Abstract
Cardiovascular disease is a major cause of morbidity and mortality in patients with chronic kidney disease (CKD). Such cardiovascular risk is not limited to those with advanced renal disease, as even patients with early stages of renal dysfunction show increased risk of cardiovascular morbidity and mortality. However, there is a remarkable lack of reliable information regarding the threshold of renal function, increasing the risk of long-term outcomes among patients who underwent multivessel coronary revascularization. Moreover, as patients with mildly decreased renal function were not differentiated from those with normal renal function in most clinical trials on coronary revascularization, there are insufficient data on the prognostic impact and comparative outcomes after percutaneous or surgical revascularization for complex or multivessel CAD in patients with early renal dysfunction.
This review aimed to highlight a recent publication that studied the long-term prognostic impact of baseline renal function, especially mildly decreased renal function on the outcomes after multivessel coronary revascularization.
Keywords
Coronary artery disease, Coronary-artery bypass grafting, Glomerular filtration rate, Percutaneous coronary intervention, Renal function
Abbreviations
CABG: Coronary Artery Bypass Grafting; CAD: Coronary Artery Disease; CI: Confidence Intervals; CKD: Coronary Kidney Disease; HR: Hazard Ratio; PCI: Percutaneous Coronary Intervention; eGFR: Estimated Glomerular Filtration Rate; CKD-EPI: Chronic Kidney Disease Epidemiology Collaboration
Introduction
Chronic kidney disease (CKD) has numerous direct negative effects on the cardiovascular system [1-3]. The effects of decreased renal function on cardiovascular disease are attributed to coexisting classical cardiovascular risk factors (e.g., diabetes, hypertension, smoking, dyslipidemia, and advanced age) and non-classical CKD-related risk factors (e.g., mineral and bone disease abnormalities, anemia, inflammation, and oxidative stress) [4]. A subclinical atherothrombotic process based on oxidative stress, inflammation, and endothelial dysfunction has been observed to exist in the early stages of renal dysfunction [5]. These metabolic changes progress with renal impairment, eventually leading to more advanced atherogenic burden at the systemic level [6]. For risk stratification in patients with multivessel coronary artery disease (CAD), understanding the relative effect of coronary revascularization according to the stage of renal dysfunction is crucial. However, the differences in the clinical features and outcomes of patients undergoing coronary revascularization according to the varying degree of renal dysfunction remain unknown. Moreover, as patients with mildly decreased renal function corresponding to the borderline between the CKD and normal renal function stage were under-represented in most of the clinical trials on myocardial revascularization, there are insufficient data on the characteristics and long-term prognosis of patients with mildly decreased renal function after percutaneous or surgical revascularization [7-9]. Resultingly, the level of renal function at which cardiovascular risks begin to increase has not been clearly elucidated.
By reviewing a recent published manuscript titled, “Prognostic Impact of Mildly Impaired Renal Function in Patients Undergoing Multivessel Coronary Revascularization” [10], we summarized the prognostic impact of baseline renal function in patients who underwent multivessel coronary revascularization, focusing more on patients with mild renal dysfunction.
Study Population, Kidney Function Assessment, and Outcome Definition
This study was a registry-based analysis on consecutive patients with multivessel CAD who underwent either isolated coronary artery bypass graft (CABG) surgery or percutaneous coronary intervention (PCI) with drug-eluting stents between January 1, 2003 and April 30, 2018, at Asan Medical Center (Seoul, Korea) [11]. A total of 10,883 eligible patients with multivessel disease who underwent coronary revascularization were identified, of whom 6,730 (61.8%) patients underwent PCI with drug-eluting stents and 4,153 (38.2%) patients underwent isolated CABG.
