortex and nerve cells due to the accumulation of soluble Aβ and neurofibrillary tangles (NFT), eventually deplete the demand for high energy molecules adenosine triphosphate (ATP), ROS/RNS (reactive oxygen species/reactive nitrogen species) lead to mitochondrial dysfunction. Proinflammatory cytokines and chemokines including TNF, NLP-1 (5-[3-(2-nitro-l-imidazoyl)-propyl]-phenanthridinium bromide), Mitogen-activated protein kinase 1 (MAPK 1), glia maturation factor (GMF), c-Jun NH2-terminal kinase (JNK) etc. are responsible for early endocannabinoid (ECS) dysregulation [23]. Germ free (GF) mice showed altered immunity with increased susceptibility to various immunological disorders and infections. Microbiota oriented immunological functions are carried out from a range of immune receptors like toll-like receptors (TLRs), and microbes to maintain mucosal barrier function explains its relations with different organs of the body [24]. AD is linked to microbiota, phosphorylated tau protein, amyloid -β, and activation of NLR family pyrin domain containing 3 (NLRP3) inflammasome pathway [25]. NLRPs are activated during microbiome’s disruption and rotavirus infection [10]. Bacterial peptidoglycan activates nucleotide-binding oligomerization domain (NOD) and through NOD-like receptors (NLR) during viral infection triggers caspase 1 apoptosis. NLRP 1-3 in microglia activates apoptosis-associated speck-like protein containing a caspase recruitment domain (ASC) and procaspase 1 to pyroptosis or apoptosis. NLRP3 activated by various agonists, TLRs, LPS, nigericins, TNFs, K+, Ca2+ and mitochondrial dysfunctions are involved in the neurodegenerative inflammations and cause neuronal cells’ damage. Microglial cells NLRPs activation directly refers to the accumulated amyloid β, 2-Synuclein protein, superoxide dismutase (SOD), and ATP. Proinflammatory IL-1β and gasdermin D (GSDMD) increase cell swelling leading to pyroptosis. Therefore, NLRPs is a biomarker and can be exploited to modulate the brain immunity by altering ROS/LYN/SYK/PI3K/Ca++/K+ Pathway [25]. Mitochondrial function, ATP release and ROS activity has been ascribed to be impaired in the MS. The reduced activity of electron transport led to degeneration of grey and white matter of brain. The enhancing activity of mitochondria through the activation of peroxisome proliferator-activated receptor γ coactivator 1-alpha (PGC-1alpha) prevented neurodegeneration in mice model [26]. Cellular and molecular alterations were revealed in the brain of AD mouse model has shown the plaques surrounded by DAM cells linked to Aβ, whereas the astrocytes and dendritic cells (DCs) also present around the space secreting the tau proteins. In addition to PD the other neurological disorders like MS, HD, and frontal temporal dementia have also shown the microglial cell involvement [16,27].
Neurotransmitters
Gut microbiota-based neurotransmitters
It has been speculated that microbiome’s regulations are highly linked with neurotransmitters’ functions. Serotonin level was positively associated with Proteobacteria. The nor-epinephrine was positively associated with Bacteroides and negatively linked to Firmicutes, hence, reflecting the functional correlation among these neurotransmitters and microbiota. However, Serotonin, (5-hyroxytryptamine) 5-HT cannot pass through BBB under normal circumstances, but gut derived 5-hydroxytryptophan and N-acetyl serotonin and melatonin can cross the BBB. In serotonergic regulations the spore forming bacteria like Turicibacter sanguinis is largely involved with reuptake of 5-HT. It is still unresolved how this particular bacterial species is involved in metabolic process. Dysregulation of this pathway implicates the development of AD, PD etc. [16,28]. Bile acids have the capability to modulate Th1 cells and DCs and also able to pass through BBB to prime immunological response. Primary bile acids such as cholic acids and chenodeoxycholic acids are effective in preventing early plaque formation. Tauroursodeoxycholic acid (TUDCA) exhibits anti-inflammatory and anti-apoptotic effects, suggesting that bile acids can differentiate into various forms. Although, no evidence has clarified the linkage of bile acids to cause neuropathy. Nonetheless, the disturbance in bile acid levels is a suggestive of considering it as a biomarker in the diagnosis of neuropathy. The aliphatic nature of bile acids and their conjugation with other drugs and nanoparticles offers the potential benefits in pharmaceutical drug development, permeation, and prolonging drug half-life [12,16,29,30]. Stress-related secretion of catecholamines in the gut, such as norepinephrine, can stimulate the growth of enteric pathogens and to intensify their virulence properties e.g., Campylobacter jejuni [29]. Neurotransmitters like GABA, serotonin, melatonin, histamine, and acetylcholine represent biologically active forms of catecholamines. Primary form of bile acids often observed in reduced quantity in antibiotics dependent patients [16]. The neurotransmitter GABA is also considered as postbiotic produced by Bacteroides, Bifidobacterium, and Lactobacillus. GABA has a significant role all the way through Gut-Brain axis to effectively regulate cognitive functions, learning and memory, sensory processing, circadian rhythm, vigilance state. Bacterial decarboxylase converts glutamate to GABA to control neurons’ regulations [16,31]. Glutamate decarboxylase enzyme attains the catalytic reaction in presence of cofactor-pyridoxal -5-phosphate, inorganic phosphate, phosphocreatine, magnesium, ADP-ATP, with vitamin B6, maintain the balance between glutamate and GABA. GABA producing microbes mainly present in fermented foods, dairy products milk and cheese [31]. Central and peripheral activities e.g., cognition, voluntary actions, pleasure and motivation etc. are being regulated by the neurotransmitter dopamine. Thus, balancing the vagus nerve stimulation, immune system modulation, hypothalamus-pituitary-adrenal axis, microbial metabolites, and postbiotics’ action [32]. Phenyl alanine to tyrosine subsequently to L-DOPA are converted into Dopamine in the presynaptic region of nerves using activated enzymes secreted through microbiota hydroxylases and aromatic L-amino decarboxylase [32]. Various microbial species were found to establish their effects in post synaptic nerve ending by increasing/decreasing the dopamine binding e.g., Bacteroides uniformis and Prevotella copri. Similarly, Enterococcus faecalis and E. faecium are the producers of hydroxylase & decarboxylase. Notably, Lactobacillus plantarum increases the dopamine binding and Ruminococcus decreases the hydroxylase activity [32]. Deficiency in dopamine causes dopamine disorders which could be a cause of reduction in homovallic (HVA) acid [32,33]. Butyrate is emphasized to control the neurons deficit and increase the level of dopamine. Conversely, some studies revealed the opposite effect of sodium butyrate to induce neurotoxicity [33]. Butyrates are the big contributors to change the abundance of Bacteroides uniformis and Prevotella copri [32]. Intestinal inflammations linked to degradation of mucin membrane, more LPS, α-synuclein accumulation, with decreased neuroprotective butyrate and bile, these pathological changes were observed during PD, AD, and dementia. Dopaminergic neurons are largely affected by the vagus nerve implicating neurodegeneration. Furthermore, microglial activation increases neuroinflammation while reducing BBB and ROS generation is negatively correlated to BDNF, Monoamine oxidase B (MAO-B) and tyrosine hydroxylase [32]. The excitatory signals generated in neurons allow cholinergic neurotransmitters directly linked to neurodegenerative diseases. Vagus nerve activation secrete acetylcholine in visceral organs to activate α 7nAchRs (α acetylcholine receptors) in macrophages to control proinflammatory cytokines, thus, promoting Janus kinase 2/signal transducer and activator of transcription 3 (JAK2/STAT3) pathway through nucleus and inhibiting TNF-α, IL-6, and nuclear factor-κB (NF-κB) induction. Therefore, activating the phosphoinositide-3-kinase (PI3K/Akt). During the degeneration process inflammasomes tend to inhibit PI3K and exacerbate the secretion of proinflammatory cytokines including TNFα, IL-6 and IL-1β. Treatment with galantamine activates the nicotinic acetylcholine receptors (nAChRs) prevent the ulcer formations and reduce the inflammatory mediators [33]. Acetylcholine neurotransmitter modulates the neurons and was first reported to be secreted by Bacillus acetylcholine. Lactobacillus plantarum, Bacillus subtilis, Escherichia coli, and Staphylococcus aureus are the producers of acetylcholine. Bacillus subtilis is the biggest producer of acetylcholine than E. coli and S aureus. Acetyl choline is synthesized from the acetyl CoA and enzyme acetyl transferase [34]. The choline transporter like protein mediates the choline transportation to the brain to perform essential functions in central nervous system (CNS) could be a rate limiting step in the cholinergic neuronal activity [35,36]. Glutamate is a neurotransmitter that regulates the functions of Gut and brain under the normal or diseased state. These amino acids activate the receptors of vagus nerve and splanchnic afferents conveying the sensory input to brain to regulate efferent functions i.e., excitatory and inhibiting [37]. It is speculated that the saprophytic microflora could involve in regulations of neuroactive molecules along with neurotransmitters, neuromodulators, Brain tryptophan (5-HT precursor), tyrosine (dopamine and nor adrenalins) and glutamine (glutamate/GABA synthesis). The GF mice model study revealed the lower level of these neurotransmitters [37]. Microbiota directly and indirectly influences glutamatergic pathway via Gut-Brain axis by controlling the L-tryptophan metabolism. The essential amino acid L-tryptophan synthesizes the 5HT, Kyn and indole derivatives under the microbiota’s control (directly or indirectly). Kyn is diverted into Kyn A and Quinolinic acid pathway. The latter with N-methyl-D-Aspartate (NMDA) and Glu receptor create a neurotoxic proinflammatory condition. While the former KynA has the ability to minimize the excitotoxic effect in the cells, thus, contributing to the neuroprotective role through CNS to enteric nervous system (ENS) and vice versa (Gut brain axis regulations). Dietary sources like cheese, sea food, and vegetables are Glutamate rich. [37]. L-glutamate and GABA are mainly known for their role as the main neurotransmitter to affect the excitatory/inhibitory actions through post synaptic regulations via glial cells [38]. LAB strains belonging to Lactobacillus plantarum, Lactobacillus paracasei, Lactobacillus lactis, and Bifidobacterium spp. can synthesize Glutamate. Glutamate formation can also be induced in the nerve cells. Therefore, prokaryotes and eukaryotes can synthesize GABA by enzymatic action of glutamate decarboxylation (GAD) [38]. Evidently, glutamate is being synthesized in neurons that cannot pass the BBB. Glutamate can be stored in the vesicle during excessive production within the cells, hence, prolong the neuronal cell death. The presynaptic neurons convert Glutamate to GABA efficiently under the normal regulations of both enzymes’ glutamate dehydrogenase [38].
