Abstract
Purpose: According to the literature, 80% of patients undergoing cataract surgery suffer from ocular surface disease (OSD). Along with postoperative refractive surprise, the onset of OSD is the main cause of dissatisfaction in cataract surgery patients, accounting for 35% of cases. These patients are characterized by persistent dysregulation of ocular surface para-inflammation, leading to chronic low- grade inflammation with significant consequences on daily activities and work productivity. This study aims to investigate the effects of topical desonide sodium phosphate 0.025% eye drops on the signs and symptoms of OSD in patients undergoing cataract surgery.
Methods: A multi center randomized controlled trial was conducted. Cataract patients without ocular comorbidities were consecutively enrolled and randomized into two groups. Both groups underwent phacoemulsification with standard postoperative therapy (levofloxacin 0.5% eye drops six times a day for 5 days and dexamethasone sodium phosphate 0.15% eye drops four times a day for 1 week and then twice a day for 1 week). Additionally, the study group received lubricating eye drops containing xanthan gum 0.2% and desonide sodium phosphate 0.025% three times a day, while the control group received hyaluronic acid 0.15% three times a day. At patient enrollment (T0), 1 day (T1), 2 weeks (T2), and 1 month after surgery (T3), signs and symptoms of OSD and tear function tests were recorded.
Results: OSDI scores significantly worsened at T1 in both groups (study: 28.4 ± 2.1 to 75.7 ± 5.7; control: 26.3 ± 1.8 to 68.0 ± 6.9; p<0.001) but improved more rapidly in the study group, reaching 12.0 ± 3.0 at T3 vs. 30.9 ± 5.4 in controls (p<0.001). T-BUT improved from 6.0 ± 0.8 s at T1 to 11.9 ± 1.0 s at T3 in the study group, outperforming the control group (4.9 ± 0.9 s to 7.8 ± 0.5 s, p<0.001). Schirmer’s test showed better recovery in the study group (18.2 ± 1.3 mm to 16.3 ± 1.3 mm) compared to controls (16.8 ± 1.1 mm to 6.8 ± 1.3 mm, p<0.001). No adverse events or intraocular pressure changes were observed.
Conclusions: The addition of desonide sodium phosphate 0.025% with xanthan gum 0.2% significantly improved OSD signs and symptoms post-cataract surgery, offering an effective, well-tolerated treatment with a favorable safety profile.
Introduction
Cataract surgery is one of the most frequently performed surgical procedures worldwide, with over 20 million procedures conducted annually [1]. The continual advancements in technology and refinement of surgical techniques have significantly elevated patient expectations regarding postoperative visual outcomes [2]. Despite the high success rates, up to 80% of patients experience ocular surface disease (OSD) after cataract surgery, which remains a prominent factor affecting patient satisfaction [3]. Along with postoperative refractive surprise, the onset of OSD is the main cause of dissatisfaction in cataract surgery patients, accounting for 35% of cases. This elevated percentage of dissatisfaction after cataract surgery, due to OSD, is linked to the phenomenon of 'unhappy 20/20', where patients, despite achieving an excellent refractive outcome (20/20 best corrected visual acuity), remain highly dissatisfied [4,5]. OSD, characterized by tear film instability and corneal epithelial dysfunction, is often accompanied by chronic low-grade inflammation [6]. This inflammation, or "para-inflammation," is an adaptive response to restore tissue homeostasis following stressors such as surgical trauma. If uncontrolled, however, this response may lead to chronic inflammation, exacerbating symptoms such as dry eye, foreign body sensation, and visual disturbances, ultimately impacting the patient's quality of life and work productivity [7]. Managing ocular surface para-inflammation is essential to improve postoperative outcomes and prevent long-term complications. The application of topical corticosteroids has been widely studied for its efficacy in reducing inflammation and improving tear film stability [8]. Desonide sodium phosphate, a low-potency corticosteroid, offers a promising therapeutic approach due to its anti-inflammatory properties and favorable safety profile, minimizing the side effects commonly associated with stronger corticosteroids [9]. Xanthan gum, a mucoadhesive natural polymer, has been used to enhance the stability of the tear film and increase the residence time of active ingredients on the ocular surface [10].
