Abstract
Objective: Myeloproliferative neoplasms (MPN) are occasionally associated with autoimmune manifestations. The prevalence of positive direct antiglobulin test (DAT) in patients with JAK2V617F mutation is not yet known.
Methods: We conducted a cohort study of all consecutive patients with JAK2V617F mutation who underwent DAT at presentation from 2010 to 2022. We also looked for the prevalence of autoimmune diseases (AIDs) and relation to thrombosis.
Results: Fifty nine patients formed the cohort, 22 females and 37 males, with a mean age of 65 years. The median follow-up time was 90 months. A positive-DAT was found in 6 to 7% of essential thrombocythemia (ET) and polycythemia vera (PV) patients, 60% of primary myelofibrosis (PMF) patients and 50% of myelodysplasia/myeloproliferation (MDS/MPN) patients with or without anemia. Autoimmune manifestations were present in 13% of patients with PV and 50% of those with MDS/MPN features. Overall, 25% of patients had autoimmune phenomena including the 15% with positive-DAT.
Conclusion: In patients with JAK2V617F mutation, those with PMF and MDS/MPN subtypes are most likely to display a positive-DAT results and autoimmune manifestations (≥ 50%). Patients with PV and ET are less likely to exhibit autoimmune manifestations (≤ 10%). No correlation with the occurrence of thrombosis was seen.
Keywords
Myeloproliferative neoplasm, JAK2V617F, Direct antiglobulin test, Autoimmune diseases
Introduction
Autoimmune manifestations have been described in myeloid neoplasms. Myelodysplastic syndrome (MDS) has been epidemiologically associated with AIDs in up to 20% of cases and chronic myelomonocytic leukemia (CMML) in up to 30% of cases. MPN has been occasionally complicated by AID or autoimmune cytopenia [1,2]. Various cases of rheumatoid arthritis, dermatomyositis, periarteritis nodosa, multiple sclerosis, inflammatory bowel disease, and primary biliary cirrhosis have been reported in MPN [3,4]. Autoimmune hemolytic anemia and pure red blood cell aplasia are occasionally observed. Overlapping conditions such as chemotherapy, bone marrow infiltration, and transfusions may be confounding factors challenging the diagnosis of autoimmune cytopenia in MPN. The hematologic malignancies’ therapies such as interferon, fludarabin, tyrosine kinase inhibitors or immunotherapy have been implicated in the development of autoimmune manifestations [5-7].
The prognostic significance of the coexistence of AID in MPN patients is still debated. Although, reports have linked the presence of systemic vasculitis in MDS/MPN patients to a shorter survival [8,9].
JAK2 is a tyrosine kinase that promotes cytokine production in immune, hematopoietic cells. The JAK2V617F is the most frequently detected mutation in Philadelphia-negative myeloproliferative neoplasms. It promotes a pro-inflammatory state that is associated with a higher risk of immune mediated diseases and thromboembolic complications [10-12].
In order to look for autoimmune comorbidity and to quantify the prevalence of positive DAT in MPN patients at presentation we conducted a cohort study of all consecutive patients with JAK2V617F mutation.
Materials and Methods
All consecutive patients with MPN who tested positive for JAK2V617F mutation and who underwent the DAT at presentation from 2010 to 2022 were the subject of our longitudinal cohort study. For the purposes of this analysis, this cohort was divided into 6 groups according to the type and degree of myelofibrosis on bone marrow biopsy and the cell morphology on bone marrow aspiration and peripheral blood smear. The clinical and hematological data enabled a clear-cut distinction between ET, PV, PMF, secondary myelofibrosis (ET/MF and PV/MF) and Hybrid states of MDS/MPN. The hematological diagnoses were confirmed according to the 2016 WHO classification and diagnostic criteria for MPN. All patients have had a DAT performed at diagnosis upon physician request even in the absence of hemolysis. Demographic data, detailed history, and inflammation markers including LDH, CRP, rheumatoid factor, and indirect bilirubin levels were collected at diagnosis. Specific autoimmune markers were selectively tested according to the personal history and clinical data for each patient. Also, the follow-up clinical and biological data were regularly collected and the overall survival was evaluated for the different groups. We also search for a possible correlation between the occurrence of thrombosis and the presence of autoimmune manifestation. The JAK2V617F mutation was detected using highly specific real time PCR technique and the DAT was performed with a gel technique using BIO-RAD ID-Card “DC-Screening I” consisting of five different mono-specific AHG reagents; anti-IgG, antiIgA, anti-IgM, anti-C3c (all rabbit), and anti-C3d (Monoclonal cell line C139-9) suspended in gel, and the negative control was used. The t-test was used whenever required to compare the means of different small groups.
