Abstract
Storage and periodic elimination of urine requires coordinated activity of the bladder and the urethral outlet. This coordination is provided by a complex neural control system in the brain and spinal cord. Experimental studies in animals show that urine storage is modulated by reflex mechanisms located in the sacral spinal cord, whereas urine release is mediated by pontine micturition center in the brainstem. Detachment of spinal neural circuits of the lower urinary tract from supraspinal structures after spinal cord injury (SCI) impairs reflex voiding and results in detrusor-sphincter-dyssynergia (DSD). In patients and animals with DSD, the bladder content cannot be released due to persistent contraction of the urethra. However, reflex voiding slowly recovers in chronic SCI animals due to plasticity of intraspinal mechanisms. A recently found mid-lumbar circuit synaptically connected to sacral segments through descending axons of propriospinal neurons is a likely candidate to lead restoration of reflex voiding. In this review I discuss available data on propriospinal neurons of the lower urinary tract and propose a model to explain neural mechanism of bursting contractions of the external urethral sphincter in rats and mice during voiding.
Keywords
Spinal cord, External urethral sphincter, Bladder, Trans-synaptic tracing, Bursting, Recurrent inhibition
Neural Control of the Lower Urinary Tract
The lower urinary tract (LUT) includes the bladder (BL) and urethra. The LUT has two functions: accumulation and storage of urine and elimination of urine from the body through micturition. These functions require tight coordination of the two organs to prevent undesirable leakage of the waste product and to allow for a timely and comfortable emptying of the bladder.
The urethra contains three types of muscles: (a) the outer layer of striated muscles representing the external urethral sphincter (EUS) which extends from the bladder neck to 20-80% of the total urethral length and is much thicker in males compared to females [1-3], (b) a thick inner layer of the longitudinal smooth muscle and (c) a thin outer layer of the circular smooth muscle [4]. The longitudinal layer of the smooth muscle (SM) is ten times thicker than the surrounding circular smooth muscle [5]. The smooth muscles of the urethra are contracted during storage due to activation of α-adrenoceptors by norepinephrine released from the sympathetic hypogastric nerve and are relaxed during voiding via NO mechanism mediated by the parasympathetic pelvic nerve [6-8]. There is still no consensus on the role of smooth muscle in autonomic reflexes. However, the predominant concept is that contraction of the longitudinal urethral smooth muscle is important for the long-term maintenance of the tone at the initial stage of urine accumulation (the guarding reflex), when pressure in the bladder is relatively low. The striated muscle of the EUS contributes more to urethral tone later, when bladder pressure increases [9-11]. Despite very sparse research on the SM, it seems that it alone cannot maintain high urethral pressure coordinated with increasing bladder pressure to prevent incontinence. Intracellular microelectrode recordings in vitro have shown that smooth myocytes can spontaneously generate slow (~1 sec) Ca2+-dependent “spikes” separated by quiescent periods of ~5-10 sec. These events were uncoordinated among muscle cells and did not spread across the tissue [12]. However, it is possible that some number of random but concomitantly occurring asynchronous electrical events and the associated contractions, sum to form an overall tonic contraction. Experiments with Ca2+-sensitive fluorescent indicators also have shown asynchronous intracellular Ca2+ transients with no correlation between any particular muscle fibers and the muscle contraction [13]. While distinct Ca2+ events could be imaged within individual cells, there was no intercellular propagation of a Ca-wave within smooth muscle bundles [14]. The asynchronous spontaneous activity in the smooth muscle can be modulated by autonomic neurotransmitters such as nitric oxide, adenosine triphosphate, and noradrenaline [15,16]. Release of nitric oxide (NO, the primary inhibitory transmitter in the urethra) is mediated through the pelvic nerve originating from the parasympathetic preganglionic neurons in sacral/lower lumbar spinal segments. Release of norepinephrine (NE, the primary excitatory transmitter) results from activation of the hypogastric nerve, originating from sympathetic preganglionic neurons in the upper lumbar segments [6,17]. The lack of coordination between myocytes of the smooth muscle makes it unlikely to perform fast and coordinated reflective voiding contraction observed in micturition reflex. However, integrated contraction of longitudinal fibers results in the overall shortening and thickening of the SM. The latter expands both inside and outside. Expansion to the inside closes the lumen, whereas expansion to the outside stretches a thin (and likely weak) layer of circular smooth fibers along with the more robust EUS striated muscle surrounding the urethra. It is the EUS tonic activity that can effectively counteract the outside expansion of SM and maintain the closure of the lumen. Furthermore, when EUS contracts it uses the thickened smooth muscle as a plug to keep the urethral lumen tightly closed when BL pressure increases [18]. Thus, the smooth muscle of the urethra plays a significant role in the guarding reflex. In the micturition reflex it plays a supportive role, whereas the striated muscle of the external urethral sphincter is the main contributor to contraction of urethra at high bladder pressure as well as to relaxation of urethra during voluntary or reflexive voiding.