Nowadays, the growing awareness of ocular symptoms, driven by patients’ increasing expectations for not only clear vision but also ocular comfort, underscores a critical gap in current postoperative management strategies. Standard therapy alone appears insufficient to fully control OSD-related symptoms, suggesting the need for adjunctive treatments that specifically target ocular surface para-inflammation and promote tear film stability. Therefore, the aim of this study is to investigate whether the addition of desonide sodium phosphate 0.025%, combined with xanthan gum 0.2%, can improve postoperative OSD outcomes in cataract surgery patients. We hypothesize that this combination therapy will provide superior control of ocular surface inflammation and discomfort compared to standard therapy alone, ultimately enhancing patient satisfaction in the era of increasingly demanding visual expectations.
Methods
A multicenter, randomized controlled trial was conducted in adherence with the tenets of the Declaration of Helsinki, following approval from the Internal Ethics Committee of the Campus Bio-Medico University Hospital Foundation, Rome. Written informed consent was obtained from all participants prior to enrollment. Between January and June 2023, 40 eyes of 40 patients with diagnosis of senile cortico-nuclear cataract were consecutively enrolled. Inclusion criteria included patients aged ≥ 50 years with a diagnosis of senile cortico-nuclear cataract. Exclusion criteria included ocular and systemic comorbidities that could potentially affect postoperative outcomes, such as diabetes mellitus, a prior diagnosis of Dry Eye Disease, corneal dystrophies, or autoimmune disorders. Additional exclusion criteria included the use of medications that influence ocular surface homeostasis, known hypersensitivity to any of the study medications, contact lens wear, and the presence of active ocular infections or inflammation.
Participants were randomized into two groups of 20 patients (study group, n=20 eyes and control group, n=20 eyes) each using a computer-generated randomization sequence.
All patients underwent uneventful phacoemulsification performed by the same experienced surgeon to ensure consistency across procedures. The surgeries were carried out using the Centurion® Vision System (Alcon Laboratories, Inc., Fort Worth, TX, USA). A standard technique was applied, beginning with a 2.2 mm clear corneal incision followed by continuous curvilinear capsulorhexis and hydrodissection. Nucleus fragmentation was performed using the stop-and-chop technique. Throughout the procedure, ProviscÒ (Alcon, Fort Worth, TX, USA) was used as the ophthalmic viscoelastic device (OVD). After successful nucleus removal, posterior chamber intraocular lenses were implanted. The same surgical protocol was strictly followed for all patients to reduce procedural variability. Both groups received a standard post-operative therapy consisting of levofloxacin 0.5% eye drops administered 6 times daily for 5 days to provide broad-spectrum antibacterial coverage, and dexamethasone sodium phosphate 0.15% eye drops administered 4 times daily for one week, then reduced to twice daily for the following week.
The study group received standard postoperative therapy in addition with lubricating eye drops containing xanthan gum 0.2% and desonide sodium phosphate 0.025%, administered three times daily. The control group received standard postoperative therapy plus eye drops containing hyaluronic acid 0.15%, also administered three times a day.
Patients were evaluated at four specific time points: preoperatively (T0), one day postoperatively (T1), two weeks postoperatively (T2), and one month postoperatively (T3), see Figure 1. Assessments included the Ocular Surface Disease Index (OSDI) questionnaire to evaluate subjective symptoms of OSD, such as dryness, foreign body sensation, and visual disturbances. Tear Breakup Time (T-BUT) was measured to assess tear film stability, and Schirmer’s test without anesthesia was conducted to evaluate basal tear production. Intraocular pressure (IOP) was measured using Goldmann applanation tonometry to monitor for any corticosteroid-induced elevations.