Results
The cohort analyzed consists of 59 patients, aged between 18 and 93 years with a median age of 65 years. There were 22 females and 37 males. The median follow-up time was 90 months (12 to 130 months). All demographic, clinical and hematological data are summarized in Table 1. The patients were divided into 6 groups: 22 ET, 28 PV with or without secondary myelofibrosis, 5 PMF and 4 MDS/MPN.
|
Table 1. Patient characteristics, clinical presentation, direct antiglobulin test positivity, presence of autoimmune manifestations, history of thrombosis and progression. |
|||||||
|
|
ET |
PV |
PMF |
ET/MF |
PV/MF |
MDS/MPN |
Total (%) |
|
Total (%)
|
16 (26.8%) |
26 (44%) |
5 (8.4%) |
6 (10.1%) |
2 (3.4%) |
4 (6.7%) |
59 |
|
Sex F/M
|
5/11 |
10/16 |
4/1 |
1/5 |
1/1 |
1/3 |
22/37 |
|
Mean age (Years)
|
53.3 [18-93] |
53.6 [20-84] |
68.4 [57-78] |
72 [67-77] |
69 [59-79] |
72.5 [67-78] |
65 [18-93] |
|
Positive-DAT |
1 (6.2%) |
2 (7.4%) |
3 (60%) |
1 (16.5%) |
- |
2 (50%) |
9 (15.2%) |
|
Rheumatoid Arthritis |
- |
2 (7.4%) |
- |
- |
- |
2 (50%) |
4 (6.6%) |
|
Fibromyalgia |
- |
1 (3.7%) |
- |
- |
- |
- |
1 (1.7%) |
|
Dermatomyositis |
- |
1 (3.7%) |
- |
- |
- |
- |
1 (1.7%) |
|
Development of CLL |
- |
1 (3.7%) |
- |
- |
- |
- |
1 (1.7%) |
|
Monoclonal gammopathy |
1 (6.2%) |
|
- |
- |
- |
- |
1 (1.7%) |
|
Arterial thrombosis |
6 (37.1%) |
7 (36.8%) |
1 (20%) |
3 (50%) |
2 (100%) |
1 (20%) |
20 (33.9%) |
|
Deep vein thrombosis |
4 (25%) |
8 (26.9%) |
1 (20%) |
- |
1 (50%) |
- |
14 (23.6%) |
|
Splanchnic vein Thrombosis |
1 (6.2%) |
2 (7.4%) |
1 (20%) |
- |
1 (50%) |
- |
5 (8.5%) |
|
AML transformation |
- |
- |
- |
- |
- |
1 (25%) |
1 (1,7%) |
|
Splenomegaly |
9 (56.1%) |
16 (61.4%) |
5 (100%) |
6 (100%) |
2 (100%) |
4 (100%) |
42 (71%) |
|
WBC (G/l)
|
14.2 [12-20] |
18.2 [15-20] |
8.5 [1.2-12] |
11.7 [9-16] |
18.2 [15-20] |
10.3 [1-17] |
|
|
Platelets (G/l) |
950 [500-1800] |
550 [80-950] |
167 [20-300] |
790 [600-950] |
550 [400-1200] |
77 [30-230] |
|
|
Hemoglobin level (g/dl) |
11.4 [7.5-20] |
16,3 [9.8-22] |
9.8 [2-12] |
14 [9.5-16] |
14 [12-16] |
6.7 [3-19] |
|
Nine patients (15.2%) were found to have a positive-DAT with warm autoantibodies mainly in patients with PMF and MDS/MPN. Autoimmune phenomena were found in 15 patients (25.4%).
All positive-DAT patients had splenomegaly at presentation. There was no statistically significant correlation between positive-DAT status and the younger age, the gender, or the hemoglobin serum level, although 2 of the positive-DAT patients had severe anemia. There was no correlation between the presence of venous or arterial thrombosis and DAT positivity (Table 2).