The BL and EUS muscles normally work in a highly coordinated reciprocal manner. During urine accumulation the smooth BL muscle is relaxed, whereas EUS is tonically contracted. On the contrary, during voiding the BL muscle contracts and EUS relaxes. Neural control of the bladder and urethra have been intensely studied and documented (reviewed in [17,19-24]). In mammals, all spinal output neurons for the LUT are located in the lower lumbar and upper sacral segments of the spinal cord. Motoneurons for the EUS (EUS-MNs) in rats and mice create a compact cluster called Onuf’s nucleus in the ventral horn of L6-S1 segments (Figure 1). In cats Onuf’s nucleus is in S1-S2 segments and in humans it is in S1-S3 segments [19]. Efferent signals from EUS-MNs reach the striated muscle of the EUS directly via pudendal nerve. The smooth muscle of the bladder receives indirect input from preganglionic parasympathetic neurons (PPNs) located in the intermediolateral nucleus (IML) of the same segments. Via the pelvic nerve axons of PPNs reach the ganglion cells, which represent actual motoneurons of the BL smooth muscle. Cellular elements of intraspinal circuits for LUT control can be revealed using trans-synaptic retrograde labeling with pseudorabies virus (PRV) encoding for a fluorescent marker [25,26]. PPNs and EUS-MNs are considered the first order spinal neurons retrogradely labeled from the target organs. Apart from PPNs and EUS-MNs, the retrograde transsynaptic tracing either from BL or from EUS muscles has revealed populations of local intrasegmental interneurons synaptically connected to the output neurons, and, therefore representing the second order cells. In the rat, a compact pool of these interneurons was found in the dorsal commissure (DCM) of L6-S1 segments above the central canal (CC) [25-28] (Figure 1). Interestingly, interneurons presynaptic to EUS-MNs are intermingled with interneurons presynaptic to PPNs and occupy the same area in DCM [26,27]. In addition, it is likely that not all local interneurons retrogradely labeled by PRV establish synaptic contacts with EUS-MNs or/and PPNs. Some of them may represent the third order cells of the LUT circuit being infected through the second order cells.
Figure 1: Schematic of innervation of the bladder (BL) and external urethral sphincter (EUS) from lumbo-sacral segments of the rat spinal cord (connections with supralumbar structures are not included). Intermediolateral nucleus (IML) contains parasympathetic preganglionic neurons (PPNs, red septagons) innervating the smooth bladder muscle. Onuf’s nucleus contains motoneurons (EUS-MNs, green triangles) innervating striated muscle of the external urethral sphincter. Dorsal commissure (DCM) in L6-S1 segments contains local interneurons presynaptic either to BL, or to EUS, or to both. Lumbar spinal coordinating center (LSCC) in L3-L4 segments contains propriospinal neurons (PNs) presynaptic either to BL, or to EUS, or to both. Axons of propriospinal neurons descend within ventral column to L6-S1 segments where they ramify in the lower portion of ventral horn. Possible targets of PNs may be not only somata and/or dendrites of EUS-MNs, but also other LUT-related neurons. The central canal is depicted by a hollow circle.
Infection and labeling of the 3rd order interneurons presynaptic to 2nd order cells is practically inevitable because of variations in trans-synaptic spread of PRV through neuronal chains and variations in expression of a virus-encoded fluorescent marker. However, separation of labeled neurons of 2nd and 3rd order was possible using noticeably graded differences in the fluorescence intensity between cells in a narrow time window following virus injection into the muscle. In P30-P35 rat spinal interneurons of the 2nd and 3rd orders traced from the EUS were found above the central canal in the DCM of L6-S1 segments; interneurons of the 2nd and 3rd orders traced from the BL wall were located in essentially the same area [26]. These overlapping distributions of interneurons imply synaptic interactions between EUS and BL circuits. Indeed, a substantial number of neurons in L6-S1 DCM showed double labeling after simultaneous injections of two different tracers into BL and EUS muscles, revealing cellular elements involved in interaction and, possibly, coordination of BL and EUS functional states [26,27]. In the DCM double-labeled cells presynaptic to both EUS-MNs and to BL- PPNs comprise ~20% of all EUS- related 2nd order cell and ~24% of all BL-related 2nd order cells [26]. Thus, there is a complex spinal network supporting LUT functions. The current concept of LUT regulation suggests that urine accumulation and storage (guarding reflex) is controlled by the L6-S1 intraspinal circuit. However, this circuit alone was found to be insufficient for organization of reflex voiding or deliberate voiding. Despite the smooth muscle of the bladder wall slowly contracts via autonomic control, its contractions alone are not sufficient for voiding. Urine release is ultimately gated by the EUS, which is normally constricted, but relaxes to allow urine flow when the BL is full and micturition reflex turns on. Relaxation of the EUS is difficult or impossible in patients and animals after spinal cord injury (SCI) because SCI disrupts communication of lumbar-sacral segments with supraspinal structures taking part in LUT regulation [29-32]. Trans-synaptic PRV tracing has identified populations of neurons involved in control of the BL and EUS in the brainstem pontine micturition center, periaqueductal grey, raphe nucleus, locus coeruleus, hypothalamus, thalamus and some areas of the neocortex [25,27,28,33-36]. The main supraspinal structure controlling the switch from urine storage to elimination is the pontine micturition center (PMC) [37]. The PMC contains two major groups of neurons. One of them expresses the corticotropin releasing hormone (CRT+ cells) [38-40]. Optogenetic stimulation of these cells leads to bladder contraction and increase of intravesical pressure [41]. The other group of PMC neurons expresses estrogen receptor 1 (ESR1+ cells) and projects down to L6-S2 in mice and rats. Their axons profoundly ramify within the dorsal commissure. Optogenetic stimulation of ESR1+ neurons results in EUS bursting and efficient voiding in anesthetized and behaving animals [42]. Thus, disruption of PMC-to-L6/S1 connections seems to be critical for organization of reciprocally coordinated contractions and relaxations of the BL and EUS.