Given that serum creatinine is considered as an insensitive indicator of renal function and mild renal impairment exists despite normal or near-normal creatinine levels [12], the estimated glomerular filtration rate (eGFR) was used for the assessment of renal dysfunction in the current study. The eGFR at baseline was measured using the CKD Epidemiology Collaboration (CKD-EPI) creatinine equation that includes age, race, and sex as surrogates for the non-GFR determinants of serum creatinine [13]. According to the baseline eGFR, 3,735 (34.3%) patients were considered to have normal renal function (stage I), while 5,125 (47.1%), 1,497 (13.8%), 198 (1.8%), and 331 (3.0%) patients were deemed to have mild (stage II), moderate (stage III), severe (stage IV), and end-stage renal dysfunction (stage V), respectively. The primary outcome was the composite of death, spontaneous myocardial infarction, or stroke. All the outcomes were assessed according to standard endpoint definitions [14].
Baseline Characteristics and Clinical Outcomes According to the Renal Function
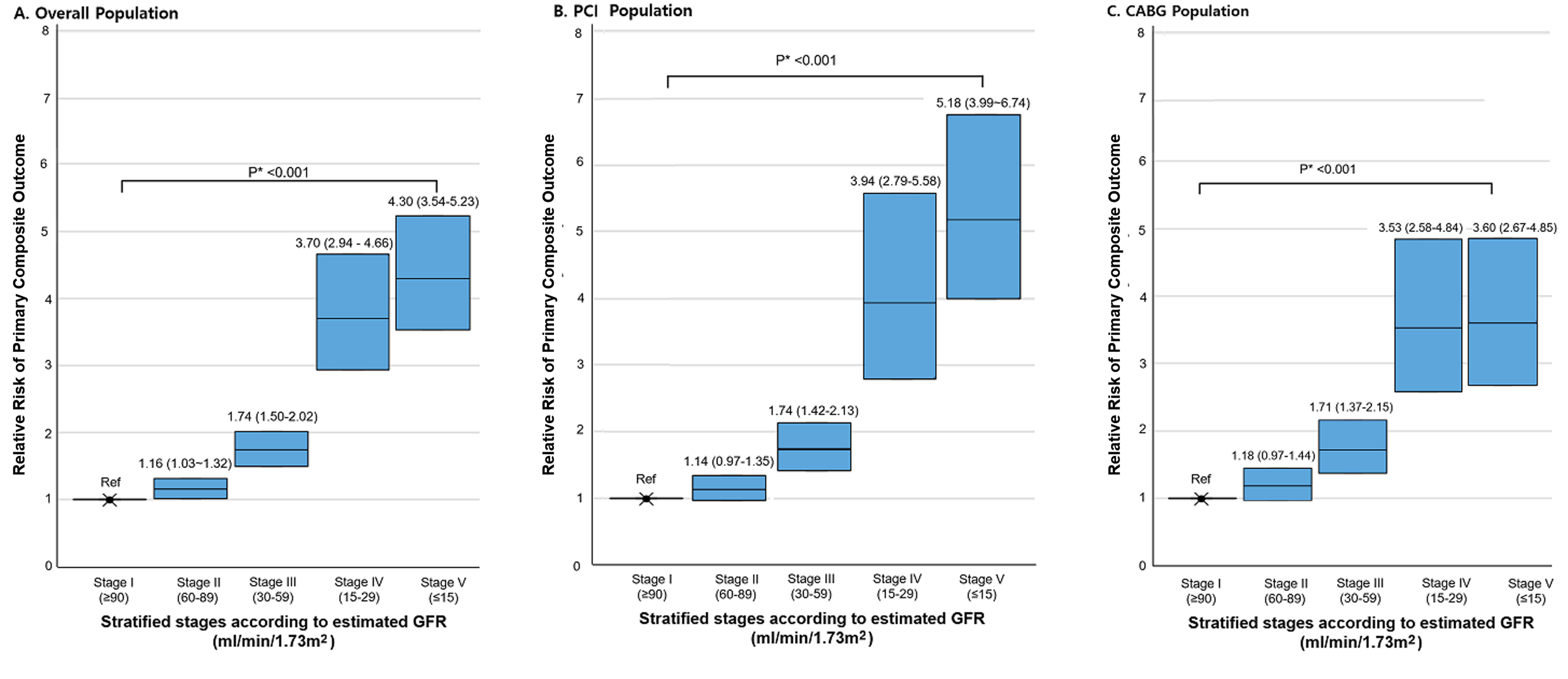
In general, patients with more severe renal dysfunction showed a higher prevalence of clinical risk factors (such as old age and left ventricular dysfunction) and comorbidities (such as diabetes, hypertension, previous stroke, and previous myocardial infarct). The prevalence of three-vessel disease and left main disease was also higher in patients with more severe renal dysfunction. The difference in baseline demographics according to the stage of renal function was more prominent in the advanced renal dysfunction group (stage IV/V). In the overall population, the risk of the primary composite outcome of death, spontaneous MI, or stroke proportionally increased, especially in patients with more severe renal dysfunction (stage IV and V) with respect to the predicted probability per 1,000 person-year of events (Figure 1A). This increased risk of primary composite outcome associated with decreased renal function was not different in each stratum of PCI or CABG (Figure 1B and 1C).