Figure 1. Schematic representation of the timeline illustrating the various time points for clinical visits (T0-T3) and the surgical procedure.
Data were analyzed using SPSS version 25.0 (IBM Corp., Armonk, NY, USA). Continuous variables were expressed as mean ± standard deviation (SD). Intra-group comparisons were performed using the paired Student’s t-test, while inter-group comparisons employed the Mann-Whitney U test due to the non-parametric nature of the data. A p-value of less than 0.05 was considered statistically significant.
Results
Both groups were comparable in terms of age and gender distribution, with no significant differences observed in baseline ocular parameters, including the Ocular Surface Disease Index (OSDI), Tear Breakup Time (T-BUT), Schirmer’s test, and intraocular pressure (IOP) (see Table 1, p>0.05). The mean age was 68 ± 7 years in the study group and 70 ± 6 years in the control group. Detailed values for means, standard deviations, and 95% confidence intervals (CIs) for all parameters are reported in Table 1.
|
|
Visit |
Study group (mean ± SD [95% CI]) |
Control group (mean ± SD [95% CI]) |
inter-group p-value |
Study group intra-group p-value |
control group intra-group p-value |
|
OSDI |
T0 |
29.27 ± 5.18 [26.85-31.69] |
27.04 ± 4.64 [28.87-29.21] |
0.16 |
- |
- |
|
T1 |
75.76 ± 5.68 [73.10-78.42] |
68.68 ± 6.93 [65.44-71.92] |
0.001 |
<0.001 (vs T0) |
<0.001 (vs T0) |
|
|
T2 |
21.44 ± 5.13 [19.19-2.69] |
34.09 ± 5.79 [33.54-36.64] |
< 0.001 |
<0.001 (vs T1) |
<0.001 (vs T1) |
|
|
T3 |
11.57 ± 3.37 [9.99-13.15] |
26.89 ± 5.41 [24.36-29.42] |
< 0.001 |
<0.001 (vs T2) |
<0.001 (vs T2) |
|
|
TBUT |
T0 |
10.85 ± 1.06 [10.35-11.35] |
10.67 ± 0.94 [10.23-11.11] |
0.568 |
- |
- |
|
T1 |
6.02 ± 0.82 [5.63-6.41] |
4.92 ± 0.92 [4.48-5.36] |
0.0003 |
<0.001 (vs T0) |
<0.001 (vs T0) |
|
|
T2 |
7.85 ± 0.97 [7.43-8.27] |
5.78 ± 0.97 [5.34-6.22] |
<0.001 |
<0.001 (vs T1) |
0.008 (vs T1) |
|
|
T3 |
11.85 ± 1.04 [11.36-12.34] |
7.77 ± 0.48 [7.53-8.01] |
<0.001 |
<0.001 (vs T2) |
<0.001 (vs T2) |
|
|
Schirmer |
T0 |
15.13 ± 1.26 [14.56-15.70] |
15.24 ± 0.86 [14.84-15.64] |
0.758 |
- |
- |
|
T1 |
18.20 ± 1.31 [17.61-18.69] |