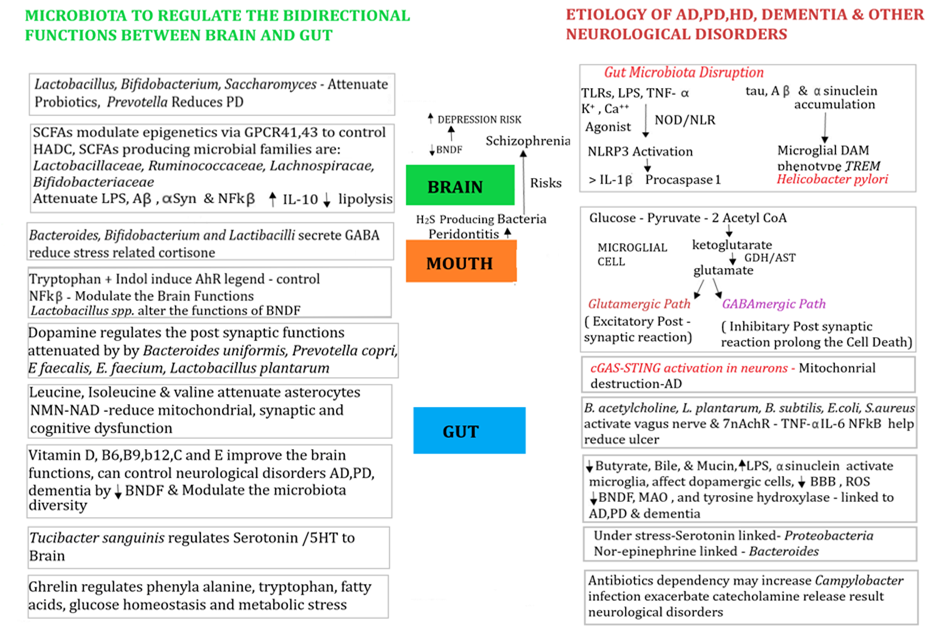
Figure 1. Representation of bidirectional molecular and cellular signaling of immune regulation determines the fate of neurological disorders. Microglial cells play a major role in translating signaling through post synaptic cells under stressful conditions could result in accumulation of tau, Aβ and α-synucleins accumulation may change the microglial cells into DAM phenotypes. Some bacterial families such as, Lactobacillaceae, Ruminococcacea, Lachnospiraceae, and Bifidobacteriaceae increase the microbiota’s stability to improve the signals to secrete GABA, IL-10, reduce mitochondria, synaptic dysfunctions. Vitamins reduce BNDF and moderate diversity.
Ghrelin hormone and microbiota regulations
Orexigenic hormone Ghrelin may have a role in central regulation of homeostasis and non-homeostasis state to control food intake. Bacteroidetes, Firmicutes, Actinobacteria, Fusobacteria, and Proteobacteria, in the stomach influence the ghrelinergic regulations. Entero-endocrine cells in GI tract absorb GI peptides, microbiota secrete these peptides via utilizing/breaking down the various nutrients. The anorexigenic peptides stimulate phenylalanine, tryptophan, and fatty acids. G protein coupled receptors (GPCRs) are meant to regulate the ghrelin receptors (GHSR). Additionally, ghrelin is also involved in glucose homeostasis and manages metabolic stress. A body of evidence has revealed the uninterrupted involvement of gut associated metabolisms could be related to psychiatric disorders [16,39]. Prevotella spp. abundance also increases the plasma ghrelin [32]. Bacteroides/Firmicutes ratio was greater in patients infected with Helicobacter pylori and undergoing antibiotic treatment has shown lower Ghrelin level in plasma. On the other hand, Fermicutes/Bacteroides ratio increase referred to abundance of Faecalibacterium increasing glucagon level with decreased ghrelin level. Fecal transplant in rats increase the Lactobacilli with higher ghrelin level. These are the opportunities to discover the role of ghrelin in health [40]. Bifidobacterium and Lactobacillus are supposed to increase the Ghrelin’s efficacy before it gets transferred to the other parts through host cells [40]. Therapeutic SCFAs indirectly affect the ghrelin secretion, thus influencing the ghrelinergic signaling. LPS and TLR provide the reduced Ghrelin response and under oxidative stress it is increased. Microbiota does have the implications of processing carbohydrates and produce signals that can impact mental health and obesity [40].
Microbiome Interact through Gut-Brain Regulations
Host microbiota in human body is located in nasal cavity, oral cavity, lungs, skin, gut, urinary tract, and vagina [14]. Advancements and implementation of sequencing and non-cultured methods has refined the regulatory pathways from Gut to Brain, to reveal the remarkable role of microbiota to regulate cell activities. To contextualize the regular and irregular bidirectional [16] or multidirectional pathways among multiple organs could determine the poor or good prognosis of disease. Gut microbiota including Bacteroides vulgatus and Campylobacter jejuni affect the glutamate metabolism and metabolite to 2-keto glutamic acid. Corynbacterium glutamicum, Brevibacterium lactofermentum, and Brevibacterium avium convert L-glutamate to D-glutamate [14,41,42]. Non-pathogenic bacteria with glutamate racemace including Corynebacterium glutamicum, Brevibacterium lactofermentum, Brevibacterium avium, Mycobacterium smegmatis, and Bacillus subtilis convert L-glutamate to D glutamate, warrant further studies to establish this claim. However, Corynebacterium glutamicum (in vitro) convert the food into glutamate [14,16,41,42]. To better understand the bidirectional relationship between Gut and Barin, the healthy mice are inoculated with fecal matter obtained from unhealthy stressed mice eventually produced the symptoms of anxiety and HPC neurogenesis [43]. Lactobacillus, Bifidobacteria, and Saccharomyces species including other prebiotics can attenuate the commensals and flourish their growth in the gut to regulate the homeostasis. Also improve the depression and neuropsychiatric symptoms [43,44]. Post traumatic stress disorder (PTSD) was associated with decreased level of sp_HMT_914,332,871 and noxia levels, coupled with increased level of Bacteroidetes [45,46]. Prevotella has been associated to reduce the symptoms of PD [3,46-49]. Clostridium bolteae, Ruthenibacterium lactatiformans, and Akkermansia spp. found in increased levels in MS patients. Continuous presence of Akkermansia ameliorates the autoimmune encephalomyelitis associated with reduction of RORγt+ and IL-17- producing γδT cells [50]. On the other hand, LPS administered mice downregulate synaptic communication and enhance the proinflammatory cytokine production [51]. Noticeable impact of using adeno-associated viral (AAV) carriers with immunogens could increase the α-Syn accumulation, might provide clues to design further strategies to use AAV carriers to minimize the adverse impact [46-49]. Therefore, the disrupted microbiota has the ability to downregulate the innate pathways and dysregulate the immune response [10]. Altered microbiota substituted with Helicobacter pylori may induce inflammatory genotoxic effects lead to tumor formation. However, the microbiota activity including commensals reduce the defective immune signals and increase the efficacy of immunotherapies against tumor in glioma model [10,48]. Helicobacter pylori and Escherichia coli increase the tau and Aβ load (AD), α-Syn aggregation affecting the motor neurons performance (PD), thus, impacting the proinflammatoty and genotoxic ability (MS). However, Bifidobacterium and Lactobacillus reduce the dopaminergic impact and neurodegeneration events. Therefore, microbiota is capable of inducing neurotransmitters like serotonin, dopamine and GABA to regulate the response between Gut and Brain [16,48] and produce positive impact improving PD.