After spinal cord injury rostral to the lumbar level the bladder initially becomes areflexic, whereas the EUS remains tonically active resulting in complete urinary retention. In a few weeks post-SCI bladder overactivity slowly develops, but voiding is usually inefficient due to simultaneous contractions of the BL and EUS [29,43]. With longer post-SCI time, the ability to void recovers, and filling of the bladder to some threshold level initiates opening of the urethra and release of urine. This restoration of reflex voiding indicates unmasking of previously dormant or development of new intraspinal circuit capable of at least partial compensation of detrusor-sphincter-dyssynergy.
In rats, EUS relaxation is not persistent during voiding. In spinal intact (SI) animals it is represented by a series of short pauses between rhythmically occurring EMG bursts. These alternating bursts-pauses indicate intermittent synchronized contractions of many motor units in the EUS periodically interrupted by a complete block of their activity [17,44,45]. This series of rhythmic contractions of the circular striated muscle works like a peristaltic pump forcefully expelling urine from urethra and increasing efficiency of voiding. In chronically spinalized animals, recovery of reflex voiding correlates with re-appearance of EUS bursting. Therefore, EUS bursting is a good indicator of recovery after SCI in rats and mice [46-48]. However, despite wide usage of EUS bursting in neurourological experiments, the neural mechanism of this phenomenon is still unclear. EUS-MNs themselves are incapable of generating bursts of spikes [49]. To explain occurrence of rhythmic EMG-bursting during ejaculation in mammals as well as during voiding in rats some authors suggest presence of an intraspinal central pattern generator by analogy with the circuitry supporting coordinated locomotion [50,51]. However, no evidence of such a rhythm-generating network or pacemaking neurons presynaptic to EUS-MNs and activated during voiding has been demonstrated.
Thus, BL-EUS coordinating mechanisms clearly survive after complete removal of supraspinal control. Nevertheless, it is still unclear what are these remaining mechanisms and what intraspinal structures are involved in compensation of supraspinal deficiency. It seems that these mechanisms slowly emerge after SCI following re-balancing of neuronal activity or rewiring in existing circuits and possible recruitment of some additional circuits [29].
Propriospinal Neurons of the LUT
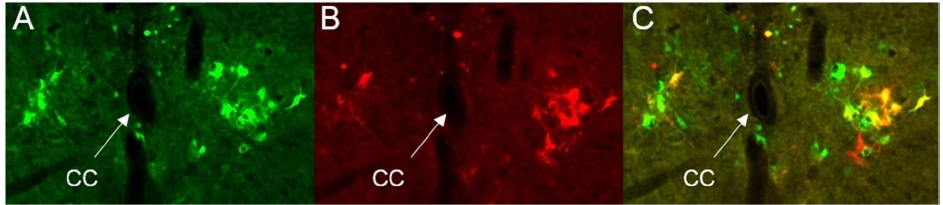
The likely candidate to an additional circuit which is able to substitute the lacking supraspinal afferentation is the recently found small population of interneurons located near the central canal in L3-L4 segments and projecting their axons into L6-S1 [26,52]. Intraspinal neurons projecting through a few or many spinal segments to provide intersegmental communication are called propriospinal neurons (PNs). They participate in complex motor reflexes and have been found to play a critical role in motor control and sensory processing [53-55]. Furthermore, they form indirect CNS-to-spinal cord connections which are phylogenetically older than direct connections from the motor cortex to spinal motoneurons. These seemingly redundant relict connections may again play a role after an evolutionary newer system is impaired. Recent studies demonstrate that propriospinal neurons are actively involved in restoration of locomotion after spinal cord injuries [56-59]. Therefore, it is plausible that the LUT system conserved an auxiliary mechanism subserving BL-EUS coordination. It was found that in rats and mice full transection of the spinal cord above L3-L4 lumbar segments does not prevent restoration of reflex voiding and accompanying EUS reflex bursting, but voiding and bursting do not recover after transection in or below L3-L4 [44]. Therefore, L3-L4 segments have been hypothesized to contain an auxiliary EUS-related circuit which somewhat substitutes loss of afferentation from supraspinal structures. Functional importance of an upper lumbar circuit for sphincter relaxation was also shown in experiments with epidural stimulation above L3 in rats, which in combination with a serotonergic receptor agonist elicited bursting in EUS and facilitated voiding [60]. Involvement of L3-L4 in micturition was also indicated by significant increase in the number of neurons expressing c-fos in the L3-L4 medial gray observed in experiments with urogenital reflex [61]. The hypothetical L3-L4 LUT-related circuit was named the spinal lumbar coordinating center (LSCC) [44]. Its existence was anatomically confirmed in recent tracing experiments with pseudorabies virus expressing either green or red fluorescent proteins (PRV-GFP or PRV-RFP) [26,52]. To our surprise simultaneous injections of PRV-RFP in the bladder wall and PRV-GFP in the EUS revealed that LSCC consists not only of EUS-related PNs, but also of a comparable number of propriospinal neurons traced from the BL. Above and lateral to the central canal in L3-L4 segments we found horseshoe-shaped populations of labeled propriospinal interneurons monosynaptically targeting either PPNs, or EUS-MNs (Figure 2). In the L3-L4 LSCC the EUS- and BL-related interneurons occupied the same area near the central canal, similar to that in the L6-S1 DCM. Some neurons in this area demonstrated double-labeling, indicating involvement in both circuits (yellow cells in Fig.2). In the LSCC double-labeled propriospinal neurons simultaneously presynaptic to EUS-MNs and to BL-PPNs comprise ~22% of all EUS-PNs and ~17% of all BL-PNs [26]. Injection of the anteretrograde tracer AAV-GFP into LSCC has shown that descending axons of PNs converge into the ventral column, reach L6-S1 and form a dense arborization in the ventral horn around Onuf’s nucleus [26]. Apart from PNs which are the 2nd order neurons, there is an even greater number of 3rd order interneurons local to L3-L4 and involved in control of the LUT [26]. Assuming that after chronic SCI a synaptic input from the LSCC to L6-S1 becomes strengthened or re-organized, one may consider the LUT-related population of spinal interneurons as the anatomical substrate for post-SCI recovery of reflex voiding.