16.84 ± 1.11 [16.34-17.34] |
0.001 |
<0.001 (vs T0) |
<0.001 (vs T0) |
|
|
T2 |
17.01 ± 2.15 [15.99-18.03] |
9.16 ± 1.36 [8.55-9.77] |
<0.001 |
0.042 (vs T1) |
<0.001 (vs T1) |
|
|
T3 |
16.30 ± 1.34 [15.69-16.91] |
6.79 ± 1.36 [6.18-7.40] |
<0.001 |
0.283 (vs T2) |
<0.001 (vs T2) |
|
|
IOP |
T0 |
14.91 ± 0.55 [14.66-15.16] |
15.86 ± 0.41 [15.67-16.05] |
0.756 |
- |
- |
|
T1 |
15.83 ± 0.42 [15.63-16.03] |
15.09 ± 0.41 [14.89-15.29] |
0.985 |
0.065 (vs T0) |
0.068 (vs T0) |
|
|
T2 |
15.92 ± 0.50 [15.66-16.18] |
13.93 ± 0.59 [13.63-14.23] |
0.525 |
0.074 (vs T1) |
0.071 (vs T1) |
|
|
T3 |
13.90 ± 0.41 [13.71-14.09] |
15.41 ± 0.51 [15.16-15.66] |
0.554 |
0.069 (vs T2) |
0.069 (vs T2) |
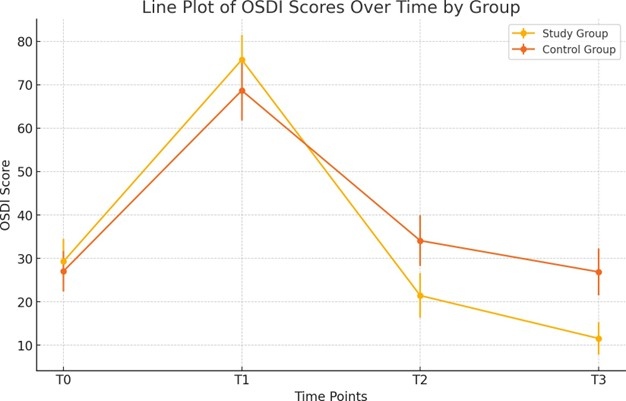
At the first postoperative evaluation (T1), both groups exhibited a significant increase in OSDI scores compared to baseline (T0), reflecting a temporary worsening of ocular surface symptoms immediately after surgery. Specifically, OSDI scores increased from 29.27 ± 5.18 to 75.76 ± 5.68 in the study group (p<0.001) and from 27.04 ± 4.64 to 68.68 ± 6.93 in the control group (p<0.001) (Figure 2). From T1 to T3, OSDI scores decreased significantly in both groups, with the study group showing a more pronounced improvement, decreasing to 11.57 ± 3.37 (p<0.001), compared to 26.89 ± 5.41 in the control group (p<0.001). The reduction in OSDI scores was significantly greater in the study group at both T2 and T3 (p<0.001).
Figure 2. Trend of OSDI scores at various time points for both the study and control groups.
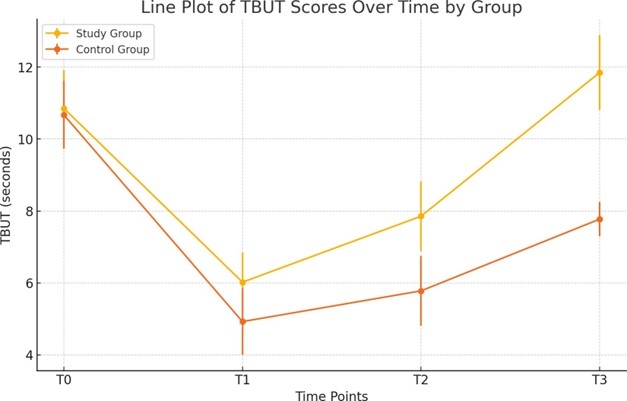
Similarly, T-BUT showed a significant initial decrease in both groups at T1, dropping from 10.85 ± 1.06 to 6.02 ± 0.82 in the study group (p<0.001) and from 10.67 ± 0.94 to 4.92 ± 0.92 in the control group (p<0.001) (Figure 3). However, from T1 to T3, T-BUT improved significantly in both groups, with the study group increasing to 11.85 ± 1.04 (p<0.001) and the control group to 7.77 ± 0.48 (p<0.001). The difference in improvement between the two groups was statistically significant at both T2 and T3 (p<0.001).
Figure 3. Variations of TBUT values across different time points for the study and control groups.