Microbial Metabolites Promote Physiological Activities
Clostridium and Eubacterium convert bile into secondary bile (deoxycholic acid and lethocholic acid) bind to the tGR5 receptor to stimulate the secretion of incretin, insulin and also promote energy expenditure [52]. Additionally, secondary bile promotes several physiological and biological functions i.e., metabolism, immunological functions, and resistance against intestinal pathogens [16]. Ursodeoxycholic acid has anti-inflammatory impact on Farnesoid X receptors, GPCR both in microglia and neuron cells [16]. Ursodeoxycholic acid reduces, apoptosis, ROS, TNF-α, NO, IL-1β in neuropschyatric models and increase glutathione in AD, PD and HD. Therefore, beneficial effects of taurodeoxycholic acid can be utilized for neurodegenerative, neurological, and neuropsychiatric disorders [53]. Chinese decoction diminished the plaque pathology, suppress the dysbiosis and control the taurohydroxycholic acid [16]. Trimethylamine N-oxide (TMAO) is derived from dietary choline, betaine, and L-carnitine. TMAO contributes towards neurodegenerative diseases and able to pass through BBB reducing microglial and astrocytes activation that also enhance Aβ pathology and tau deposition [54].Tryptophan and indole are amine precursors of serotonin that help modulate the central brain functions and health by controlling neuroinflammation and neurodegeneration. Specific-pathogen-free (SPF) mice showed higher concentrations of indole derivatives in gut, serum, and brain [16,54]. It has given clues for dietary tryptophan is being processed as biosynthetic precursor utilized by microbiota to release its active forms. Indole works with Ahr, which is a transcription factor that negatively regulate NF-κB. Ahr gene deletion in astrocytes drives CNS inflammation and neurodegeneration of astrocytes and microglia. Moreover, transforming growth factor (TGF) and Vascular endothelial growth factor (VEGF) B also regulate the pathogenic activities of astrocytes in MS models [22,55]. Poly unsaturated fatty acids (PUFAs) in the presence of Bacteroides that convert the biologically active compounds to activate the microglial cells. PUFA metabolism results in increased concentration of amino acids and Leukotrienes B4, Prostaglandin E2 and hydroxy heptadecanoic acid may exacerbate the AD pathology by microglial cells’ activation [56]. Branched chain amino acids like leucine, isoleucine, and valine also attenuate the microglial phenotype into anti-inflammatory M2 phenotype. Reduced plasma level of BCAA is directly correlated to the disease severity found to be associated with reduction of Prevotella copri. Moreover, L. plantarum restores the intestinal integrity and astrocyte functions to reduce dementia [16,57]. Nicotinamide Ribonucleotide (NMN) and NAD+ (precursor) benefit to regulate the cell signaling to reduce neuroinflammation, mitochondrial abnormalities, synaptic dysfunctions, and cognitive impairments, thus, regulate the energetic, genotoxic and infectious stress to reduce AD featured brain functions. NMN also increases the abundance of Lactobacillus and Bacteroides [58]. Transgenic rats’ model of AD was reported to deficit in NAM than normal rats, hence, indicating it as a biomarker for AD. But the ultimate contribution of NAM in curing AD is still in question [59]. Microbial metabolites like SCFAs are known to regulate the anti-neuroinflammatory and anti-neurodegenerative effects, that also control the physiological, immune, intestinal, cholesterol and glucose homeostasis in the body [3]. Besides, SCFA also plays a significant role in epigenetic regulations via GPCR 43, 41 (FFAR-2) endogenously to control the histone deacetylase (HDAC) enzyme [49]. Nevertheless, SCFAs increase the GPCR 41 in Aβ stimulated BV2 cells (glial cells) and stop the ERK (MAPK)/JNK/NF-κB inflammatory pathways, which induces CD11b and COX in mice [16,60]. Acetate and butyrate moderate LPS response to reduce the proinflammatory response in diseased state. SCFA producing microbial species boosts the prebiotic interventions reducing Aβ and α-synuclein, therefore, ameliorating the impacts of AD and PD via motor cells function and microglial activity [16]. Butyrate also influences cardiometabolic health, reduction in blood pressure and prothrombic factor as well. Evidently, increasing the Treg and CD4+ T-cells in circulation to reduce NF-κB and enhance IL-10 to prepare homeostatic environment. Fermicutes with Lactobacillaceae, Ruminococcaceae, Lachnospiraceae are the contributors to synthesizing SCFAs. SCFAs also reduce lipolysis and decrease lipid plasma level [16,60]. Microbial families like Bifidobacteriaceae and Probiotics- L. planatarum, L. paracasei, L. rhamnosus use hydrolases to utilize polysaccharides and produce butyrate. Enzymes such as transferase, phosphorylase, and butyrate kinase use 2 molecules of Acetyl CoA to form Butyrate [16]. Inulin, starch, and Raffinose are effective prebiotics that can be used for butyrate production. SCFA-butyrate provides special benefits like change redox state, glucose D metabolism, cancer cells apoptosis, HDCAs inhibition and regulate p21. Propionate stimulates GPR-43 [60].
Oral Microbiome
The oral cavity constitutes the second largest microbiota of the body. Actual microbial disruptions start from the oral cavity that led to the progression of autoimmune disorders and psychiatric disorders. Actinobacteria, Bacteroides, Firmicutes, Fusobacteria, and Proteobacteria are the dominant microbes. Patients with poor oral hygiene with already existing periodontal conditions increase the chances of harboring neuropsychiatric disorders 1.45 times higher than other patients. BD patients with higher risk often have Aggregatibacter actinomycetemcomitans and Porphyromonas gingivalis. Additionally, H2S producing bacteria raises the risks for schizophrenia [14,61].
Neurotransmitters to Implicate the Function of Microglial Cells
Neurotransmitters such as serotonin, dopamine, GABA, kynurenic acid, SCFAs, D-amino acids, bile acids are effectively involved in altering the behavior of microglial cells. D-amino acids were linked to reduced blood glutamate level in AD patients compared to healthy controls. D glutamate higher level is associated with more cognitive impairment in AD, whereas D-alanine higher range is aligned with behavioral symptoms [14]. GABA receptors are involved in regulating the neurotransmitters through CNS that directly regulate the physiological and psychological functions through microglial and synaptic neuronal cells. L. rhamnosus reduced the stress related corticosterone, anxiety and depression related symptoms. Vagotomized mice are devoid of all neurochemical and behavioral functions, therefore, healthy gut microbiome and brain constitute the modulatory pathway [62]. PD associated neuropathy, more precisely synucleinopathy contextualized with accumulation of α-Synuclein is determined by Synuclein inclusion bodies in the neurons of Meissner plexus joining the gastric mucosa. These are the susceptible enteric neurons affecting CNS [46- 49]. rAAV-alpha syn model revealed the over expression of α-Syn inclusion bodies in E46K, and A53T mediated through rAAV2/9 and α-Syn at the 12 weeks of rAAV/α-Syn administration reached at maximal level, where from actual neurodegeneration begins. On the other hand, the contractual administration of empty rAAV had not given evidence of neurodegeneration. P1K1 gene alteration also failed to reproduce dopaminergic neurodegeneration [46-48]. Dysregulatory functions of microglial cells evidently assess the fate of neurodegenerative disorders. The neuronal functions along with physiological, neurogenesis, angiogenesis and BBB integrity, and synaptic functions are coordinated through microglial cells. The activity is always associated with phagocytosis, apoptosis and removal of cell debris, that eliminates tau, Aβ, and α-Synuclein. However, the activity of these cells decreases over time i.e., old age results in accumulation of toxic compounds responsible for cognitive decline [16]. The regulations of microglial cells are implicated through tyrosine kinase, 2-apolipoprotein, spleen tyrosine kinase, complement, sialic acid, immunoglobulin like lectins, and observed in various disease outcomes. tau, Aβ, Huntingtin, TDP-43, superoxide dismutase (SOD) always fused in sarcoma. Additionally, prolonged exposure to Aβ autophagy led to an increase in lysosomal dysfunction [16,63,64]. In PD model, α-Synuclein inhibits the microglial autophagy with TLR4 sensor with P38 MAPK pathway to exacerbate the AKT-mTOR (mammalian target of rapamycin) signaling leading to autophagy [16]. Apoliporotein E assists the accumulation of Aβ with consecutive increase in late AD pathology [63]. It is important to understand the actual functions of microglial cells and their broad communication with other body parts would shed some light to combat the poor disease prognosis [63].
Microbial Metabolites Implicate the Function of Microglial Cells
N-methyl-D-aspartate receptors (NMDAR) are responsible for the synaptic transmission signaling to metabolize D-glutamate to blockade Mg2+ and Ca2+ permeability essential for excitatory neurotransmission in CNS [14,41,42]. NMDAR may stop pro-apoptotic signaling with apoptotic peptide activating factor cAMP and PUMA in cytoplasm element binding protein O (FOXO) amd p53. Aβ peptide also plays a key role in regulating the activity dependent synaptic vesicle release [14,41,42]. D-amino acid oxidase and amino acids regulate NMDAR; hence, disrupting NMDA could result in AD development. The increased activity of D-amino oxidase enzyme (DAO) is associated with deficit in cognition lowering the D-glutamate level [41]. Microglial activities are profiled by two mechanisms contributing to the neuropathology in the brain. The DAM cells downstream the path of TREM2 and CD33, thus, increase the Aβ pathology. Which in turn activate PI3K-AKT, Glycogen synthase kinase-3β (GSK-3β) via mTOR to establish Aβ pathology – by disruption of SYK (spleen tyrosine kinase) signaling. However, the removal of gene TREM2R47H (Human allele) from mice microglial cells helps these regain the activity into proinflammatory state. More discoveries need to be conducted on this front to leverage the activity restoration [16,65]. cGAS signaling activity is higher in acute inflammation, but it assists in accumulation of more Aβ, aberrant cellular and mitochondrial DNA and tau in the chronic state with low grade of inflammation specified by continuous STING-GAS signaling [16, 65]. The microglial cells dysregulated with use of antibiotics and deprivation of microbial metabolite SCFAs, were found to restore the microglial homeostasis by its consumption. The family Ruminococcaceae abundance to be targeted by bacteriophage myoviridae increase the isoamylamine (IAA) in elderly mice. IAA decrease modulate the neuronal cells and their cognitive deficit in aged mice [16,63]. The alterations of gut microbiota and microglial cells’ development is largely influenced by their functions in the development of neurological diseases. Microbiology, immunology, and neurology altogether hold the potentials to develop microglial associated target drugs [63].