Figure 1: Predicted probability of primary composite outcome by renal function group. Data are shown as the number of primary composite outcomes (death from any cause, spontaneous myocardial infarction, or stroke) events as well as the event rate per 1,000 person-year. *P value for trend represents the likelihood of proportional trend between each stage and predicted probability of the primary composite outcome.
The multivariable-adjusted hazard ratios (HRs) for outcomes according to the stage of renal function in the overall population are shown in Figure 2A and those in each stratum of PCI and CABG are shown in Figure 2B, 2C. With the stage I group as the reference, the adjusted HRs for primary composite outcome proportionally increased according to increasing levels of renal dysfunction (HRs for stage II, stage III, stage IV, and stage V: 1.16, 1.74, 3.70, and 4.30, respectively; P-for-trend <0.001). This pattern was consistent in each stratum of the PCI and CABG groups.
Figure 2: Risks of the primary composite outcome by renal function group. Hazard ratios (HRs) and 95% confidence intervals (CIs) for stratified stages according to the baseline renal function as compared with the group with an eGFR above 90 mL per minute per 1.73 m2 (stage I). *P value for trend represents the likelihood of proportional trend between each stage and the relative risk of the primary composite outcome.
Linear Association between eGFR and Outcome
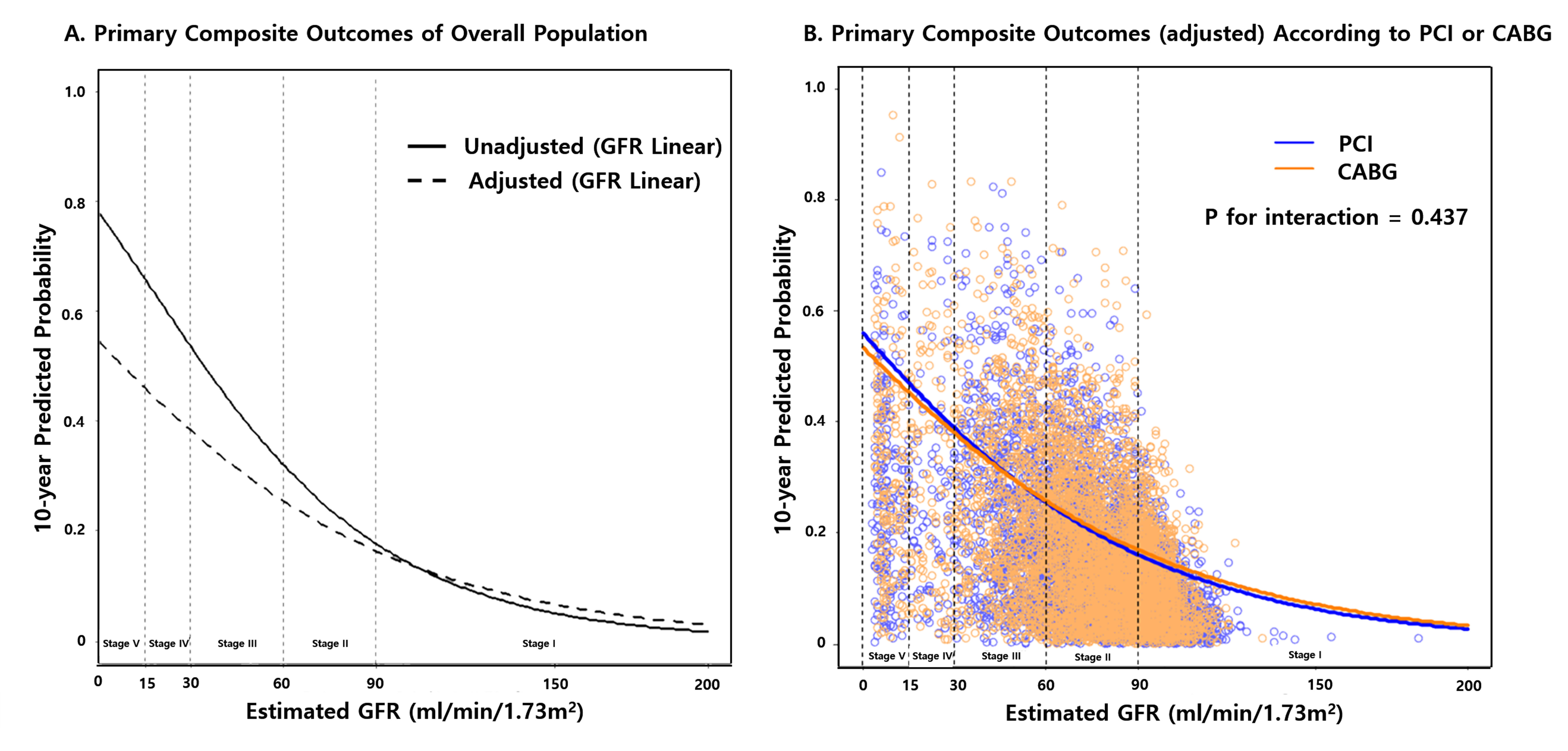
Using the linear model, we calculated the 10-year probability of the primary composite outcome according to the baseline eGFR (as a continuous variable). In the crude model, the probability of primary composite outcome substantially increased below the eGFR value of approximately 60 ml/min/1.73 m2. In multivariable models, although the effect of eGFR on outcome was slightly diminished, the inverse relationship of eGFR with the risk of 10-year probability of the primary outcome was consistent (Figure 3A). This association was similar in each stratum of PCI and CABG (Figure 3B).
Figure 3: Linear predicted probability of primary composite outcome by the level of renal function. Unadjusted curve (left panel) shows the risk incorporating each individual’s value for 17 covariates in the published article. The adjusted curve (right panel) shows the predicted probability of primary composite outcome with the eGFR value on the X-axis, assuming that the continuous variables, such as age, BMI, and LVEF had the mean value, the sex was male, and the remaining categorical risk factors were null. The linear model included GFR as a continuous variable in a Cox regression analysis.
Prognostic Impact of Mild or Moderate Renal Dysfunction on the Clinical Outcome
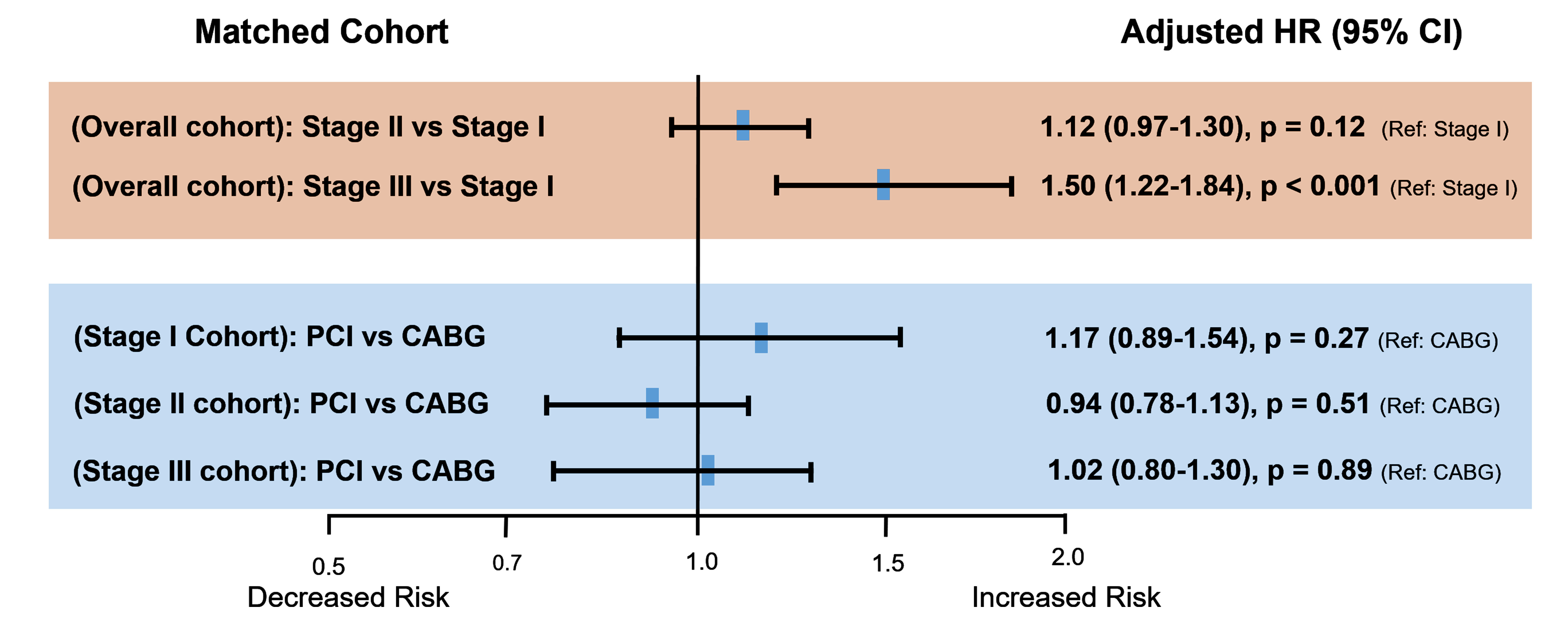