Figure 2: Trans-synaptically traced interneurons in the upper L4 of a P35 male rat. Concomitant injections of PRV-GFP in the EUS (A) and PRV-RFP in the bladder wall (B) resulted in three main types of labeling: purely green neurons included only in the EUS circuit, purely red cells included only in the bladder circuit and cells expressing green and red fluorescent proteins (yellow color in C=A+B) for cells involved in both circuits.
Studies of detailed structure and physiological properties of the LSCC are in their initial stage. Presence of LUT-related L3-L4 propriospinal neurons synaptically connected to targets in L6-S1 segments was demonstrated in juvenile rats of P18-P20 and P30-P35 [26,52]. Propriospinal neurons of the EUS circuit were also found in L3-L4 segments of adult rats and mice (unpublished observations). It is still unknown whether the LSCC is present in other species like cats, monkeys or humans. In rats some PNs not only project caudally to L6-S1, but also have axonal collaterals spreading locally in the LSCC [52]. In the spinal cord slices (in vitro preparation) PNs receive mono- or polysynaptic excitatory inputs from distances up to 500-700 µm laterally (in transverse slices) and from up to 1 mm in rostro-caudal direction (in horizontal or parasagittal slices). Inhibitory inputs to PNs in these preparations are rare and weak. Besides, evoked IPSPs faded after a few stimuli were applied in the nearby grey matter and there were no IPSPs from longer distances. The majority of EUS-PNs responded by tonic firing to intracellular depolarizing current pulses, and only a few EUS-PNs were defined as phasic or single-spiking cells. None of PNs showed pacemaking or bursting activity [52]. Thus, LSCC contains neither intrinsically bursting neurons, nor a sufficiently powerful internal inhibitory loop to form network oscillations and generate a burst-like rhythmic excitatory output. Therefore, LSCC cannot serve as a central pattern generator for inducing rhythmic bursts in EUS-MNs. It is more likely that LSCC generates a tonic excitatory barrage of various intensity modulating activity of L6-S1 circuits. This descending barrage is probably strengthened in chronic SCI animals. In post-SCI mice C-fiber afferent pathways become hyperexcitable, demonstrating decreased spike thresholds and increased firing rate of sensory neurons compared with SI mice [62,63], which increases impact of the nociceptive C-fiber input from BL to the spinal cord. The deficiency of supraspinal signaling usually leads to sprouting, enhancement of remaining synaptic contacts and creation of additional contacts within local circuits [64-67]. Hence, it is plausible that post-traumatic plastic changes of LUT-related circuits lead to significantly enhanced excitatory input from LSCC to EUS-MNs, to PPNs and probably to some local neurons in L6-S1. Our tracing experiments combined with immunolabeling by a specific marker for GABA- and glycinergic cells Pax2 have shown that only ~ 8% of all PRV-labeled EUS-related PNs in LSCC could be classified as inhibitory neurons [26]. Observation of infrequent and weak inhibitory inputs to PNs and a low percentage of inhibitory neurons among them lead to a conclusion that LSCC is sensitive to excitation, but cannot be easily inhibited. Furthermore, the percentage of Pax2+ local interneurons in the DCM was ~3%, i.e., even lower than in the LSCC [26]. The latter implies that inhibitory neurons blocking EUS-MNs activity and causing relaxation of the EUS are located not in the DCM, but somewhere else. A possible location of these neurons is the Onuf’s nucleus itself or its closest vicinity. A reason why they have not been found earlier may be the fact that viral trans-synaptic tracing does not allow reliable separation of the first order neurons (i.e., EUS-MNs) from presynaptic to them adjacent neurons of the second order if these cells packed together into in a seemingly inseparable compact cluster.
Hypothetical Spinal Circuit of EUS Bursting
We hypothesize that the burst-generating mechanism in rats as well as EUS-relaxing mechanism in cats and humans is located in L6-S1, S1-S2 and S1-S3 respectively. The proposed model (Figure 3) suggests that in healthy rats lumbo-sacral burst-generating circuit is dormant until a strong excitatory signal comes from the PMC at the peak of bladder contraction.