Discussion
GABA plays an important role for glutamate conversion in endocrine, microglial, and neuronal synaptic cells. It is imperative to determine the efficacy of GABA producing probiotics may play a significant role in moderating the early intervention for mental health [31]. Tryptophan, an amino acid derived from the diet, can cross the blood-brain barrier and induce serotonin synthesis [66]. Hence, it was discovered as an essential nutrient to maintain the brain immunity, lacking tryptophan from diet results in autoimmune inflammation altered encephalitogenic T cell response. This effect is medicated via gut microbiome, but not directly from host sensors [67]. SCFAs regulate the serotonin synthesis in enterochromaffin cells, thereby affecting the gut-brain interconnection to influence the modulation of disrupted microglial communication [68]. SCFAs-GPR (GPR41 and GPR43) in the sympathetic and autonomic nervous system interact to coordinate cognitive activities. SCFA metabolites from probiotics increase the HVA production improve the dopamine effects. Autism in children shows reduced levels of acetic acid, propionic acid, and butyric acid with hyper serotonergic state and dopamine disorder (HVA decreased) [33,32]. Bifidobacterium controls the growth of Clostridium meant to decrease the severity of autism [32,33]. The excessive secretion of glutamate creates an ‘imbalanced state’ can induce neuronal toxicity with increased calcium ions uptake, ultimately leading to nerve cell degeneration. Extrapulmonary sequelae associated with neuroplasticity were resolved in 10 months in PACS patients [6]. Elevated LPS levels could also reactivate the Epstein-Barr virus. The microthrombosis formation disrupts the intracellular junctions [6]. Individuals with MS are reported to have reduced circulating Aryl Hydrocarbon (AHR). However, GM catalyzes the tryptophan into indole derivatives serve as AHR ligand to suppressing the NF-kB to stop CNS inflammation, via controlling the cytochrome signaling 2 (SOCS2). Anti-inflammatory IFNβ and dietary tryptophan are AHR agonists. Antibiotics treated mice supplemented with tryptophan derivative indole, indoxyl -3-sulfate, indole-3-propionoic acid and indole-3-aldehyde and enzyme tryptophanase could restrict the inflammation [16,21,22]. Restricted inflammation also occurred via TRAIL+ associated apoptosis of astrocytes (T-cells). Many HD models explain no relationship between microbiota and microglia [19]. The gene C9orf72 in HD with hexanucleotide repeat mainly drives the disease pathogenesis that favors the accumulation of toxins in nerve cells that flare up the inflammation related to dysfunctional autophagy and lysosomal pathway [16,17]. The FMT treated mice that were given synergistic antibiotics were discovered to restore microglial functions in PD and improve the expression of microglial receptors like ApoE [15,16]. The glial cells are primarily affected by the inflammatory events developed through brain regulating with other body organs, thereby, stimulating the astrocytes to receive signals depending upon the behavior of affected microglial cells [19]. Studies have shown treating small animals with synergistic antibiotics ABX i.e., kanamycin, gentamicin, colistin, metronidazole, and vancomycin reduce the Aβ plaque formation and increase the microglial function, clearly demonstrating that microbiota does play a role in regulation of microglial homeostasis. However, individual effect of antibiotic was not revealed [15]. High fat diet fed obese mice given fibers impeded the microglial dysfunction while increasing the social behavior in offsprings [48]. The oxygen toxicity with gradual loss of mitochondrial functions establishes as ROS state conferring the DNA damage and mutations develop neurogenerative disorders to decrease the life expectancy. Thereafter, poor communication between microbiota and brain brings more damage. However, healthy lifestyles including nutritious foods, SOD, vitamin A, C, E etc. can increase life expectancy by slowing down these processes. Therefore, microbiome does play a role in altering the immune signaling depending upon the status of utilizing these therapeutics [16,69]. The administration of Lactobacillus rhamnosus IMC 501 in a Zebrafish Model has been shown to alter the expression of BDNF in the hippocampus. This alteration is involved in enhancing the serotonin secretion. Serotonin is primarily produced in the gut when tryptophan is metabolized by common gut microflora, and it plays a crucial role in regulating brain functions. Furthermore, an increase in the levels of Firmicutes and a decrease in Protobacteria may support the modulation of endogenous neuroactive molecules. Therefore, the beneficial effects of these probiotics serve as antidepressants by affecting the vagus nerve, which mediates communication between the gut and the brain [70]. Bifidobacterium infantis and Bifidobacterium longum NCC 3001 help normalize gastrointestinal inflammation and anxiety-like behaviors by stimulating the vagus nerve and optimizing cytokine production [71,72]. The clinical trials on the improvement of cognitive behaviors considering the patients suffering from various neurological diseases are presented in (Table 1).Bifidobacterium bifidum, Lactobacillus plantarum, Lactobacillus longum, Lactobacillus rhamnosus, Lactobacillus gasseri, Bifidobacterium breve, and Lactobacillus casei have been used to modulate the gut microflora in various respiratory diseases [73]. Bifidobacterium treated COVID-19 patients (mid to severe stage) discovered with increased IgG and IgM response and decreased IL-6 levels and found to cut short the hospital stays [9,74]. Probiotics are generally considered safer to use in vulnerable populations. Probiotics-associated bacteremia and fungemia are rarely observed. Considering the fact, it is crucial to perform quality checks when using specific probiotics in known immunocompromised hosts or critically ill patients. Probiotic usage has been shown to reduce the burden of various respiratory diseases like COVID-19. Prebiotics such as, fructooligosaccharides’ or non-digestible galactooligosaccharides’ formulations elevate the level of BDNF and expression of NMDA subunit receptors in rat models [66,75]. Fecal microbiome transplantation has been used to control recurrent infections with Clostridium difficile and may also contribute to the treatment of psychopathological conditions [12,13]. The routine use of dietary interventions and decoction-derived Chinese medicines can also enhance safe immune regulation to prevent patients from progressing to critical conditions [3]. The detection of altered microbiota, associated metabolites, and immunological markers to determine the disease prognosis is a meaningful strategy. Interventional probiotics, prebiotics, and synbiotics are useful for patients from mild to severe stage, suggesting their use for personalized treatment.
|
Assigned Participants |
Probiotic Intervention |
Time |
Microbiome /Bioinformatics Analysis |
Pathological and Molecular Biomarkers |
Outcome |
Reference |
|
63 healthy adults (≥ 65 yrs old) |
Bifidobacterium bifidum BGN4
Bifidobacterium longum BORI |
12 weeks |
↓ abundance of inflammatory bacteria such as, Eubacteriales, Allisonella, Closteridiales
|
↑ Blood brain derived neurotrophic factor (BDNF) significantly
|
Improved Cognitive function |
Kim CS et al., 2021 [81] |
|
40 participants with AD |
Bifidobacterium longum subsp. infantis BLI-02, B. breve Bv-889, B. animalis subsp. lactis CP-9, B. bifidum VDD088, and Lactobacillus plantarum PL-02 Control group received- 1 × 107 CFU/day, Treatment group received-1 × 1010 CFU/ day |
12 weeks |
↑ abundance of Bifidobacterium, Lactobacillus, Ruminococcus, Clostridium, and Akkermansia ↓Megamonas level |
↑ BDNF by 36% (7115.1-9678.5 pg/mL) ↓ IL-1β No change- IL-10 ↑ SOD superoxide dismutase antioxidant ↓ Cortisol |
No statistical difference in results, but a molecular trend towards less cognitive decline
|
Hsu Y-C et al., 2024 [82] |
|
42 ELD (mid- 40 Yrs to elderly ≥70 Yrs old)
|
Daily Consumption of MS Prebiotic |
12 weeks |
Proteobacteria viz., E. coli and Shigella spp. were substituted with Bifidobacteria in ELD |