|
Table 2. Positive-DAT status associated autoimmune diseases (AID) and pattern of thrombosis in the cohort patients with JAK2V617F mutation. |
|||||||
|
|
ET |
PV |
PMF |
ET/MF |
PV/MF |
MDS/MPN |
Total (%) |
|
Arterial Thrombosis |
|
|
|
|
|
|
|
|
CVA |
3 (18%) |
5 (19%) |
1 (20%) |
1(15%) |
1 (50%) |
- |
11 (18.5%) |
|
MI |
2 (12.5%) |
2 (7.7%) |
- |
2 (33.3%) |
- |
- |
14 (24.5%) |
|
Mesenteric Infarction |
1 (6%) |
1 (3.5%) |
- |
- |
- |
- |
2 (3.5%) |
|
Venous Thrombosis |
|
|
|
|
|
|
|
|
DVT |
4 (25%) |
7 (27%) |
1 (20%) |
- |
- |
1 (25%) |
13 (22.5%)) |
|
PE |
- |
1 (3.8%) |
- |
- |
- |
- |
1 (1.5%) |
|
SVT |
2 (12.5%) |
2 (7.5%) |
1 (20%) |
- |
1 (50%) |
- |
6 (10%) |
|
CVT |
- |
1 (3.8%) |
- |
- |
- |
- |
1 (1.5%) |
|
|
|
|
|
|
|
|
|
|
Positive-DAT / No thrombosis |
- |
- |
2 (40%) |
- |
- |
1 (25%) |
3 (5%) |
|
Positive-DAT + Arterial Thrombosis |
1 (6%) |
1 (3.8%) |
- |
- |
- |
- |
2 (3.5%) |
|
Positive-DAT + Venous Thrombosis |
- |
3 (11.5%) |
2 (40%) |
- |
- |
1 (25%) |
6 (10%) |
|
AID / No thrombosis |
- |
3 (11.5%) |
- |
- |
- |
2 (50%) |
5 (9.5%) |
|
AID + Arterial or venous thrombosis |
- |
2 (7.5%) |
- |
- |
- |
- |
2 (3.5%) |
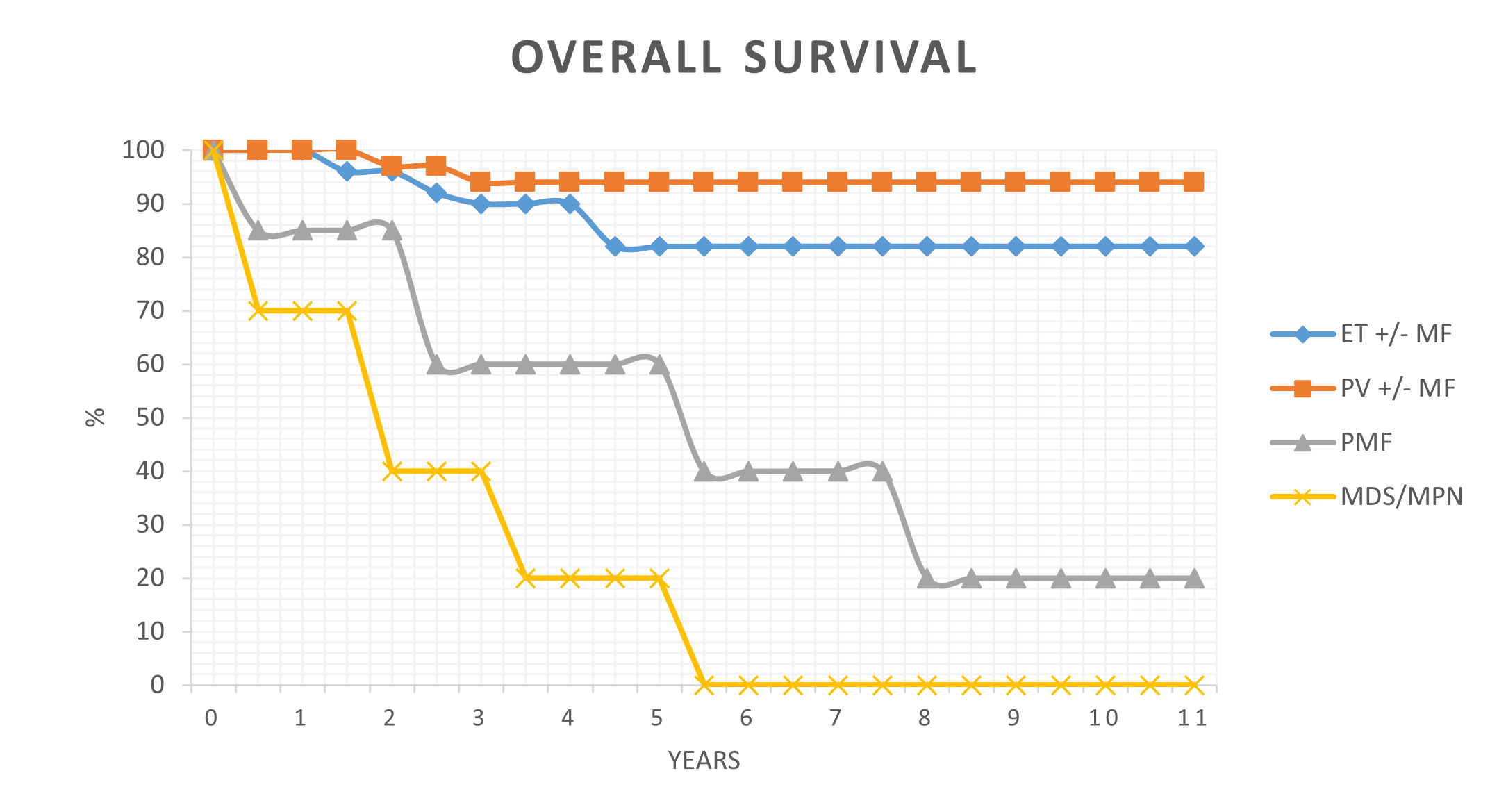
Among the 4 cases of MDS/MPN, there was one CMML, one atypical chronic myeloid leukemia (aCML) and 2 unclassifiable. All of them had autoimmune manifestations; 50% positive-DAT and 50% rheumatoid arthritis. The CMML patient developed multiple arterial aneurysms in the left axilla and the thorax causing his death. The aCML patient developed acute myeloid leukemia (AML) transformation. These patients had a shorter overall survival (Figure 1). Among the 5 patients with PMF, 3 patients had positive-DAT (60%), but no patient developed clinical AID. Among the 26 patients with PV, 6 patients (22.2%) developed autoimmune manifestations such as positive-DAT (7.4%), rheumatoid arthritis (7.4%), fibromyalgia (3.7%), and dermatomyositis (3.7%). One PV patient developed chronic lymphocytic leukemia (CLL). Two ET patients out of 16 free of secondary melofibrosis were DAT-positive (6.2%) and one ET patient developed monoclonal gammopathy. The occurrence of positive-DAT, autoimmune manifestation, and thrombosis is displayed in Tables 1 and 2. The strength of DAT reaction was correlated with the presence of overt hemolysis for either IgG or complement.