In this model of LUT-related spinal circuit (Figure 3), the sensory input from the bladder correlating with BL filling is delivered via Aδ sensory fibers into the dorsal horn. In a spinal intact (SI) animal it is distributed to PPNs, EUS-MNs, DCM and PMC through corresponding interneurons (Fig.3A). Since the DCM consists predominantly of excitatory interneurons presynaptic to the EUS [26], it is well suited to serve as an amplifier for sensory afferentation from the bladder as well as for the descending supraspinal excitatory input from the PMC. Therefore, during urine accumulation the DCM is increasingly activated by afferentation from the bladder. In turn, DCM sends amplified excitatory barrage to Onuf’s nucleus. An essential component of the model is the existence of a specific recurrent inhibitory loop created by axonal collaterals of EUS-MNs to some Renshaw-like inhibitory interneurons (InhINs). These InhINs can be activated only at the highest level of EUS-MNs’ tonic firing. Their axons project back onto somata or dendrites of EUS-MNs. As the voiding contraction of the BL starts, intravesical pressure rises faster and steeper than during storage, which results in sharp rise of Aδ -afferentation. The latter leads to involvement of more and more EUS-MNs and to the increase of intensity of the summated tonic firing [46,68]. We hypothesize that in SI animals at the peak of BL pressure an additional excitatory flow arrives from the PMC to DCM. After amplification by DCM this flow runs further to EUS-MNs sharply boosting their tonic firing up to the level when an inhibitory feedback loop is activated. As soon as the InhINs’ pool starts firing, all EUS-MNs become silent for a period ~100 ms, i.e., for duration of an average pop-IPSP. During this inhibitory pause the EUS is relaxed and a portion of urine is released. Without excitatory drive from EUS-MNs and without another source of excitation, all InhINs become inactive at the same time. With disappearance of feedback inhibition high intensity tonic EUS-MNs’ firing is resumed because it is persistently fed by the strong input from the DCM. However, this rebound firing of EUS-MNs cannot last long, because InhINs are synchronously activated again by the rebound volley from EUS-MNs. Repetition of the cycle creates a series of population bursts in the EMG of the EUS lasting as long as the sufficient combined drive from DCM and LSCC persists. The concept of alternating populational excitation/inhibition based on multiple random feedback inhibitory connections is widely used to explain network oscillations in the CNS. The EEG-waves in the cortex, theta-bursts in the hippocampus or rhythms in other brain structures are produced by this mechanism. In the spinal cord, networks are relatively small and clusters of specialized neurons are more compact. The consequence of this is higher frequency and more prominent expression of excitatory/inhibitory oscillations as it is observed, for instance, in studies of locomotion. It is likely, that in the LUT-related circuit with a small specialized network of a limited number of elements, expression of alternating excitation and inhibition reaches its extreme by generating succession of theta-bursts divided by periods of total silence.
Another version of this circuit implies non-uniformity of EUS-MN population. In the cat half of EUS-MNs had recurrent axonal collaterals, which terminated within Onuf’s nucleus and the ventral border of lamina VIII [69]. These EUS-MNs were significantly larger than those without collaterals. Since larger neurons usually have lower input resistance and larger rheobase, they would need stronger excitatory input to start firing spikes, and they can be recruited into firing activity later than low-threshold EUS-MNs. Thus, if such EUS-MNs are present in rats and mice, they may be responsible for activating feedback inhibition by turning on only after an additional input from PMC adds to afferentation from the BL. Without an additional input from PMC after spinal cord injury above L3-L4, the tonic activity of EUS-MNs in acute SCI rats is present, but it is insufficient to elicit InhINs and produce EUS bursting. In this case feedback inhibition cannot occur and the EUS remains contracted (Figure 3B). In later post-SCI time after development of plastic changes, the spinal LUT system becomes capable of coordinating BL and EUS muscles (Figure 3C). Reflex voiding recovers due to enhanced input from C-fibers, sprouting in L3-L4 and L6-S1, and most importantly due to enhancement of propriospinal excitatory input from the LSCC. The LSCC input does not target the DCM. Instead, it directly targets EUS-MNs though enhanced descending propriospinal connections and adds the lacking portion to insufficient excitatory input from DCM.
Thus, the model suggests that restoration of bursting in rats may occurs due to reorganized feeding of the recurrent inhibitory circuit with increased impact of propriospinal neurons. The model explains why the intact L3-L4 circuit is essential for restoration of reflexive voiding: destruction of LSCC eliminates the descending propriospinal input to EUS-MNs and does not allow activation of InhINs. This is why after transection of the spinal cord above L3-L4 voiding and EUS bursting restore, but after transection at L4 reflexive voiding cannot recover [46].
To explain why in cats and humans voiding is not accompanied by EUS bursting, the inhibitory circuit in the model should be slightly modified. In Figure 3 Dashed arrows designate hypothetical direct synapsing of InhINs by LSCC PNs. If such direct connections PNs-to-InhINs exist, there are two scenarios: (1) the LSCC input concomitantly excites both InhINs and EUS-MNs, and (2) LSCC input targets predominantly InhINs, while its inputs to EUS-MNs are weak and ineffective. In the first scenario the input from LSCC to InhINs must overwhelm the joint LSCC+DCM input to EUS-MNs. Therefore, activation of LSCC at the peak of BL pressure would induce a long-lasting block in the whole EUS-MNs’ pool. In the second scenario a sufficiently strong LSCC input just activates InhINs’ firing. In both cases suppression of EUS-MNs activity will persist until the LSCC input weakens. Thus, feedforward but not feedback inhibition will produce EUS relaxation and EUS bursting will not occur. Such modifications of the LUT neural network during evolution of species is more likely to happen than a less probable option of completely different arrangement of the LUT circuits in different species. Unfortunately, no studies of the LUT spinal circuits in human or chimpanzee have been done. Therefore, we can only assume that some features of their structure and function may be analogous to that in other mammals.