Although the severity was less than patients with advanced renal dysfunction, patients with mildly decreased renal function were also older and had a relatively higher prevalence of comorbidities and more extensive CAD compared to patients with normal renal function. To rigorously control the baseline characteristics of patients according to each stage of renal function and different revascularization strategies of PCI and CABG, propensity-score matching was used to assemble a cohort of patients with similar baseline characteristics. In the propensity-matched cohort, the adjusted risk for primary composite outcome was not significantly different between the stage I and stage II groups (HR: 1.12; 95% CI: 0.97–1.30; P = 0.12). However, the risk of primary composite outcome was significantly higher in the stage III group than in the stage I group (HR: 1.50; 95% CI: 1.22–1.84) (Table 1 and Figure 4). The relative effect of PCI over CABG for the primary outcome was similar in the matched cohort of each renal function group (HR: 1.17; 95% CI: 0.89–1.54 for stage I, HR: 0.94; 95% CI: 0.78–1.13 for stage II, and HR: 1.02; 95% CI: 0.80–1.30 for stage III) (Table 2 and Figure 4).
Figure 4: Risks of the primary composite outcomes in matched cohort. Compared with the normal baseline renal function, mildly decreased baseline renal function was not significantly associated with an increased risk of major adverse cardiovascular events, and there was no significant difference in the clinical outcome according to the revascularization methods of PCI and CABG.