Figure 1. Overall survival of patients with essential thrombocythemia (ET +/-MF), polycythemia vera (PV +/- MF), primary myelofibrosis (PMF) and myelodysplastic syndrome/myloproliferative neoplasm hybrid state (MDS/MPN).
There was one young woman suffering from dermatomyositis and treated occasionally with corticosteroids, for many years before developing high platelet count and high hemoglobin level revealing an MPN.
Discussion
Autoimmune conditions may cause inflammatory driven tumorigenesis that could lead to the development of MPN. AID therapies using corticosteroids, non-steroidal anti-inflammatory agents or immunotherapy may also play a role in developing MPN [1-7]. Epidemiological studies conducted by SEERs and Swedish cancer registry described the AIDs as a risk factor for developing MPN [13- 15] while other studies described AID as coincidental comorbidities in MPN [16,17]. JAK2V617F hematopoietic clones may develop many years prior to MPN diagnosis with highly variable levels of clonal expansion [18]. In our series, all patients have been tested for JAK2 mutation because of suspicious clinical and/or biological presentation and therefore they all had overt MPN. Those who presented AID were diagnosed with this condition prior to MPN diagnosis and JAK2V617F mutation testing. Twenty five percent of patients presented autoimmune phenomena including 15% positive-DAT with or without AIHA.
The JAK2 mutation is a signal transmitter downstream of major cytokine receptors [19-21] which promote the activation of “signal transducers and activators of transcription” (STAT) that translocate from the membrane to the nucleus in order to regulate the transcription of target genes. The “mitogen-activated protein kinases” (MAPK) as well as the “protein kinase b pathway” (PI3K/AKT) execute the downstream signaling of JAK and promote proliferation, differentiation, and cytokine production in immune and hematopoietic stem cells [22,23]. Additional molecular alterations such as TET2, ASXL-1, SETBP1 and IDH1/2 have been found to be involved in MPN pathogenesis, initiation of leukemic transformation or inflammation and immune response [24,25].