Figure 3: The model of the intraspinal circuit supporting reflex voiding in the rat. A – Neural control of the EUS in intact animal. Excitatory synaptic connections are designated by arrows and inhibitory synapses are designated by filled circles. The midbrain pontine micturition center (PMC) delivers descending signals to the dorsal commissure (DCM) in L6-S1 spinal segments. Excitatory neurons of DCM are presynaptic to parasympathetic preganglionic neurons (PPNs) located in the intermediolateral nucleus (IML) and to motoneurons of the external urethral sphincter (EUS-MNs) located in Onuf’s nucleus. Excitatory input from the bladder is polysynaptically transmitted via sensory neurons in the dorsal horn to PPNs and to DCM. When DCM is sufficiently excited by the combined input from the BL afferents and the PMC, it is capable to increase excitation level of EUS-MNs to the point of activation of IhnINs. Feedback inhibition of EUS-MN pool by InhINs interrupts tonic firing of the former, creates a short pause in their tonic firing and synchronizes the following rebound excitation in the entire Onuf’s nucleus. B – Impaired control of the EUS after SCI. Transection of the spinal cord above lumbar segments eliminates supraspinal input from the PMC. At the initial post-SCI stage afferentation from the BL is insufficient to adequately activate DCM interneurons and initiate recurrent inhibition; thinner lines of pathways designate weakened synaptic inputs from DCM to EUS-MN and from EUS-MN to InhIN. However, EUS-MNs generate persistent tonic firing sufficient to EUS contraction, so that the EUS cannot relax despite high pressure in the bladder. C – Restoration of reflexive voiding in chronic SCI animal. Enhanced descending excitatory input from LSCC directly to EUS-MNs compensates for the lack of PNC input. Enhancement of synaptic inputs is depicted by thickened lines of corresponding pathways.
The above model predicts existence of recurrent inhibition arranged by a set of interneurons located in the immediate vicinity of Onuf’s nucleus. It also suggests a reason for diversity of EUS function during voiding in different species. Complete EUS relaxation without bursting requires a powerful source of tonic excitatory input to InhINs, which in SI animals may come as a PMC signal amplified by the spinal excitatory network. EUS bursting occurs when this input is not strong enough to maintain persistent InhINs’ firing, so that InhINs require an additional excitatory input from EUS-MNs’ axonal collaterals to be activated. Both modes of activity can be realized on the same anatomical arrangement with slight changes in the LUT-related network. The difference is only in the number and efficacy of synaptic inputs from recurrent vs. direct excitation of inhibitory neurons targeting EUS-MNs. Future experiments will either confirm or reject this hypothetical organization of interneuronal LUT-related circuit.
References
2. DeLANCEY JO. Correlative study of paraurethral anatomy. Obstetrics & Gynecology. 1986 Jul 1;68(1):91-7.
3. Abelson B, Sun D, Que L, Nebel RA, Baker D, Popiel P, et al. Sex differences in lower urinary tract biology and physiology. Biology of Sex Differences. 2018 Dec;9(1):1-3.
4. Keegan KA, Nanigian DK, Stone AR. Female urethral stricture disease. Current Urology Reports. 2008 Sep 1;9(5):419-23.
5. Huisman AB. Aspects on the anatomy of the female urethra with special relation to urinary continence. InFemale Stress Incontinence 1983 (Vol. 10, pp. 1-31). Karger Publishers.
6. Yoshimura N, Chancellor MB. Neurophysiology of lower urinary tract function and dysfunction. Reviews in Urology. 2003;5(Suppl 8):S3.
7. Andersson KE, Pascual AG, Persson K, Forman A, Tøttrup A. Electrically-induced, nerve-mediated relaxation of rabbit urethra involves nitric oxide. The Journal of Urology. 1992 Jan;147(1):253-9.
8. Andersson KE, Wein AJ. Pharmacology of the lower urinary tract: basis for current and future treatments of urinary incontinence. Pharmacological Reviews. 2004 Dec 1;56(4):581-631.
9. Sergeant GP, Hollywood MA, Thornbury KD. Spontaneous activity in urethral smooth muscle. InSmooth Muscle Spontaneous Activity 2019 (pp. 149-167). Springer, Singapore.
10. Brading AF. The physiology of the mammalian urinary outflow tract. Experimental Physiology. 1999 Jan;84(1):215-21.
11. Rother P, Löffler S, Dorschner W, Reibiger I, Bengs T. Anatomic basis of micturition and urinary continence. Surgical and Radiologic Anatomy. 1996 Sep 1;18(3):173-7.
12. Callahan SM, Creed KE. Electrical and mechanical activity of the isolated lower urinary tract of the guinea-pig. British Journal of Pharmacology. 1981 Oct;74(2):353.
13. Hashitani H, Suzuki H. Properties of spontaneous Ca2+ transients recorded from interstitial cells of Cajal‐like cells of the rabbit urethra in situ. The Journal of Physiology. 2007 Sep;583(2):505-19.