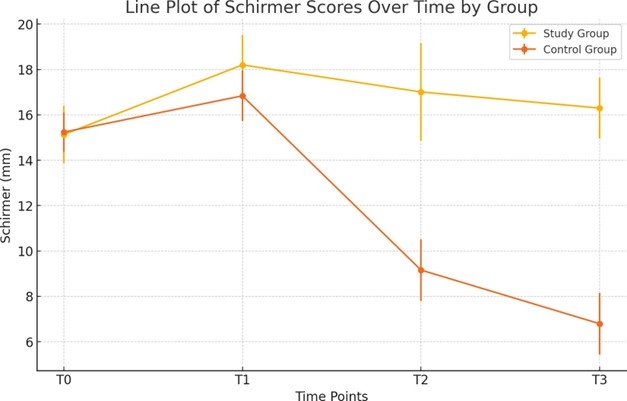
Schirmer’s test values initially increased at T1 in both groups, from 15.13 ± 1.26 to 18.20 ± 1.31 in the study group (p<0.001) and from 15.24 ± 0.86 to 16.84 ± 1.11 in the control group (p<0.001) (Figure 4). From T1 to T3, the study group showed a slight decrease to 16.30 ± 1.34 (p=0.283), while the control group experienced a significant reduction to 6.79 ± 1.36 (p <0.001). The between-group comparison showed significantly better results for the study group at both T2 and T3 (p<0.001).
Figure 4. Changes in Schirmer test measurements at various time points for both the study and control groups.
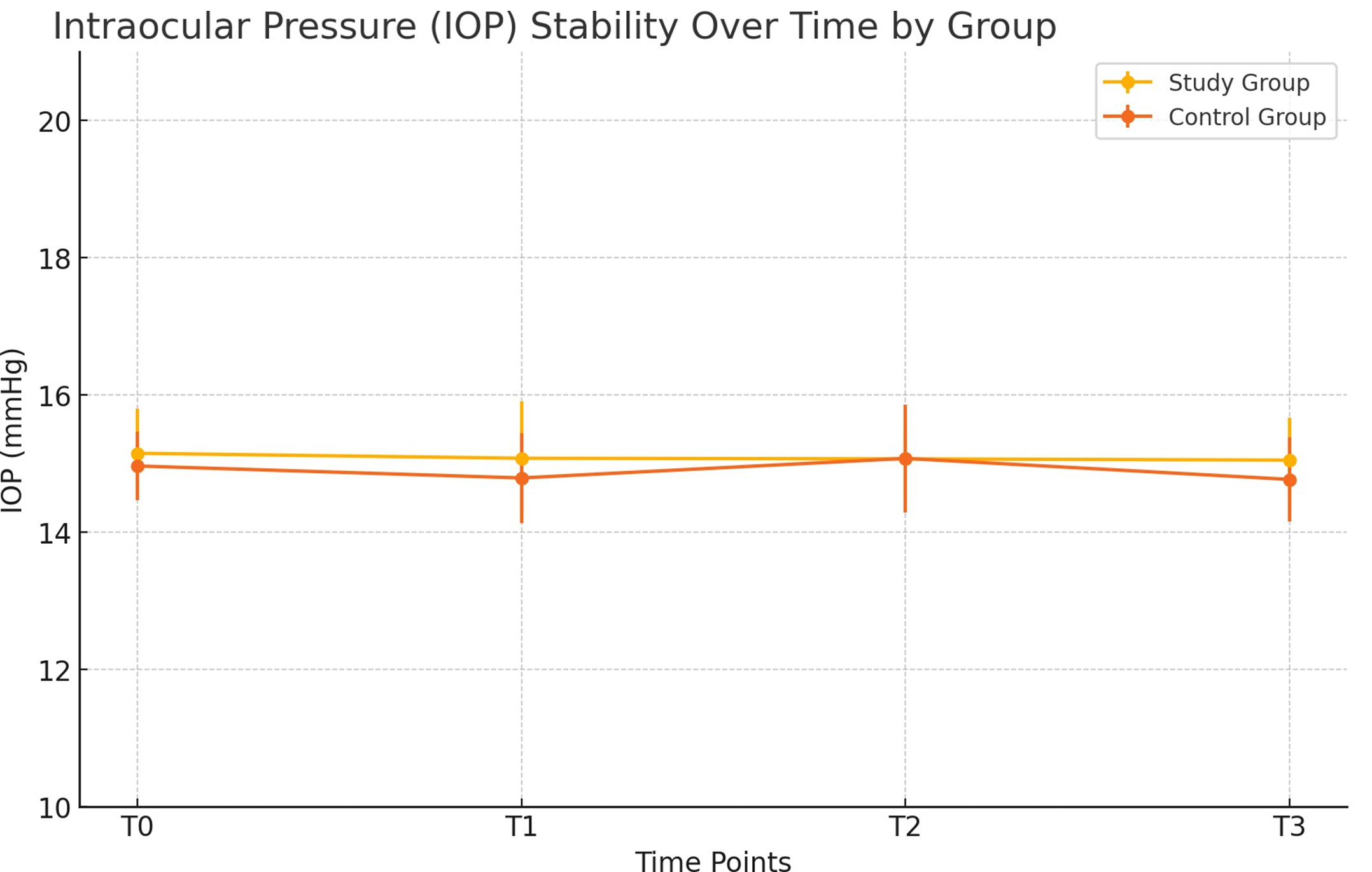
Throughout the study period, no significant changes in IOP were observed in either group (p>0.05), with values ranging from 14.91 ± 0.55 at baseline to 13.90 ± 0.41 at T3 in the study group, and from 15.86 ± 0.41 to 15.41 ± 0.51 in the control group (Figure 5). These results indicate that the use of low-potency corticosteroid desonide sodium phosphate did not induce corticosteroid-related IOP elevations. Additionally, no adverse events or complications related to the study medications were reported in either group, confirming the safety and tolerability of the treatments administered.
Figure 5. Variation in IOP levels over time for the study and control groups.
Discussion
The findings of this study reveal that the addition of desonide sodium phosphate 0.025% eye drops, combined with xanthan gum 0.2%, to standard postoperative therapy significantly enhances the recovery of ocular surface health and tear film stability in patients undergoing cataract surgery. The study group exhibited faster and more pronounced improvements in ocular surface disease (OSD) signs and symptoms compared to the control group, suggesting a beneficial role for this combination therapy in managing post-surgical OSD. These results align with existing evidence on the importance of addressing dry eye symptoms following cataract surgery to enhance patient outcomes [11,12]. Desonide, a low-potency corticosteroid, is widely recognized for its anti-inflammatory properties and favorable safety profile. Unlike other corticosteroids, which may increase intraocular pressure (IOP) and present other adverse effects, desonide exerts a milder yet effective anti-inflammatory action [13]. It works by modulating the inflammatory response at the ocular surface, thereby helping to prevent tissue trauma and chronic inflammation that can result from surgical trauma [14]. Due