↑ SCFA significantly ↑ level of Bifidobacteria, Prevotella, Alistipes, Desulfovibrio, Mogibacterium, Sporobacter |
N/A |
Alfa M et al., 2018 [83] |
|
32 AD Patients
|
No treatment/disease prognosis |
N/A |
↓ Fermicutes
↑ Proteobacteria in AD patients |
↓ SCFAs in AD patients |
Microbial cause of Poor prognosis
|
Liu P et al., 2019 [84] |
|
8 patients with NC decline/ MCI, and AD |
Pre orthopedic surgery patients-to determine microbiome load |
N/A |
↑ Bacteroides and Fermicutes in MCI than SCD ↓ abundance of Christensenellaceae in SCD than in aMCI
|
↑↓ SCFA level varied in 3 groups ↑ Plasma LPS and ↑ CRP increased in SCD ↓ Lipid synthesis in AD ↑ plasma occluding in MCI patients
|
Microbiome to corelate with Cloudin, LPS, inflammatory factor, neuropsychological assessment, and cognition |
Duan M et al., 2021 [85] |
|
14 CI patients |
No treatment/ disease prognosis |
N/A |
↓ trend of abundance in Fermicutes, Ruminococcaceae, Faecalibacterium in all patient groups |
↓Anti-inflammatory Faecalibacterium in SCD and NC SCD were positive in amyloid |
Altered microbiome correlate with pathophysiology of AD |
Sheng C et al., 2021 [86] |
|
32 Aβ amyloid+ with CN+, 32 Aβ amyloid+ with CN-, 22 with CI 11 patients with Aβ amyloid+ MCI/ AD, 11 Aβ amyloid+ with AD |
No probiotic / disease prognosis |
N/A |
↑ Bacteroides enriched ↓ Firmicutes and class Deltaproteobacteria in CN+ patients |
↓ SCFA in CN+, CN- and CI patients
AB amyloid -ve CN normal group- family Desulfovibrionaceae genus Bilophila and Faecalibacterium |
AB amyloid and microbiome is a biomarker for AD |
Sheng C et al., 2022 [87] |
|
83 with CFS and FMS
|
Lactobacillus casei strain Shirota, Bifidobacterium infantis 35624 in CFS patients
|
8 weeks |
Changed fecal composition |
↓ Inflammatory biomarkers |
↓ Anxiety |
Roman P et al., 2018 [88] |
|
42 with MCI (>60 yrs old) |
21 Patients given probiotic mixture included Lactobacillus plantarum BioF-228, Lactococcus lactis BioF-224, Bifidobacterium lactis CP-9, Lactobacillus rhamnosus Bv-77, Lactobacillus johnsonii MH-68, Lactobacillus paracasei MP137, Lactobacillus salivarius AP-32, Lactobacillus acidophilus TYCA06, Lactococcus lactis LY-66, Bifidobacterium lactis HNO19, Lactobacillus rhamnosus HNO01, Lactobacillus paracasei GL-156, Bifidobacterium animalis BB-115, Lactobacillus casei CS-773, Lactobacillus reuteri TSR332, Lactobacillus fermentum TSF331, Bifidobacterium infantis BLI-02, and Lactobacillus plantarum CN2018 Active culture >2 × 1010 CFU/g |
12 weeks |
↑ Abundance of Faecalibacterium prausnitzii and affects tryptophan metabolism ↑ Relative abundances of Blautia, Lachnospiraceae, Muribaculaceae, Haemophilus, Coprococcus, Ruminococcus, Anaerostipes, Erysipelotrichaceae, Prevotellaceae, and Pantoea |
↑ BDNF level in probiotic group |
↑ Memory cognition function and sleep quality |
Fei et al., 2023 [89] |
|
78 Patients with DM (>65 yrs old) |
Lactiplantibacillus plantarum OLL2712 |
12 weeks |
↓ Inflammatory microflora Lachnoclostridium, Monoglobus, and Oscillibacter genera |
Induction of IL-10 |
Computer assisted cognitrax revealed the protective effects against memory function |
Sakurai K et al., 2022 [90] |
|
169 CD-( 52-75yrs old) |
Lactobacillus rhamnosus GG (LGG) |
12 weeks |
Prevotella ruminicola, Bacteroides thetaiotaomicron, and Bacteroides xylanisolvens taxas were correlated with MCI. |
↓ Abundance of Prevotella and Dehalobacterium in MCI group |
↑ Cognitive success with Psycobiotics’ intervention |
Aljumaah MR et al., 2022 [91] |
|
60 AD patients |
Patients diagnosed- cognitive and metabolic variations were on probiotic supplemented group took 200 ml/day probiotic milk containing Lactobacillus acidophilus, Lactobacillus casei, Bifidobacterium bifidum, and Lactobacillus fermentum 2 × 109 CFU/g for each Other group had milk only |
12 weeks |
N/A |
Plasma malonedialdehyde, CRP, insulin resistance, beta cell function, triglycerides are varied in probiotic group But no change was observed in oxidative stress inflammation and fasting glucose, lipid profile |
MMSE mini mental state examination- state improved in probiotic group (~28%) than control (~-5%). Significantly positive impact on cognitive symptoms |
Akbari E et al., 2016 [92] |
|
79 AD patients |
27 Patients on Selenium200 (μg/day) plus probiotic containing Lactobacillus acidophilus, Bifidobacterium bifidum, and Bifidobacterium longum, 2 × 109 CFU/day each 26 Patients on selenium (200 μg/day) |
12 weeks |
N/A |
↓ hs-CRP, insulin, homeostasis model of assessment-insulin resistance, LDL/HDL-cholesterol with Selenium supplementation ↑ Glutathione and the quantitative insulin sensitivity check index |
AD improved cognitive function and metabolic profile |
Tamtaji et al., 2019 [93] |
|
60 AD patients |
30 Patients on Lactobacillus and Bifidobacterium genera (Lactobacillus acidophilus, Lactobacillus casei, Lactobacillus fermentum, and Bifidobacterium bifidum) total dosage of 3 × 109 CFU |
12 weeks |
N/A |
No significance difference observed in TNF-α, IL-6 and IL-10, oxidants, and antioxidants factors in probiotic group. |
↑ Score of TYM Cognitive in patients with mild AD |
Agahi A et al., 2018 [94] |
|
61 MC patients (50-80 yrs old) |
Bifidobacterium breve A1 supplementation -2 Capsules appx. > 2 × 1010 CFU |
12 weeks |
N/A |
No significant difference in CRP and other blood parameters |
↑ MMSE score |
Kobyashi Y et al. 2019 [95] |
|
115 MCI partients
|
55 Patients on B. breve MCC1274, 2 × 1010 CFU
|
24 weeks |
↓ Actinobacteria and Bifidobacterium in lower MMSE subgroup ↑ Abundance of Prevotella , Clostridiaceae, Ruminococcaceae, and Phascolarctobacterium in lower subgroup |
N/A |
↑ MMSE score |
Asaoka D et al., 2022 [96] |
|
79 MCI participants (50-79 yrs old) |
Bifidobacterium breve A1, 2 × 1010 CFU |
16 weeks |
N/A |
N/A |
↑ RBANS score in probiotic group |
Xiao J et al., 2020 [97] |
|
133 potential participants (55-85 yrs old)
|
DW2009 is a mixture of fermented soybean powder and Lactobacillus plantarum C29 freeze-dried powder, 1.25 × 1010 CFU/g |
6-12 weeks |
↑ Level of Bifidobacterium, Clostridium and Lactobacillus spp. |
↑ Serum BDNF levels for DW2009 group was 412.7 pg/mL |
↑ Cognitive success in MCI probiotic group |
Hwang Y-H et al., 2019 [98] |
|
Abbreviations: AD: Alzheimer’s Disease; aMCI patients: Amnestic Mild Cognitive Impairment; NCD/ MCI: Neurocognition with Cognitive Decline/ Mild Cognitive Impairment; SCD: Subjective to Cognitive Decline; CI: Cognitive Impairment; CN+: Cognitive Normal; CN-: Cognitive impaired; MCI: AD Mild Cognitive Impaired; CFS: Chronic Fatigue Syndrome; FM: Fibromyalgia Syndrome; DM: Declining Memory; CM: Community Dwelling; MC: Memory Complaints; N/A: Not Available. |
||||||
Conclusion
Oral microbial displacement is implicated for many systemic diseases like cardiovascular disease, metabolic, respiratory and cognitive disorders due to excessive inflammation. Periodontitis might be speculated to be responsible for neurodegenerative diseases. Biofilm forming bacteria increase the pathogen load to disrupt the normal microbial flora leading to developing immune and endocrine dysregulation. Virulent toxins and pathogens enter the vascular and neural pathways. It is imperative to diagnose patients suffering from dental disease could be prone the develop neurological implications, thus, indicating the more collaborations between dentists and neurologists. The appropriate therapeutics that will be designed based on microbiome structure could help develop the new methodologies to design personalized medicines. There is utmost need to develop the virome with genes alterations impacting the microglial developments. Developing innovative and non-invasive therapies in the management of neurodegenerative diseases could be a useful strategy for early development of disease. Virome and genetic studies can also unveil the relationship between microbiome alterations to affect cognitive behaviors. Microbe-related interventions not only serve to correct the gut signals, but also have the potential to regulate brain signals while enhancing the efficacy of drugs used during infection [3,74]. Byproducts of dietary carbohydrates utilized by commensals stimulate the sympathetic nervous system, modulate immune signals through the endocrine system, and improve microglial activities after passing through the BBB [12].