Genome-wide association studies (GWAS) for disease exploration have identified more than 200 single nucleotide polymorphisms (SNPs) and somatic mutation of JAK-STAT pathway genes that are involved in the pathogenesis of autoimmune diseases and hematological malignancies [26,27].The inhibition of JAK-STAT pathway could reduce the symptoms and the complications in MPN patients as well as in rheumatologic diseases [28-31]. JAK2V617F mutation arises from hematopoietic stem cell clones and is not strictly committed to myeloid cells. Experimental models suggest that it influences the lymphocyte functions [32,33]. In this respect, AID and MPN may occur as a result of JAK2 mutation regardless of the timing.
Sixty percent of patients with PMF in our series (3/5) and 50% of those with MDS/MPN (2/4) had positive-DAT results. Distinguishing between PMF and autoimmune myelofibrosis has sometimes been challenging. No difference in bone marrow morphology is seen and the autoantibodies are possibly present in PMF. In a nation-wide study of 30 patients with primary autoimmune myelofibrosis, not presenting any other condition that may lead to myelofibrosis. Mertz et al. [34] found that 40% of patients developed AID mostly systemic lupus erythematous (SLE) and 50% of them had Sub-Saharan African or North African origin. In our series, all patients with bone marrow failure and displaying autoantibodies have JAK2V617F mutation and more than 50% of them had associated autoimmune manifestation.
In a Swedish population-based study [14], patients with existing autoimmune disease had increased risk of developing MPN. On the other hand, notable studies [2,24] reported up to 45% positive-DAT in patients with PMF and 15% detectable anti-platelets antibodies although the positivity of DAT and the presence of antiplatelet antibodies do not correlate with the severity of the anemia or the thrombocytopenia.
Whether or not autoimmunity causes thrombosis in patients with JAK2V617F mutation remains to be proven. We found no correlation between the occurrence of thrombosis and the presence of AID. A positive-DAT was not an indicator of a thromboembolic event. Cacciola et al. [35] studied the anti-endothelial cell antibodies (AECAs), the endothelial leukocyte adhesion molecule 1 (ELAM-1), the intercellular adhesion molecule 1 (ICAM-1) and the von Willebrand factor antigen (VWF:Ag) in 60 patients and found them elevated in patients with thrombosis compared to those without thrombosis. In addition to the thromboembolic risk of MPN, autoimmune hemolytic anemia has been associated with an increased risk of thrombosis [36,37].
A positive DAT as well as other AIDs has long been associated with myelofibrosis. In the 1970s, Boivin et al. [38] in studying 26 cases with MF found 8 positive Latex reaction cases and 6 positive-DAT. Rondeau et al. [39] in studying 67 patients with agnogenic myeloid metaplasia known as MPN with PMF found that 19% of patients had positive-DAT, 21.7% had positive-RA factor and 10,3% had anti smooth muscle antibodies and Hasselbalch et al, [40] suggested that immune hemolysis could contribute to the development of anemia in 15% of patients with PMF.
The autoimmune phenomena seen in some patients with MF may be explained by the attenuation of Treg function by SIL2Rα and induction of CD8+ T-cell proliferation, especially in JAK2V617F patients but not CALR mutation patients [41-43].
Galimberti et al. [44] found that 8% of patients with MPN had an autoimmune manifestation, typically young females with low hemoglobin level and splenomegaly. However, they found no correlation between the AID type and the MPN histotype while other studies did [45].
In our series we did not systematically look for the presence of antiphospholipid syndrome (APS) or paroxysmal nocturnal hemoglobinuria (PNH) that could have coexisted in a subclinical state in our patients with thrombosis. However, the coexistence of antiphospholipid syndrome and MPN in JAK2 mutation carriers has been described and was associated with a significantly younger age [46]. The association of MPN and paroxysmal nocturnal hemoglobinuria (PNH) which may affect up to 10% of MPN patients deserves special attention in case of unexplained anemia with or without hemolysis, recurrent thrombosis, and atypical bone marrow morphology findings [47].
Currently, the drug development targeting JAK-STAT pathway mainly focuses on cytokine or receptors antibodies, JAK inhibitors and STAT inhibitors. Understanding the association between the JAK-STAT pathway and immune regulation and disease progression will provide new treatment strategies for immune related cancers [48,49].
Idiopathic thrombopenic purpura (ITP) has been sporadically reported in patients with ET. However, routine screening for MPN driver mutations in the work-up of isolated ITP is inappropriate [50]. The same applies to high ferritin level, AIHA, high LDH and high CRP [24]. The plasma cell neoplasms with coexisting erythrocytosis have been found to be unrelated to JAK2V617F mutation [51,52]. An inflammatory profile is a significant feature of many MPN as well as B-cell neoplasm. This may be due to a disruption of the shared JAK-STAT pathway signaling. On the other hand, molecular work-up may detect up to 17% of JAK2V617F mutation in patients with stroke, abdominal and deep vein thrombosis [53]. Patients with inflammatory bowel disease and erythrocytosis may harbor this mutation in a meaningful proportion [54].