|
|
Unmatched Population |
Propensity-Score Matched |
||||
|
Hazard ratio (95% CI)* |
Hazard ratio (95% CI)* |
|||||
|
|
Stage I |
Stage II |
Stage III |
Stage I |
Stage II |
Stage III |
|
Overall Population |
|
|
|
|
|
|
|
Primary composite of death, spontaneous MI, or stroke |
1.00 |
1.60 (1.42−1.81) P<0.001 |
3.18 (2.77−3.64) P<0.001 |
1.00 |
1.12 (0.97−1.30), P=0.12 |
1.50 (1.22-1.84) P<0.001 |
|
CABG Stratum |
|
|
|
|
|
|
|
Primary composite of death, spontaneous MI, or stroke |
1.00 |
1.68 (1.39−2.02) P<0.001 |
3.05 (2.47-3.76) P<0.001 |
1.00 |
1.18 (0.93−1.48) P=0.17 |
1.47 (1.10-1.97) P=0.01 |
|
PCI Stratum |
|
|
|
|
|
|
|
Primary composite of death, spontaneous MI, or stroke |
1.00 |
1.51 (1.29−1.77) P<0.001 |
3.15 (2.62-3.78) P<0.001 |
1.00 |
1.09 (0.91−1.32) P=0.35 |
1.54 (1.14-2.09) P=0.01 |
|
*Hazard ratios are for the stage II group (mildly decreased renal function) or stage III group (moderately decreased renal function) as compared with the stage I group (normal renal function; referent group). |
||||||
|
Outcome |
Renal function |
Unmatched Population |
Propensity-Score Matched |
||
|
HR (95% CI)* |
P-value |
HR (95% CI)* |
P-value |
||
|
Primary composite of death, spontaneous MI, or stroke |
Stage I |
0.82 (0.67−1.01) |
0.06 |
1.17 (0.89−1.54) |
0.27 |
|
Stage II |
0.74 (0.64−0.84) |
<0.001 |
0.94 (0.78−1.13) |
0.51 |
|
|
Stage III |
0.84 (0.70-1.02) |
0.008 |
1.02 (0.80-1.30) |
0.89 |
|
|
*HRs are for the PCI group compared with the CABG group (reference group). |
|||||
Conclusion
In the present study, we included a large number of patients with a broad spectrum of renal function, which enabled us to perform a detailed evaluation of the clinical impact of baseline renal function after coronary revascularization. Overall, we observed substantial differences in the demographic features, comorbidities, clinical presentation, and atherosclerotic burden of CAD according to the degree of renal dysfunction. When focusing on the early stages of renal dysfunction, as renal function deteriorated from mild to moderate dysfunction, the patients were older, with more frequent comorbidities, and more advanced coronary disease. After an adjustment of baseline covariates using a propensity-score analysis, compared to patients with normal renal function, those with mild renal dysfunction did not have increased risks of the primary composite outcome of death, spontaneous MI, or stroke. By contrast, patients with moderate renal dysfunction had a higher risk of major adverse cardiovascular events.
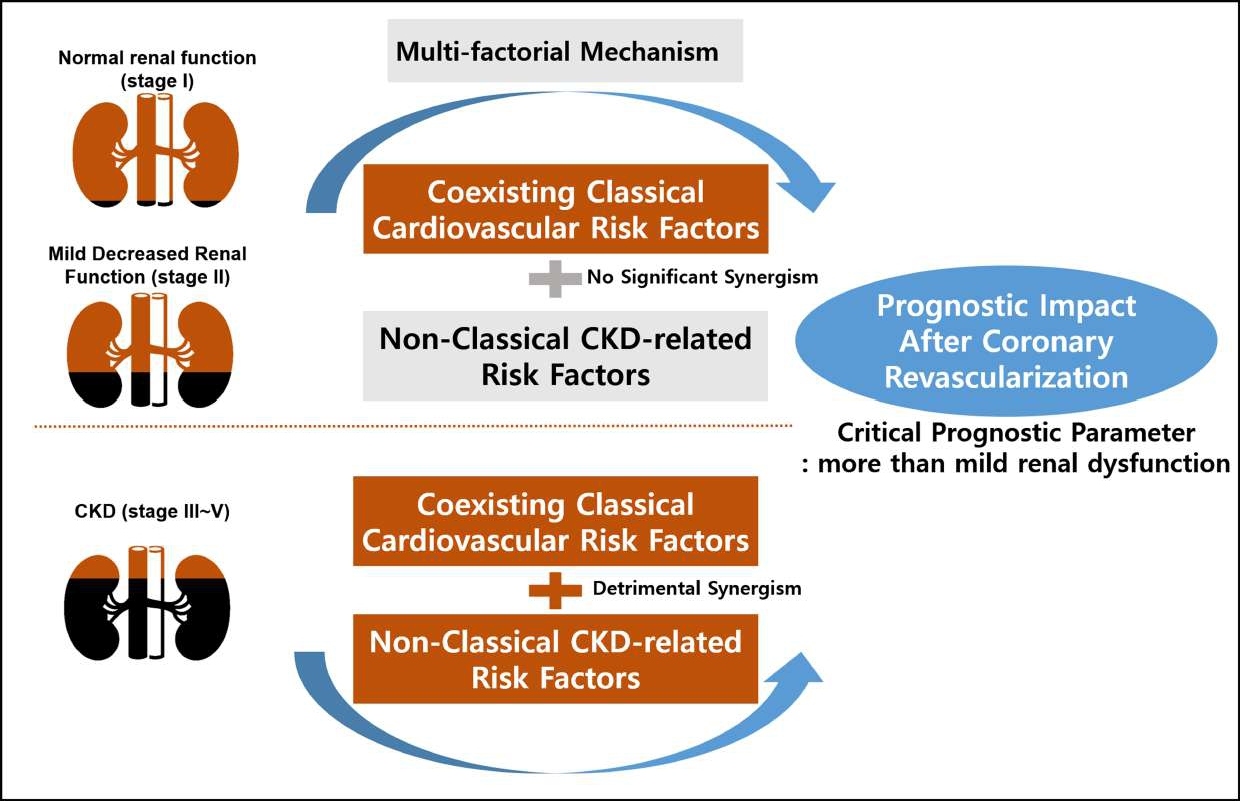