Life span antibiotics treatment to recover the microglial activity in reducing the ALS is always not true, as dysbiosis and changes in metabolites can also exacerbate the ALS conditions [18]. Long-term use of antibiotics has also been reported to be linked with psychiatric effects, even in individuals without a pre-morbid psychiatric history. Prolonged antibiotic and corticosteroid usage can propel immune dysregulation, disrupting the normal microflora and establishing abnormal communication with immune cells, TLRs, Treg cells, and immune modulatory metabolites. Consequently, the emerging stress-related pathologies may be accompanied by higher levels of proinflammatory cytokines. The resident gut microbiome plays a significant role in detoxifying drugs through the liver mitigating drug side effects [13]. 5-hydroxytryptamine (5-HT) is typically regulated through the gut microbiome, that directly implicates the fetal neuronal cell division, differentiation, and synaptogenesis. Perturbed microbiome activities in early life could have a long-lasting consequence on the neurodevelopment and mental health, potentially leading to develop the permanent disorders if not treated in a timely manner [76,77]. Normal microbial regulation is essential for programming normal social and repetitive behavior, memory, and pain signaling in the body [13]. Lachnospiracea-Dorea, Blautia are correlated with insulin resistance. On the other hand, Bacteroides and Alistipes are related to lower insulin resistance and monosaccharides. Insulin signaling would follow ERK/ MAPK and IRS/PI3K/AKT [78]. Faecalibacterium prausnitzii and Roseburia are the most important microbiota responsible for the onset and development of T2D. Bacteroides Vulgatus and Bacteroides dorei preserve the cell wall integrity and reduce LPS production. To some extent the endotoxemia occurred is predisposed to the low grade of inflammation involved in the pathogenesis of insulin resistance [79]. Lactobacillus improves IL-10, insulin sensitivity and inhibits the pro-inflammatory cytokines IL-1β, monocyte chemoattractant protein-1 (MCP-1), IL-8, Interferon γ and C-reactive protein synthesis [79]. Essentially, probiotics are capable of modulating and stabilizing microbiota. The gut microbiome influences microglial cells to modulate and function in building homeostasis. Early microglia contribute to early brain development and synaptic modeling. A few weeks after birth, the mature microglia activate themselves to recognize the essential immune response resulting in pathological state or homeostatic state [80]. Therefore, microbiota influences the Gut Brain axis directly and indirectly [38]. Vitamin D, B6, B9, B12, C and E offer significant advantages promoting the well-being of brain. Vitamin deficiency is correlated with memory impairment during late stage of neurological disorders. Vitamins target BCL-2, PINK-1 and Klotho that suppress the aging phenomenon in mice to extend the life expectancy. Therefore, vitamins improve the cognitive deficit, suppression of seizures, impede neurodegeneration, and reduce the Aβ levels. Calcitriol the active form of vitamin D, 1, 25(OH)2D has shown to benefit patients with neurological disorders like PD, AD, and MS, that is directly attributed to neurotrophin i.e., lower level of BDNF is correlated to the higher frequency of depression and cognitive decline. However, the excessive intake of vitamin A can cause adverse effects. Multiple signaling pathways could be effective as antioxidants are involved during multivitamin usage approach [99,100]. Evidently, nutrition does play a significant role in modulating the diversity of gut microbiota to produce protective immune signals e.g., vitamin A, B2, D, E and beta carotene, whereas, vitamin A, B2, B, C, K and D also increase the SCFAs [101], might well also be contexed with brain related regulatory functions.
Acknowledgement
All the literature data was cited by surfing Google and PubMed. The author conveyed her sincere thanks to the expert reviewers in shaping this manuscript to deliver the work aligned with actual purpose and objectives.
References
2. WHO. Over 1 in 3 people affected by neurological conditions, the leading cause of illness and disability worldwide. Press Release. Geneva, Switzerland. 2024.
3. Liu L, Wang H, Chen X, Zhang Y, Zhang H, Xie P. Gut microbiota and its metabolites in depression: from pathogenesis to treatment. EBioMedicine. 2023 Apr;90:104527.
4. COVID-19 Mental Disorders Collaborators. Global prevalence and burden of depressive and anxiety disorders in 204 countries and territories in 2020 due to the COVID-19 pandemic. Lancet. 2021 Nov 6;398(10312):1700-12.
5. The Lancet. Long COVID: 3 years in. Lancet. 2023 Mar 11;401(10379):795.
6. Plummer AM, Matos YL, Lin HC, Ryman SG, Birg A, Quinn DK, et al. Gut-brain pathogenesis of post-acute COVID-19 neurocognitive symptoms. Front Neurosci. 2023 Sep 28;17:1232480.
7. Ranjbar M, Rahimi A, Baghernejadan Z, Ghorbani A, Khorramdelazad H. Role of CCL2/CCR2 axis in the pathogenesis of COVID-19 and possible Treatments: All options on the Table. Int Immunopharmacol. 2022 Dec;113(Pt A):109325.
8. Bohnacker S, Hartung F, Henkel F, Quaranta A, Kolmert J, Priller A, et al. Mild COVID-19 imprints a long-term inflammatory eicosanoid- and chemokine memory in monocyte-derived macrophages. Mucosal Immunol. 2022 Mar;15(3):515-24.
9. Alenazy MF, Aljohar HI, Alruwaili AR, Daghestani MH, Alonazi MA, Labban RS, et al. Gut Microbiota Dynamics in Relation to Long-COVID-19 Syndrome: Role of Probiotics to Combat Psychiatric Complications. Metabolites. 2022 Sep 27;12(10):912.
10. Kaushal A, Noor R. Association of Gut Microbiota with Inflammatory Bowel Disease and COVID-19 Severity: A Possible Outcome of the Altered Immune Response. Curr Microbiol. 2022 May 5;79(6):184.
11. Autism Spectrum Disorder: Does Autism Begin in the Womb? Research Breakthrough May Lead to New Treatment Strategies. SciTech Daily, Kobe University; 2022. https://scitechdaily.com/does-autism-begin-in-the-womb-research-breakthrough-may-lead-to-new-treatment-strategies/
12. Appleton J. The Gut-Brain Axis: Influence of Microbiota on Mood and Mental Health. Integr Med (Encinitas). 2018 Aug;17(4):28-32.
13. Rogers GB, Keating DJ, Young RL, Wong ML, Licinio J, Wesselingh S. From gut dysbiosis to altered brain function and mental illness: mechanisms and pathways. Mol Psychiatry. 2016 Jun;21(6):738-48.
14. Hashimoto K. Emerging role of the host microbiome in neuropsychiatric disorders: overview and future directions. Mol Psychiatry. 2023 Sep;28(9):3625-37.
15. Dodiya HB, Frith M, Sidebottom A, Cao Y, Koval J, Chang E, et al. Synergistic depletion of gut microbial consortia, but not individual antibiotics, reduces amyloidosis in APPPS1-21 Alzheimer's transgenic mice. Sci Rep. 2020 May 18;10(1):8183.
16. Loh JS, Mak WQ, Tan LKS, Ng CX, Chan HH, Yeow SH, et al. Microbiota-gut-brain axis and its therapeutic applications in neurodegenerative diseases. Signal Transduct Target Ther. 2024 Feb 16;9(1):37.
17. Beckers J, Tharkeshwar AK, Van Damme P. C9orf72 ALS-FTD: recent evidence for dysregulation of the autophagy-lysosome pathway at multiple levels. Autophagy. 2021 Nov;17(11):3306-22.
18. Blacher E, Bashiardes S, Shapiro H, Rothschild D, Mor U, Dori-Bachash M, et al. Potential roles of gut microbiome and metabolites in modulating ALS in mice. Nature. 2019 Aug;572(7770):474-80.
19. Tabrizi SJ, Estevez-Fraga C, van Roon-Mom WMC, Flower MD, Scahill RI, Wild EJ, et al. Potential disease-modifying therapies for Huntington's disease: lessons learned and future opportunities. Lancet Neurol. 2022 Jul;21(7):645-58.
20. Sekar A, Bialas AR, de Rivera H, Davis A, Hammond TR, Kamitaki N, et al. Schizophrenia risk from complex variation of complement component 4. Nature. 2016 Feb 11;530(7589):177-83.
21. Hubbard TD, Murray IA, Bisson WH, Lahoti TS, Gowda K, Amin SG, et al. Adaptation of the human aryl hydrocarbon receptor to sense microbiota-derived indoles. Sci Rep. 2015 Aug 3;5:12689.
22. Rothhammer V, Mascanfroni ID, Bunse L, Takenaka MC, Kenison JE, Mayo L, et al. Type I interferons and microbial metabolites of tryptophan modulate astrocyte activity and central nervous system inflammation via the aryl hydrocarbon receptor. Nat Med. 2016 Jun;22(6):586-97.
23. Olajide OJ, Suvanto ME, Chapman CA. Molecular mechanisms of neurodegeneration in the entorhinal cortex that underlie its selective vulnerability during the pathogenesis of Alzheimer's disease. Biol Open. 2021 Jan 25;10(1):bio056796.
24. Spiljar M, Merkler D, Trajkovski M. The Immune System Bridges the Gut Microbiota with Systemic Energy Homeostasis: Focus on TLRs, Mucosal Barrier, and SCFAs. Front Immunol. 2017 Oct 30;8:1353.
25. Shen Y, Qian L, Luo H, Li X, Ruan Y, Fan R, et al. The Significance of NLRP Inflammasome in Neuropsychiatric Disorders. Brain Sci. 2022 Aug 10;12(8):1057.
26. Tian H, Huang D, Wang J, Li H, Gao J, Zhong Y, et al. The role of the "gut microbiota-mitochondria" crosstalk in the pathogenesis of multiple sclerosis. Front Microbiol. 2024 Apr 29;15:1404995.
27. Zeng H, Huang J, Zhou H, Meilandt WJ, Dejanovic B, Zhou Y, et al. Integrative in situ mapping of single-cell transcriptional states and tissue histopathology in a mouse model of Alzheimer's disease. Nat Neurosci. 2023 Mar;26(3):430-46.
28. Barandouzi ZA, Lee J, Del Carmen Rosas M, Chen J, Henderson WA, Starkweather AR, et al. Associations of neurotransmitters and the gut microbiome with emotional distress in mixed type of irritable bowel syndrome. Sci Rep. 2022 Jan 31;12(1):1648.
29. Yeo XY, Tan LY, Chae WR, Lee DY, Lee YA, Wuestefeld T, et al. Liver's influence on the brain through the action of bile acids. Front Neurosci. 2023 Feb 2;17:1123967.
30. Mayer EA, Savidge T, Shulman RJ. Brain-gut microbiome interactions and functional bowel disorders. Gastroenterology. 2014 May;146(6):1500-12.