Conclusions
In conclusion, we found that a positive-DAT is present in 15% of patients with JAK2V617F mutation at diagnosis regardless of the presence of overt anemia and another 10% of them were previously diagnosed with AID. Patients with PMF and MDS/MPN subtypes are most likely to develop a positive-DAT in 60% and 50% of cases respectively. Patients with PV and ET subtypes are less likely to display autoimmune manifestations. The occurrence of thromboembolic event was not associated with the presence of autoimmune phenomena.
References
2. Barcellini W, Fattizzo B. Immune Phenomena in Myeloid Neoplasms: An "Egg or Chicken" Question. Front Immunol. 2021 Sep 29;12:751630.
3. Tabata R, Tabata C, Omori K, Nagai T. Disappearing myelodysplastic syndrome-associated hemolytic anemia in leukemic transformation. Int Arch Allergy Immunol. 2010;152(4):407-12.
4. Fujimaki K, Takasaki H, Koharazawa H, Takabayashi M, Yamaji S, Baba Y, Kanamori H, Ishigatsubo Y. Idiopathic thrombocytopenic purpura and myasthenia gravis after fludarabine treatment for chronic lymphocytic leukemia. Leuk Lymphoma. 2005 Jul;46(7):1101-2.
5. Djaldetti M, Pinkhas J, de Vries A, Kott E, Joshua H, Dollberg L. Myasthenia gravis in a patient with chronic myeloid leukemia treated by busulfan. Blood. 1968 Aug;32(2):336-40.
6. Kopp CR, Jandial A, Mishra K, Sandal R, Malhotra P. Myasthenia gravis unmasked by imatinib. Br J Haematol. 2019 Feb;184(3):321.
7. Angelopoulou F, Bogdanos D, Dimitroulas T, Sakkas L, Daoussis D. Immune checkpoint inhibitor-induced musculoskeletal manifestations. Rheumatol Int. 2021 Jan;41(1):33-42.
8. Marisavljević D, Kraguljac N, Rolović Z. Immunologic abnormalities in myelodysplastic syndromes: clinical features and characteristics of the lymphoid population. Med Oncol. 2006;23(3):385-91.
9. Hamidou MA, Boumalassa A, Larroche C, El Kouri D, Blétry O, Grolleau JY. Systemic medium-sized vessel vasculitis associated with chronic myelomonocytic leukemia. Semin Arthritis Rheum. 2001 Oct;31(2):119-26.
10. Perner F, Perner C, Ernst T, Heidel FH. Roles of JAK2 in Aging, Inflammation, Hematopoiesis and Malignant Transformation. Cells. 2019 Aug 8;8(8):854.
11. Xue C, Yao Q, Gu X, Shi Q, Yuan X, Chu Q, et al. Evolving cognition of the JAK-STAT signaling pathway: autoimmune disorders and cancer. Signal Transduct Target Ther. 2023 May 19;8(1):204.
12. Hasselbalch HC. Perspectives on chronic inflammation in essential thrombocythemia, polycythemia vera, and myelofibrosis: is chronic inflammation a trigger and driver of clonal evolution and development of accelerated atherosclerosis and second cancer? Blood. 2012 Apr 5;119(14):3219-25
13. Anderson LA, Pfeiffer RM, Landgren O, Gadalla S, Berndt SI, Engels EA. Risks of myeloid malignancies in patients with autoimmune conditions. Br J Cancer. 2009 Mar 10;100(5):822-8.
14. Kristinsson SY, Landgren O, Samuelsson J, Björkholm M, Goldin LR. Autoimmunity and the risk of myeloproliferative neoplasms. Haematologica. 2010 Jul;95(7):1216-20.
15. Hemminki K, Liu X, Försti A, Ji J, Sundquist J, Sundquist K. Subsequent leukaemia in autoimmune disease patients. Br J Haematol. 2013 Jun;161(5):677-687.
16. Guillot X, Moldovan M, Vidon C, Wendling D. Myelofibrosis-related arthritis successfully treated with hydroxyurea. Case Rep Rheumatol. 2014;2014:869743
17. Thorsteinsdottir S, Bjerrum OW, Hasselbalch HC. Myeloproliferative neoplasms in five multiple sclerosis patients. Leuk Res Rep. 2013 Jul 31;2(2):61-3.