These findings may define a threshold of GFR that is associated with worse outcomes and suggest that mildly decreased renal function is not a critical prognostic parameter after coronary revascularization. Considering the mechanisms underlying the poor outcomes of renal impairment following coronary revascularization are likely multi-factorial, such findings may be explained in part by that the detrimental synergism between coexisting classical cardiovascular risk factors and non-classical CKD-related risk factors is not yet prominent in patients with mildly decreased renal function. It was observed that the atherothrombotic process was actually subclinical in the stage of mild renal dysfunction, whereas in CKD, the systemic atherogenic process based on oxidative stress and inflammation was more enhanced with the coexisting traditional cardiovascular risk factors, resulting in a higher risk of adverse clinical events after coronary revascularization (Figure 5) [15].
Figure 5: Explanation for the baseline renal function as a critical prognostic parameter. Multiple possible explanations exist for the association between chronic kidney disease and increased risks of death and cardiovascular disease. This study showed that the degree of synergism of coexisting classical cardiovascular risk factors and non-classical CKD-related risk factors is different according to the stage of renal function.
As PCI might be more challenging with a lower probability of procedural success and a higher proportion of incomplete revascularization due to more aggressive atherosclerosis and a higher degree of calcification in patients with advanced renal disease, it is generally accepted that CABG is superior to PCI in achieving better long-term outcomes in the highest-risk CKD patients [16]. However, since most previous clinical trials did not distinguish patients with mildly decreased renal function from those with normal renal function or advanced CKD, the comparative outcome of two revascularization strategies in such borderline-risk patients remains uncertain. In our large-sized real-world study, there was no significant difference between PCI and CABG with respect to the risks of the primary composite endpoint in each group of normal, mild, or moderate renal dysfunction. Well-organized understanding of the clinical features and prognostic impact of mildly decreased renal function, and the relative effect of PCI or CABG in patients with mildly decreased renal function may be helpful for further risk-stratification and crucial to guide for appropriate revascularization choices. However, owing to the inherent limitations of an observational study, this interpretation should be considered in a conservative manner; our observed findings warrants further investigation and should be confirmed or refuted through large, randomized clinical trials with a long-term follow-up.
References
2. Mann JF, Gerstein HC, Pogue J, Bosch J, Yusuf S. Renal insufficiency as a predictor of cardiovascular outcomes and the impact of ramipril: the HOPE randomized trial. Ann Intern Med. 2001;134(8):629-36.