31. Braga JD, Thongngam M, Kumrungsee T. Gamma-aminobutyric acid as a potential postbiotic mediator in the gut-brain axis. NPJ Sci Food. 2024 Apr 2;8(1):16.
32. Hamamah S, Aghazarian A, Nazaryan A, Hajnal A, Covasa M. Role of Microbiota-Gut-Brain Axis in Regulating Dopaminergic Signaling. Biomedicines. 2022 Feb 13;10(2):436.
33. Wang Y, Li N, Yang JJ, Zhao DM, Chen B, Zhang GQ, et al. Probiotics and fructo-oligosaccharide intervention modulate the microbiota-gut brain axis to improve autism spectrum reducing also the hyper-serotonergic state and the dopamine metabolism disorder. Pharmacol Res. 2020 Jul;157:104784.
34. Rueda Ruzafa L, Cedillo JL, Hone AJ. Nicotinic Acetylcholine Receptor Involvement in Inflammatory Bowel Disease and Interactions with Gut Microbiota. Int J Environ Res Public Health. 2021 Jan 29;18(3):1189.
35. Chen Y, Xu J, Chen Y. Regulation of Neurotransmitters by the Gut Microbiota and Effects on Cognition in Neurological Disorders. Nutrients. 2021 Jun 19;13(6):2099.
36. Inazu M. Functional Expression of Choline Transporters in the Blood-Brain Barrier. Nutrients. 2019 Sep 20;11(10):2265.
37. Mazzoli R, Pessione E. The Neuro-endocrinological Role of Microbial Glutamate and GABA Signaling. Front Microbiol. 2016 Nov 30;7:1934.
38. Baj A, Moro E, Bistoletti M, Orlandi V, Crema F, Giaroni C. Glutamatergic Signaling Along The Microbiota-Gut-Brain Axis. Int J Mol Sci. 2019 Mar 25;20(6):1482.
39. Leeuwendaal NK, Cryan JF, Schellekens H. Gut peptides and the microbiome: focus on ghrelin. Curr Opin Endocrinol Diabetes Obes. 2021 Apr 1;28(2):243-52.
40. Yanagi H, Tsuda A, Matsushima M, Takahashi S, Ozawa G, Koga Y, et al. Changes in the gut microbiota composition and the plasma ghrelin level in patients with Helicobacter pylori-infected patients with eradication therapy. BMJ Open Gastroenterol. 2017 Nov 26;4(1):e000182.
41. Lin CH, Yang HT, Lane HY. D-glutamate, D-serine, and D-alanine differ in their roles in cognitive decline in patients with Alzheimer's disease or mild cognitive impairment. Pharmacol Biochem Behav. 2019 Oct;185:172760.
42. Chang CH, Lin CH, Lane HY. D-glutamate and gut microbiota in Alzheimer’s disease. Int J Mol Sci. 2020;21:2676.
43. Siopi E, Galerne M, Rivagorda M, Saha S, Moigneu C, Moriceau S, et al. Gut microbiota changes require vagus nerve integrity to promote depressive-like behaviors in mice. Mol Psychiatry. 2023 Jul;28(7):3002-12.
44. Bowland GB, Weyrich LS. The Oral-Microbiome-Brain Axis and Neuropsychiatric Disorders: An Anthropological Perspective. Front Psychiatry. 2022 Mar 30;13:810008.
45. Levert-Levitt E, Shapira G, Sragovich S, Shomron N, Lam JCK, Li VOK, et al. Oral microbiota signatures in post-traumatic stress disorder (PTSD) veterans. Mol Psychiatry. 2022 Nov;27(11):4590-8.
46. Gómez-Benito M, Granado N, García-Sanz P, Michel A, Dumoulin M, Moratalla R. Modeling Parkinson's Disease With the Alpha-Synuclein Protein. Front Pharmacol. 2020 Apr 23;11:356.
47. Braak H, de Vos RA, Bohl J, Del Tredici K. Gastric alpha-synuclein immunoreactive inclusions in Meissner's and Auerbach's plexuses in cases staged for Parkinson's disease-related brain pathology. Neurosci Lett. 2006 Mar 20;396(1):67-72.
48. Liu X, Li X, Xia B, Jin X, Zou Q, Zeng Z, et al. High-fiber diet mitigates maternal obesity-induced cognitive and social dysfunction in the offspring via gut-brain axis. Cell Metab. 2021 May 4;33(5):923-38.e6.
49. Liu J, Li H, Gong T, Chen W, Mao S, Kong Y, et al. Anti-neuroinflammatory Effect of Short-Chain Fatty Acid Acetate against Alzheimer's Disease via Upregulating GPR41 and Inhibiting ERK/JNK/NF-?B. J Agric Food Chem. 2020 Jul 8;68(27):7152-61.
50. Cox LM, Maghzi AH, Liu S, Tankou SK, Dhang FH, Willocq V, et al. Gut Microbiome in Progressive Multiple Sclerosis. Ann Neurol. 2021 Jun;89(6):1195-211.
51. Zhang J, Ma L, Chang L, Pu Y, Qu Y, Hashimoto K. A key role of the subdiaphragmatic vagus nerve in the depression-like phenotype and abnormal composition of gut microbiota in mice after lipopolysaccharide administration. Transl Psychiatry. 2020 Jun 9;10(1):186.
52. Wu W, Chen Z, Han J, Qian L, Wang W, Lei J, et al. Endocrine, genetic, and microbiome nexus of obesity and potential role of postbiotics: a narrative review. Eat Weight Disord. 2023 Oct 20;28(1):84.
53. Huang F, Pariante CM, Borsini A. From dried bear bile to molecular investigation: A systematic review of the effect of bile acids on cell apoptosis, oxidative stress and inflammation in the brain, across pre-clinical models of neurological, neurodegenerative and neuropsychiatric disorders. Brain Behav Immun. 2022 Jan;99:132-146.
54. Zhang Y, Wang G, Li R, Liu R, Yu Z, Zhang Z, et al. Trimethylamine N-oxide aggravated cognitive impairment from APP/PS1 mice and protective roles of voluntary exercise. Neurochem Int. 2023 Jan;162:105459.
55. Rothhammer V, Borucki DM, Tjon EC, Takenaka MC, Chao CC, Ardura-Fabregat A, et al. Microglial control of astrocytes in response to microbial metabolites. Nature. 2018 May;557(7707):724-28.
56. Xia Y, Xiao Y, Wang Z-H, Liu X, Alam AM, Haran JP, et al. Bacteroides Fragilis in the gut microbiomes of Alzheimer’s disease activates microglia and triggers pathogenesis in neuronal C/EBPβ transgenic mice. Nature Communications. 2023;14:5471.
57. Fu Y, Wang Y, Ren H, Guo X, Han L. Branched-chain amino acids and the risks of dementia, Alzheimer's disease, and Parkinson's disease. Front Aging Neurosci. 2024 Apr 10;16:1369493.
58. Zhao X, Kong M, Wang Y, Mao Y, Xu H, He W, et al. Nicotinamide mononucleotide improves the Alzheimer's disease by regulating intestinal microbiota. Biochem Biophys Res Commun. 2023 Aug 30;670:27-35.
59. Dalmasso MC, Arán M, Galeano P, Perin S, Giavalisco P, Martino Adami PV, et al. Nicotinamide as potential biomarker for Alzheimer's disease: A translational study based on metabolomics. Front Mol Biosci. 2023 Jan 6;9:1067296.
60. Fusco W, Lorenzo MB, Cintoni M, Porcari S, Rinninella E, Kaitsas F, et al. Short-Chain Fatty-Acid-Producing Bacteria: Key Components of the Human Gut Microbiota. Nutrients. 2023 May 6;15(9):2211.
61. Coelho JMF, Miranda SS, da Cruz SS, Dos Santos DN, Trindade SC, Cerqueira EMM, et al. Common mental disorder is associated with periodontitis. J Periodontal Res. 2020 Apr;55(2):221-28.
62. Bravo JA, Forsythe P, Chew MV, Escaravage E, Savignac HM, Dinan TG, et al. Ingestion of Lactobacillus strain regulates emotional behavior and central GABA receptor expression in a mouse via the vagus nerve. Proc Natl Acad Sci U S A. 2011 Sep 20;108(38):16050-5.
63. Raulin AC, Doss SV, Trottier ZA, Ikezu TC, Bu G, Liu CC. ApoE in Alzheimer's disease: pathophysiology and therapeutic strategies. Mol Neurodegener. 2022 Nov 8;17(1):72.
64. Oliveras-Salvá M, Macchi F, Coessens V, Deleersnijder A, Gérard M, Van der Perren A, et al. Alpha-synuclein-induced neurodegeneration is exacerbated in PINK1 knockout mice. Neurobiol Aging. 2014 Nov;35(11):2625-36.
65. Ennerfelt H, Frost EL, Shapiro DA, Holliday C, Zengeler KE, Voithofer G, et al. SYK coordinates neuroprotective microglial responses in neurodegenerative disease. Cell. 2022 Oct 27;185(22):4135-52.e22.
66. Rogers GB, Bruce KD. Challenges and opportunities for faecal microbiota transplantation therapy. Epidemiol Infect. 2013 Nov;141(11):2235-42.
67. Sonner JK, Keil M, Falk-Paulsen M, Mishra N, Rehman A, Kramer M, et al. Dietary tryptophan links encephalogenicity of autoreactive T cells with gut microbial ecology. Nat Commun. 2019 Oct 25;10(1):4877.