18. McKerrell T, Park N, Chi J, Collord G, Moreno T, Ponstingl H, et al. JAK2 V617F hematopoietic clones are present several years prior to MPN diagnosis and follow different expansion kinetics. Blood Adv. 2017 Jun 12;1(14):968-971.
19. Koschmieder S, Mughal TI, Hasselbalch HC, Barosi G, Valent P, Kiladjian JJ, et al. Myeloproliferative neoplasms and inflammation: whether to target the malignant clone or the inflammatory process or both. Leukemia. 2016 May;30(5):1018-24.
20. O'Shea JJ, Kontzias A, Yamaoka K, Tanaka Y, Laurence A. Janus kinase inhibitors in autoimmune diseases. Ann Rheum Dis. 2013 Apr;72 Suppl 2(0 2):ii111-5.
21. Jang YN, Baik EJ. JAK-STAT pathway and myogenic differentiation. JAKSTAT. 2013 Apr 1;2(2):e23282.
22. O'Shea JJ, Murray PJ. Cytokine signaling modules in inflammatory responses. Immunity. 2008 Apr;28(4):477-87.
23. Perner F, Perner C, Ernst T, Heidel FH. Roles of JAK2 in Aging, Inflammation, Hematopoiesis and Malignant Transformation. Cells. 2019 Aug 8;8(8):854.
24. Barcellini W, Iurlo A, Radice T, Imperiali FG, Zaninoni A, Fattizzo B, et al. Increased prevalence of autoimmune phenomena in myelofibrosis: relationship with clinical and morphological characteristics, and with immunoregulatory cytokine patterns. Leuk Res. 2013 Nov;37(11):1509-15.
25. Tefferi A, Gilliland DG. Oncogenes in myeloproliferative disorders. Cell Cycle. 2007 Mar 1;6(5):550-66.
26. Hu X, Li J, Fu M, Zhao X, Wang W. The JAK/STAT signaling pathway: from bench to clinic. Signal Transduct Target Ther. 2021 Nov 26;6(1):402.
27. Greenfield G, McMullin MF, Mills K. Molecular pathogenesis of the myeloproliferative neoplasms. J Hematol Oncol 14, 103 (2021).
28. Vannucchi AM, Kiladjian JJ, Griesshammer M, Masszi T, Durrant S, Passamonti F, et al. Ruxolitinib versus standard therapy for the treatment of polycythemia vera. N Engl J Med. 2015 Jan 29;372(5):426-35.
29. Harrison C, Kiladjian JJ, Al-Ali HK, Gisslinger H, Waltzman R, Stalbovskaya V, et al. JAK inhibition with ruxolitinib versus best available therapy for myelofibrosis. N Engl J Med. 2012 Mar 1;366(9):787-98.
30. Winthrop KL. The emerging safety profile of JAK inhibitors in rheumatic disease. Nat Rev Rheumatol. 2017 Apr;13(4):234-243.
31. Schwartz DM, Bonelli M, Gadina M, O'Shea JJ. Type I/II cytokines, JAKs, and new strategies for treating autoimmune diseases. Nat Rev Rheumatol. 2016 Jan;12(1):25-36.
32. Mullally A, Lane SW, Ball B, Megerdichian C, Okabe R, Al-Shahrour F, et al. Physiological Jak2V617F expression causes a lethal myeloproliferative neoplasm with differential effects on hematopoietic stem and progenitor cells. Cancer Cell. 2010 Jun 15;17(6):584-96.
33. Nishanth G, Wolleschak D, Fahldieck C, Fischer T, Mullally A, Perner F, et al. Gain of function in Jak2V617F-positive T-cells. Leukemia. 2017 Apr;31(4):1000-1003.
34. Mertz P, Chalayer E, Amoura Z, Cathebras P, Chiche L, Coestedoat N, et al. Clinical spectrum and therapeutic management of auto-immune myelofibrosis: a nation-wide study of 30 cases. Haematologica. 2021 Mar 1;106(3):871-874.
35. Cacciola R, Gentilini Cacciola E, Vecchio V, Cacciola E. Impact of Anti-Endothelial Cell Antibodies (AECAs) in Patients with Polycythemia Vera and Thrombosis. Diagnostics (Basel). 2022 Apr 25;12(5):1077.