to its low potency, desonide minimizes the risk of side effects, including steroid-induced IOP elevation, which was a critical consideration in this study’s design to ensure patient safety while achieving effective inflammation control. Xanthan gum, on the other hand, is a polysaccharide with excellent mucoadhesive and viscosity-enhancing properties. It is commonly used in ophthalmic formulations to increase the contact time of eye drops with the ocular surface, thereby improving drug bioavailability. This prolonged retention allows for a more sustained therapeutic effect and improved patient comfort, particularly beneficial in the postoperative period when the ocular surface is highly susceptible to dryness and inflammation [7]. The combination of desonide with xanthan gum may enhance therapeutic outcomes by both reducing inflammation and maintaining tear film stability, ultimately supporting faster recovery of the ocular surface. In the immediate postoperative period (T1), both groups experienced a worsening in OSDI scores, as expected due to the natural inflammatory response and tear film instability following surgery. However, in subsequent assessments, distinct differences emerged. The OSDI scores in the study group improved significantly, demonstrating a more rapid recovery of comfort and symptom relief, whereas the control group showed only a modest improvement. This suggests that the adjunctive use of desonide and xanthan gum was effective in addressing inflammation and discomfort associated with postoperative OSD. The Schirmer’s test results further supported these observations. In the control group, tear production diminished progressively after T1, reflecting the onset of postoperative dry eye syndrome, a common complication in cataract surgery patients. In contrast, the study group showed an initial increase in Schirmer values postoperatively, stabilizing at levels comparable to or even better than baseline, indicating effective management of the ocular surface. Tear Breakup Time (TBUT) results also highlight the efficacy of the adjunctive treatment in the study group. While TBUT initially decreased in both groups post-surgery due to tear film instability, the study group experienced a more significant recovery in TBUT over time, ultimately exceeding baseline values by T3. This suggests that the anti-inflammatory and mucoadhesive effects of desonide and xanthan gum may contribute to enhanced tear film stability, a critical factor in reducing dry eye symptoms and improving visual comfort.