68. Mayer EA, Tillisch K, Gupta A. Gut/brain axis and the microbiota. J Clin Invest. 2015 Mar 2;125(3):926-38.
69. Hashim HM, Makpol S. A review of the preclinical and clinical studies on the role of the gut microbiome in aging and neurodegenerative diseases and its modulation. Front Cell Neurosci. 2022 Nov 3;16:1007166.
70. Borrelli L, Aceto S, Agnisola C, De Paolo S, Dipineto L, Stilling RM, et al. Probiotic modulation of the microbiota-gut-brain axis and behaviour in zebrafish. Sci Rep. 2016 Jul 15;6:30046.
71. Desbonnet L, Garrett L, Clarke G, Kiely B, Cryan JF, Dinan TG. Effects of the probiotic Bifidobacterium infantis in the maternal separation model of depression. Neuroscience. 2010 Nov 10;170(4):1179-88.
72. Bercik P, Park AJ, Sinclair D, Khoshdel A, Lu J, Huang X, et al. The anxiolytic effect of Bifidobacterium longum NCC3001 involves vagal pathways for gut-brain communication. Neurogastroenterol Motil. 2011 Dec;23(12):1132-9.
73. Baud D, Dimopoulou Agri V, Gibson GR, Reid G, Giannoni E. Using Probiotics to Flatten the Curve of Coronavirus Disease COVID-2019 Pandemic. Front Public Health. 2020 May 8;8:186.
74. Bozkurt HS, Bilen Ö. Oral booster probiotic bifidobacteria in SARS-COV-2 patients. Int J Immunopathol Pharmacol. 2021 Jan-Dec;35:20587384211059677.
75. Savignac HM, Corona G, Mills H, Chen L, Spencer JP, Tzortzis G, et al. Prebiotic feeding elevates central brain derived neurotrophic factor, N-methyl-D-aspartate receptor subunits and D-serine. Neurochem Int. 2013 Dec;63(8):756-64.
76. Neufeld KA, Kang N, Bienenstock J, Foster JA. Effects of intestinal microbiota on anxiety-like behavior. Commun Integr Biol. 2011 Jul;4(4):492-4.
77. Gareau MG, Wine E, Rodrigues DM, Cho JH, Whary MT, Philpott DJ, et al. Bacterial infection causes stress-induced memory dysfunction in mice. Gut. 2011 Mar;60(3):307-17.
78. Semo D, Reinecke H, Godfrey R. Gut microbiome regulates inflammation and insulin resistance: a novel therapeutic target to improve insulin sensitivity. Signal Transduct Target Ther. 2024 Feb 21;9(1):35.
79. Crudele L, Gadaleta RM, Cariello M, Moschetta A. Gut microbiota in the pathogenesis and therapeutic approaches of diabetes. EBioMedicine. 2023 Nov;97:104821.
80. Abdel-Haq R, Schlachetzki JCM, Glass CK, Mazmanian SK. Microbiome-microglia connections via the gut-brain axis. J Exp Med. 2019 Jan 7;216(1):41-59.
81. Kim CS, Cha L, Sim M, Jung S, Chun WY, Baik HW, et al. Probiotic Supplementation Improves Cognitive Function and Mood with Changes in Gut Microbiota in Community-Dwelling Older Adults: A Randomized, Double-Blind, Placebo-Controlled, Multicenter Trial. J Gerontol A Biol Sci Med Sci. 2021 Jan 1;76(1):32-40.
82. Hsu YC, Huang YY, Tsai SY, Kuo YW, Lin JH, Ho HH, et al. Efficacy of Probiotic Supplements on Brain-Derived Neurotrophic Factor, Inflammatory Biomarkers, Oxidative Stress and Cognitive Function in Patients with Alzheimer's Dementia: A 12-Week Randomized, Double-Blind Active-Controlled Study. Nutrients. 2023 Dec 20;16(1):16.
83. Alfa MJ, Strang D, Tappia PS, Graham M, Van Domselaar G, Forbes JD, et al. A randomized trial to determine the impact of a digestion resistant starch composition on the gut microbiome in older and mid-age adults. Clin Nutr. 2018 Jun;37(3):797-807.
84. Liu P, Wu L, Peng G, Han Y, Tang R, Ge J, et al. Altered microbiomes distinguish Alzheimer's disease from amnestic mild cognitive impairment and health in a Chinese cohort. Brain Behav Immun. 2019 Aug;80:633-43.
85. Duan M, Liu F, Fu H, Lu S, Wang T. Preoperative Microbiomes and Intestinal Barrier Function Can Differentiate Prodromal Alzheimer's Disease From Normal Neurocognition in Elderly Patients Scheduled to Undergo Orthopedic Surgery. Front Cell Infect Microbiol. 2021 Mar 29;11:592842.
86. Sheng C, Lin L, Lin H, Wang X, Han Y, Liu SL. Altered Gut Microbiota in Adults with Subjective Cognitive Decline: The SILCODE Study. J Alzheimers Dis. 2021;82(2):513-26.
87. Sheng C, Yang K, He B, Du W, Cai Y, Han Y. Combination of gut microbiota and plasma amyloid-? as a potential index for identifying preclinical Alzheimer's disease: a cross-sectional analysis from the SILCODE study. Alzheimers Res Ther. 2022 Feb 14;14(1):35.
88. Roman P, Carrillo-Trabalón F, Sánchez-Labraca N, Cañadas F, Estévez AF, Cardona D. Are probiotic treatments useful on fibromyalgia syndrome or chronic fatigue syndrome patients? A systematic review. Benef Microbes. 2018 Jun 15;9(4):603-11.
89. Fei Y, Wang R, Lu J, Peng S, Yang S, Wang Y, et al. Probiotic intervention benefits multiple neural behaviors in older adults with mild cognitive impairment. Geriatr Nurs. 2023 May-Jun;51:167-75.
90. Sakurai K, Toshimitsu T, Okada E, Anzai S, Shiraishi I, Inamura N, et al. Effects of Lactiplantibacillus plantarum OLL2712 on Memory Function in Older Adults with Declining Memory: A Randomized Placebo-Controlled Trial. Nutrients. 2022 Oct 14;14(20):4300.
91. Aljumaah MR, Bhatia U, Roach J, Gunstad J, Azcarate Peril MA. The gut microbiome, mild cognitive impairment, and probiotics: A randomized clinical trial in middle-aged and older adults. Clin Nutr. 2022 Nov;41(11):2565-76.
92. Akbari E, Asemi Z, Daneshvar Kakhaki R, Bahmani F, Kouchaki E, Tamtaji OR, et al. Effect of Probiotic Supplementation on Cognitive Function and Metabolic Status in Alzheimer's Disease: A Randomized, Double-Blind and Controlled Trial. Front Aging Neurosci. 2016 Nov 10;8:256.
93. Tamtaji OR, Heidari-Soureshjani R, Mirhosseini N, Kouchaki E, Bahmani F, Aghadavod E, et al. Probiotic and selenium co-supplementation, and the effects on clinical, metabolic and genetic status in Alzheimer's disease: a randomized, double-blind, controlled trial. Clinical Nutrition. 2019 Dec 1;38(6):2569-75.
94. Agahi A, Hamidi GA, Daneshvar R, Hamdieh M, Soheili M, Alinaghipour A, et al. Does severity of Alzheimer's disease contribute to its responsiveness to modifying gut microbiota? A double blind clinical trial. Frontiers in Neurology. 2018 Aug 15;9:662.
95. Kobayashi Y, Kuhara T, Oki M, Xiao JZ. Effects of Bifidobacterium breve A1 on the cognitive function of older adults with memory complaints: a randomised, double-blind, placebo-controlled trial. Beneficial Microbes. 2019 May 28;10(5):511-20.
96. Asaoka D, Xiao J, Takeda T, Yanagisawa N, Yamazaki T, Matsubara Y, et al. Effect of probiotic Bifidobacterium breve in improving cognitive function and preventing brain atrophy in older patients with suspected mild cognitive impairment: results of a 24-week randomized, double-blind, placebo-controlled trial. Journal of Alzheimer's Disease. 2022 Jan 1;88(1):75-95.
97. Xiao J, Katsumata N, Bernier F, Ohno K, Yamauchi Y, Odamaki T, et al. Probiotic Bifidobacterium breve in improving cognitive functions of older adults with suspected mild cognitive impairment: a randomized, double-blind, placebo-controlled trial. Journal of Alzheimer's Disease. 2020 Jan 1;77(1):139-47.
98. Hwang YH, Park S, Paik JW, Chae SW, Kim DH, Jeong DG, et al. Efficacy and safety of Lactobacillus plantarum C29-fermented soybean (DW2009) in individuals with mild cognitive impairment: a 12-week, multi-center, randomized, double-blind, placebo-controlled clinical trial. Nutrients. 2019 Feb 1;11(2):305.
99. Kumar S, Malviya R, Sundram S. Nutritional neurology: Unraveling cellular mechanisms of natural supplements in brain health. Human Nutrition & Metabolism. 2023 Dec 5:200232.
100. Rai SN, Singh P, Steinbusch HW, Vamanu E, Ashraf G, Singh MP. The role of vitamins in neurodegenerative disease: An update. Biomedicines. 2021 Sep 22;9(10):1284.
101. Pham VT, Dold S, Rehman A, Bird JK, Steinert RE. Vitamins, the gut microbiome and gastrointestinal health in humans. Nutrition Research. 2021 Nov 1;95:35-53.