36. Schär DT, Daskalakis M, Mansouri B, Rovo A, Zeerleder S. Thromboembolic complications in autoimmune hemolytic anemia: Retrospective study. Eur J Haematol. 2022 Jan;108(1):45-51.
37. Strickland M, Quek L, Psaila B. The immune landscape in BCR-ABL negative myeloproliferative neoplasms: inflammation, infections and opportunities for immunotherapy. Br J Haematol. 2022 Mar;196(5):1149-1158.
38. Boivin P, Bernard JF, Hakim J, Woroclans M. Anomalies immunitaires au cours de splénomégalies myéloïdes avec myélosclérose [Immunologic abnormalities in the course of myeloid splenomegaly with myelosclerosis]. Acta Haematol. 1974;51(2):91-100. French.
39. Rondeau E, Solal-Celigny P, Dhermy D, Vroclans M, Brousse N, Bernard JF, et al. Immune disorders in agnogenic myeloid metaplasia: relations to myelofibrosis. Br J Haematol. 1983 Mar;53(3):467-75.
40. Hasselbalch H, Berild D, Hansen OP. Red-cell sensitization in myelofibrosis. Scand J Haematol. 1984 Feb;32(2):179-82.
41. Barosi G. An immune dysregulation in MPN. Curr Hematol Malig Rep. 2014 Dec;9(4):331-9.
42. Barosi G, Campanelli R, Catarsi P, De Amici M, Abbà C, Viarengo G, et al. Plasma sIL-2Rα levels are associated with disease progression in myelofibrosis with JAK2V617F but not CALR mutation. Leuk Res. 2020 Mar;90:106319.
43. Wang JC, Sindhu H, Chen C, Kundra A, Kafeel MI, Wong C, et al. Immune derangements in patients with myelofibrosis: the role of Treg, Th17, and sIL2Rα. PLoS One. 2015 Mar 20;10(3):e0116723
44. Galimberti S, Baldini C, Baratè C, Fornili M, Balducci S, Ricci F, et al. Myeloid neoplasms and autoimmune diseases: markers of association. Clin Exp Rheumatol. 2022 Jan;40(1):49-55.
45. Geyer HL, Kosiorek H, Dueck AC, Scherber R, Slot S, Zweegman S, et al. Associations between gender, disease features and symptom burden in patients with myeloproliferative neoplasms: an analysis by the MPN QOL International Working Group. Haematologica. 2017 Jan;102(1):85-93.
46. Janjetovic S, Beckmann L, Holstein K, Rolling C, Thiele B, Schafhausen P, et al. Prevalence of definite antiphospholipid syndrome in carriers of the JAK2V617F mutation. Thromb Res. 2021 Feb;198:55-61.
47. Giannotta JA, Fattizzo B, Barcellini W. Paroxysmal Nocturnal Hemoglobinuria in the Context of a Myeloproliferative Neoplasm: A Case Report and Review of the Literature. Front Oncol. 2021 Nov 11;11:756589.
48. Barbui T, Finazzi G. Special issues in myeloproliferative neoplasms. Curr Hematol Malig Rep. 2011 Mar;6(1):28-35.
49. Pourcelot E, Trocme C, Mondet J, Bailly S, Toussaint B, Mossuz P. Cytokine profiles in polycythemia vera and essential thrombocythemia patients: clinical implications. Exp Hematol. 2014 May;42(5):360-8.
50. Langabeer SE. Excluding JAK2 V617F mutation analysis from the primary immune thrombocytopenia 'diagnosis of exclusion'. Blood Coagul Fibrinolysis. 2022 Dec 1;33(8):475-476.
51. Malhotra J, Kremyanskaya M, Schorr E, Hoffman R, Mascarenhas J. Coexistence of myeloproliferative neoplasm and plasma-cell dyscrasia. Clin Lymphoma Myeloma Leuk. 2014 Feb;14(1):31-6.
52. Langabeer SE. The JAK2 V617F Mutation in Plasma Cell Neoplasms with Co-existing Erythrocytosis. J Clin Diagn Res. 2015 Dec;9(12):EL01.
53. McCarthy N, McCarron SL, Langabeer SE. Prevalence of the JAK2 V617F and MPL mutations in stroke, abdominal and peripheral venous thrombosis. Acta Haematol. 2010;124(3):160-1.
54. Hesselø H, Bak M, Boysen T, Bytzer P, Hasselbalch HC. [Myeloproliferative neoplasms and chronic inflammatory bowel disease]. Ugeskr Laeger. 2020 May 5;182(22):V09190483.