Understanding ocular surface para-inflammation is crucial for interpreting our results. This mild, adaptive inflammatory response arises from minor stressors, such as surgical trauma, and helps restore tissue balance without triggering severe inflammation [15,16]. However, if left unchecked, para-inflammation can shift into a chronic state, worsening tear film instability, epithelial damage, and OSD symptoms. The timely use of desonide in our study seems to have effectively regulated this response, supporting tissue recovery and preventing the escalation into chronic inflammation, which is key to avoiding persistent dry eye symptoms and visual impairment. This regulation is particularly relevant for addressing the “unhappy 20/20” phenomenon, where patients achieve excellent visual acuity post-surgery but continue to experience discomfort and visual disturbances due to unresolved OSD. This phenomenon is well-documented in studies addressing the impact of dry eye disease on patient satisfaction [17]. These symptoms often stem from subtle inflammation that destabilizes the tear film, leading to fluctuating vision and dry eye discomfort. The improvements in OSDI and TBUT scores in the study group demonstrate the potential of combining low-potency corticosteroids with mucoadhesive agents to alleviate these lingering issues by reducing inflammation and stabilizing the tear film, this combined therapy not only improves clinical outcomes but also enhances patient comfort and satisfaction during recovery. Throughout the study, no significant IOP elevations were observed in the study group, underscoring the safety of using desonide as a low-potency corticosteroid. This is particularly reassuring for clinicians concerned about the potential for corticosteroid-induced ocular hypertension, as this study demonstrates that desonide can be effectively used without impacting IOP or increasing the risk of glaucoma in this patient population.
These findings have important implications for clinical practice. Integrating desonide and xanthan gum into standard postoperative regimens could reduce the incidence and severity of postoperative gum into standard postoperative regimens could reduce the incidence and severity of postoperative OSD, leading to improved patient comfort and satisfaction. This is particularly valuable in modern cataract surgery, where patient expectations are high, and even minor symptoms can impact perceived surgical success. Moreover, the favorable safety profile observed suggests that this approach can be safely applied without increasing the risk of corticosteroid-related complications. While this study focused primarily on objective clinical measures, future research should incorporate patient-reported outcomes and quality-of-life assessments to provide a more comprehensive evaluation of treatment efficacy. Understanding how these therapies affect daily functioning, visual comfort, and overall well-being will be essential for optimizing postoperative care strategies. Additionally, larger, long-term studies are needed to confirm the durability of these benefits and explore potential applications in other ocular surface conditions.
The limitations of this study include the relatively small sample size and short follow-up duration. Future research with larger, long-term studies will be valuable in confirming these findings and exploring the sustainability of these therapeutic benefits.
Conclusions
Postoperative administration three times a day for 1 month of desonide sodium phosphate 0.025% eye drops combined with xanthan gum 0.2% significantly improved the signs and symptoms of OSD in patients undergoing cataract surgery. This treatment is effective, well-tolerated, and has a favourable safety profile, enhancing patient satisfaction and postoperative outcomes.
AU Contribution Declaration
Design of the study (ADZ).