3. Best PJ, Lennon R, Ting HH, Bell MR, Rihal CS, Holmes DR, et al. The impact of renal insufficiency on clinical outcomes in patients undergoing percutaneous coronary interventions. J Am Coll Cardiol. 2002;39(7):1113-9.
4. Lai AC, Bienstock SW, Sharma R, Skorecki K, Beerkens F, Samtani R, et al. A Personalized Approach to Chronic Kidney Disease and Cardiovascular Disease: JACC Review Topic of the Week. J Am Coll Cardiol. 2021;77(11):1470-9.
5. Stam F, van Guldener C, Schalkwijk CG, ter Wee PM, Donker AJ, Stehouwer CD. Impaired renal function is associated with markers of endothelial dysfunction and increased inflammatory activity. Nephrol Dial Transplant. 2003;18(5):892-8.
6. Shavadia JS, Southern DA, James MT, Welsh RC, Bainey KR. Kidney function modifies the selection of treatment strategies and long-term survival in stable ischaemic heart disease: insights from the Alberta Provincial Project for Outcomes Assessment in Coronary Heart Disease (APPROACH) registry. Eur Heart J Qual Care Clin Outcomes. 2018;4(4):274-82.
7. Chonchol M, Whittle J, Desbien A, Orner MB, Petersen LA, Kressin NR. Chronic kidney disease is associated with angiographic coronary artery disease. Am J Nephrol. 2008;28(2):354-60.
8. Szummer K, Lundman P, Jacobson SH, Schön S, Lindbäck J, Stenestrand U, et al. Influence of renal function on the effects of early revascularization in non-ST-elevation myocardial infarction: data from the Swedish Web-System for Enhancement and Development of Evidence-Based Care in Heart Disease Evaluated According to Recommended Therapies (SWEDEHEART). Circulation. 2009;120(10):851-8.
9. Farkouh ME, Sidhu MS, Brooks MM, Vlachos H, Boden WE, Frye RL, et al. Impact of Chronic Kidney Disease on Outcomes of Myocardial Revascularization in Patients With Diabetes. J Am Coll Cardiol. 2019;73(4):400-11.
10. Kim TO, Kang DY, Ahn JM, Kim SO, Lee PH, Lee J, et al. Prognostic Impact of Mildly Impaired Renal Function in Patients Undergoing Multivessel Coronary Revascularization. J Am Coll Cardiol. 2022;79(13):1270-84.
11. Park DW, Kim YH, Song HG, Ahn JM, Oh J, Kim WJ, et al. Long-term comparison of drug-eluting stents and coronary artery bypass grafting for multivessel coronary revascularization: 5-year outcomes from the Asan Medical Center-Multivessel Revascularization Registry. J Am Coll Cardiol. 2011;57(2):128-37.
12. Inker LA, Astor BC, Fox CH, Isakova T, Lash JP, Peralta CA, et al. KDOQI US commentary on the 2012 KDIGO clinical practice guideline for the evaluation and management of CKD. Am J Kidney Dis. 2014;63(5):713-35.
13. Levey AS, Stevens LA, Schmid CH, Zhang YL, Castro AF, 3rd, Feldman HI, et al. A new equation to estimate glomerular filtration rate. Ann Intern Med. 2009;150(9):604-12.
14. Garcia-Garcia HM, McFadden EP, Farb A, Mehran R, Stone GW, Spertus J, et al. Standardized End Point Definitions for Coronary Intervention Trials: The Academic Research Consortium-2 Consensus Document. Circulation. 2018;137(24):2635-50.
15. Go AS, Chertow GM, Fan D, McCulloch CE, Hsu CY. Chronic kidney disease and the risks of death, cardiovascular events, and hospitalization. N Engl J Med. 2004;351(13):1296-305.
16. Neumann F-J, Sousa-Uva M, Ahlsson A, Alfonso F, Banning AP, Benedetto U, et al. 2018 ESC/EACTS Guidelines on myocardial revascularization. Eur Heart J. 2018;40(2):87-165.