14. Drumm BT, Rembetski BE, Cobine CA, Baker SA, Sergeant GP, Hollywood MA, et al. Ca2+ signalling in mouse urethral smooth muscle in situ: role of Ca2+ stores and Ca2+ influx mechanisms. The Journal of Physiology. 2018 Apr 15;596(8):1433-66.
15. de Groat WC, Yoshimura N. Pharmacology of the lower urinary tract. Annual Review of Pharmacology and Toxicology. 2001 Apr;41(1):691-721.
16. de Groat WC, Yoshiyama M, Ramage AG, Yamamoto T, Somogyi GT. Modulation of voiding and storage reflexes by activation of α1-adrenoceptors. European Urology. 1999;36(Suppl. 1):68-73.
17. de Groat WC, Yoshimura NA. Anatomy and physiology of the lower urinary tract. InHandbook of Clinical Neurology 2015 Jan 1 (Vol. 130, pp. 61-108). Elsevier.
18. Schäfer W. Some biomechanical aspects of continence function. Scandinavian Journal of Urology and Nephrology. 2001 Jan 1;35(207):44-60.
19. Blok BF, Holstege G. The central nervous system control of micturition in cats and humans. Behavioural Brain Research. 1998 May 1;92(2):119-25.
20. Danziger ZC, Grill WM. Sensory and circuit mechanisms mediating lower urinary tract reflexes. Autonomic Neuroscience. 2016 Oct 1;200:21-8.
21. De Groat WC, Wickens C. Organization of the neural switching circuitry underlying reflex micturition. Acta Physiologica. 2013 Jan;207(1):66-84.
22. Fowler CJ, Griffiths D, De Groat WC. The neural control of micturition. Nature Reviews Neuroscience. 2008 Jun;9(6):453-66.
23. Shefchyk SJ. Spinal cord neural organization controlling the urinary bladder and striated sphincter. InProgress in Brain Research 2002 Jan 1 (Vol. 137, pp. 71-82). Elsevier.
24. Shefchyk SJ. Spinal mechanisms contributing to urethral striated sphincter control during continence and micturition:“how good things might go bad”. Progress in Brain Research. 2006 Jan 1;152:85-95.
25. Nadelhaft I, Vera PL. Neurons in the rat brain and spinal cord labeled after pseudorabies virus injected into the external urethral sphincter. Journal of Comparative Neurology. 1996 Nov 18;375(3):502-17.
26. Karnup SV, De Groat WC. Mapping of spinal interneurons involved in regulation of the lower urinary tract in juvenile male rats. IBRO Reports. 2020 Dec 1;9:115-31.
27. Nadelhaft I, Vera PL. Separate urinary bladder and external urethral sphincter neurons in the central nervous system of the rat: simultaneous labeling with two immunohistochemically distinguishable pseudorabies viruses. Brain Research. 2001 Jun 8;903(1-2):33-44.
28. Nadelhaft I, Vera PL, Card JP, Miselis RR. Central nervous system neurons labelled following the injection of pseudorabies virus into the rat urinary bladder. Neuroscience Letters. 1992 Aug 31;143(1-2):271-4.
29. de Groat WC, Yoshimura N. Plasticity in reflex pathways to the lower urinary tract following spinal cord injury. Experimental Neurology. 2012 May 1;235(1):123-32.
30. Pikov V, Wrathall JR. Coordination of the bladder detrusor and the external urethral sphincter in a rat model of spinal cord injury: effect of injury severity. Journal of Neuroscience. 2001 Jan 15;21(2):559-69.
31. de Groat WC, Griffiths D, Yoshimura N. Neural control of the lower urinary tract. Comprehensive Physiology. 2011 Jan 17;5(1):327-96.
32. Stoffel JT. Detrusor sphincter dyssynergia: a review of physiology, diagnosis, and treatment strategies. Translational Andrology and Urology. 2016 Feb;5(1):127.
33. De Groat WC, Araki I, Vizzard MA, Yoshiyama M, Yoshimura N, Sugaya K, et al. Developmental and injury induced plasticity in the micturition reflex pathway. Behavioural Brain Research. 1998 May 1;92(2):127-40.
34. Sugaya K, Roppolo JR, Yoshimura N, Card JP, de Groat WC. The central neural pathways involved in micturition in the neonatal rat as revealed by the injection of pseudorabies virus into the urinary bladder. Neuroscience Letters. 1997 Feb 28;223(3):197-200.
35. Vizzard MA, Erickson VL, Card JP, Roppolo JR, de Groat WC. Transneuronal labeling of neurons in the adult rat brainstem and spinal cord after injection of pseudorabies virus into the urethra. Journal of Comparative Neurology. 1995 May 15;355(4):629-40.
36. Griffiths DJ, Fowler CJ. The micturition switch and its forebrain influences. Acta Physiologica. 2013 Jan;207(1):93-109.
37. Verstegen AM, Vanderhorst V, Gray PA, Zeidel ML, Geerling JC. Barrington's nucleus: Neuroanatomic landscape of the mouse “pontine micturition center”. Journal of Comparative Neurology. 2017 Jul 1;525(10):2287-309.