Collection, management, analysis and interpretation of the data (all).
Preparation (all), reviewing (all), approval (all) of the manuscript.
References
2. Dawn AG, Lee PP. Patient expectations for medical and surgical care: a review of the literature and applications to ophthalmology. Surv Ophthalmol. 2004 Sep-Oct;49(5):513-24.
3. Gupta PK, Drinkwater OJ, VanDusen KW, Brissette AR, Starr CE. Prevalence of ocular surface dysfunction in patients presenting for cataract surgery evaluation. J Cataract Refract Surg. 2018 Sep;44(9):1090-6.
4. Holtmann C, Geerling G. Cataract Surgery in Eyes with Ocular Surface Problems and Severe Dry Eye. In: Alió JL, Dick HB, Osher RH, Eds. Cataract Surgery: Advanced Techniques for Complex and Complicated Cases. Cham: Springer International Publishing; 2022 Jul 28. p. 93-104
5. Porela-Tiihonen S, Kokki H, Kaarniranta K, Kokki M. Recovery after cataract surgery. Acta Ophthalmol. 2016 Apr;94 Suppl 2:1-34.
6. Priyadarshini K, Sharma N, Kaur M, Titiyal JS. Cataract surgery in ocular surface disease. Indian J Ophthalmol. 2023 Apr;71(4):1167-75.
7. Aragona P, Giannaccare G, Dammino E, D'Esposito F, Genovese P, Postorino EI, et al. Observational Clinical Investigation Evaluating an Ophthalmic Solution Containing Xanthan Gum and Low Concentration Desonide Phosphate in Dry Eye Disease Treatment. Ophthalmol Ther. 2024 Oct;13(10):2559-73.
8. Khare A, Grove K, Pawar P, Singh I. Mucoadhesive polymers for enhancing retention in ocular drug delivery: A critical review. Review of Adhesion and Adhesives. 2014;2(4):467-502.
9. Kahanek N, Gelbard C, Hebert A. Desonide: a review of formulations, efficacy and safety. Expert Opin Investig Drugs. 2008 Jul;17(7):1097-104.
10. Silva B, São Braz B, Delgado E, Gonçalves L. Colloidal nanosystems with mucoadhesive properties designed for ocular topical delivery. Int J Pharm. 2021 Sep 5;606:120873.
11. Di Zazzo A, Spelta S, Micera A, De Gregorio C, Affatato M, Esposito G, et al. Prophylactic Therapy for Long-Term Ocular Discomfort After Cataract Surgery. Cornea. 2024 May 17.
12. Giannaccare G, Barabino S, Di Zazzo A, Villani E. Preventing and Managing Iatrogenic Dry Eye Disease during the Entire Surgical Pathway: A Study Focusing on Patients Undergoing Cataract Surgery. J Clin Med. 2024 Jan 27;13(3):748.
13. Musleh MG, Bokre D, Dahlmann-Noor AH. Risk of intraocular pressure elevation after topical steroids in children and adults: A systematic review. Eur J Ophthalmol. 2020 Sep;30(5):856-66.
14. Cutrupi F, Di Zazzo A, Bonini S. Representation of corneal re-innervation following refractive surgery treatments. J Fr Ophtalmol. 2024 Mar;47(3):104055.
15. Di Zazzo A, Coassin M, Surico PL, Bonini S. Age-related ocular surface failure: A narrative review. Exp Eye Res. 2022 Jun;219:109035.
16. Di Zazzo A, Micera A, Coassin M, Varacalli G, Foulsham W, De Piano M, Bonini S. InflammAging at Ocular Surface: Clinical and Biomolecular Analyses in Healthy Volunteers. Invest Ophthalmol Vis Sci. 2019 Apr 1;60(5):1769-75.
17. Cutrupi F, De Luca A, Di Zazzo A, Micera A, Coassin M, Bonini S. Real Life Impact of Dry Eye Disease. Semin Ophthalmol. 2023 Nov;38(8):690-702.