38. McFadden K, Griffin TA, Levy V, Wolfe JH, Valentino RJ. Overexpression of corticotropin‐releasing factor in Barrington’s nucleus neurons by adeno‐associated viral transduction: effects on bladder function and behavior. European Journal of Neuroscience. 2012 Nov;36(10):3356-64.
39. Valentino RJ, Wood SK, Wein AJ, Zderic SA. The bladder–brain connection: putative role of corticotropin-releasing factor. Nature Reviews Urology. 2011 Jan;8(1):19.
40. Vincent SR, Satoh K. Corticotropin-releasing factor (CRF) immunoreactivity in the dorsolateral pontine tegmentum: further studies on the micturition reflex system. Brain Research. 1984 Aug 13;308(2):387-91.
41. Hou XH, Hyun M, Taranda J, Huang KW, Todd E, Feng D, et al. Central control circuit for context-dependent micturition. Cell. 2016 Sep 22;167(1):73-86.
42. Keller JA, Chen J, Simpson S, Wang EH, Lilascharoen V, George O, et al. Voluntary urination control by brainstem neurons that relax the urethral sphincter. Nature Neuroscience. 2018 Sep;21(9):1229-38.
43. de Groat WC, Yoshimura N. Mechanisms underlying the recovery of lower urinary tract function following spinal cord injury. Progress in Brain Research. 2006 Jan 1;152:59-84.
44. Cheng CL, de Groat WC. Role of 5-HT1A receptors in control of lower urinary tract function in anesthetized rats. American Journal of Physiology-Renal Physiology. 2010 Mar;298(3):F771-8.
45. LaPallo BK, Wolpaw JR, Chen XY, Carp JS. Long-term recording of external urethral sphincter EMG activity in unanesthetized, unrestrained rats. American Journal of Physiology-Renal Physiology. 2014 Aug 15;307(4):F485-97.
46. Chang HY, Cheng CL, Chen JJ, De Groat WC. Serotonergic drugs and spinal cord transections indicate that different spinal circuits are involved in external urethral sphincter activity in rats. American Journal of Physiology-Renal Physiology. 2007 Mar;292(3):F1044-53.
47. Chang HY, Cheng CL, Chen JJ, Peng CW, De Groat WC. Reflexes evoked by electrical stimulation of afferent axons in the pudendal nerve under empty and distended bladder conditions in urethane-anesthetized rats. Journal of Neuroscience Methods. 2006 Jan 15;150(1):80-9.
48. Kadekawa K, Yoshimura N, Majima T, Wada N, Shimizu T, Birder LA, et al. Characterization of bladder and external urethral activity in mice with or without spinal cord injury—a comparison study with rats. American Journal of Physiology-Regulatory, Integrative and Comparative Physiology. 2016 Apr 15;310(8):R752-8.
49. D'Amico SC, Collins III WF. External urethral sphincter motor unit recruitment patterns during micturition in the spinally intact and transected adult rat. Journal of Neurophysiology. 2012 Nov 1;108(9):2554-67.
50. Carro-Juárez M, Rodríguez-Manzo G. Evidence for the presence of the spinal pattern generator involved in the control of the genital ejaculatory pattern in the female rat. Brain Research. 2006 Apr 21;1084(1):54-60.
51. Dolber PC, Gu B, Zhang X, Fraser MO, Thor KB, Reiter JP. Activation of the external urethral sphincter central pattern generator by a 5-HT1A receptor agonist in rats with chronic spinal cord injury. American Journal of Physiology-Regulatory, Integrative and Comparative Physiology. 2007 Apr;292(4):R1699-706.
52. Karnup SV, de Groat WC. Propriospinal Neurons of L3-L4 Segments Involved in Control of the Rat External Urethral Sphincter. Neuroscience. 2020 Jan 15;425:12-28.
53. Laliberte AM, Goltash S, Lalonde NR, Bui TV. Propriospinal neurons: essential elements of locomotor control in the intact and possibly the injured spinal cord. Frontiers in Cellular Neuroscience. 2019;13:512.
54. Cowley KC, Zaporozhets E, Schmidt BJ. Propriospinal transmission of the locomotor command signal in the neonatal rat. Annals of the New York Academy of Sciences. 2010 Jun;1198(1):42-53.
55. Jordan LM, Schmidt BJ. Propriospinal neurons involved in the control of locomotion: potential targets for repair strategies?. InProgress in Brain Research 2002 Jan 1 (Vol. 137, pp. 125-139). Elsevier.
56. Courtine G, Song B, Roy RR, Zhong H, Herrmann JE, Ao Y, et al. Recovery of supraspinal control of stepping via indirect propriospinal relay connections after spinal cord injury. Nature Medicine. 2008 Jan;14(1):69-74.
57. Cowley KC, MacNeil BJ, Chopek JW, Sutherland S, Schmidt BJ. Neurochemical excitation of thoracic propriospinal neurons improves hindlimb stepping in adult rats with spinal cord lesions. Experimental Neurology. 2015 Feb 1;264:174-87.
58. Gerasimenko Y, Roy RR, Edgerton VR. Epidural stimulation: comparison of the spinal circuits that generate and control locomotion in rats, cats and humans. Experimental Neurology. 2008 Feb 1;209(2):417-25.
59. Tohda C, Kuboyama T. Current and future therapeutic strategies for functional repair of spinal cord injury. Pharmacology & Therapeutics. 2011 Oct 1;132(1):57-71.
60. Abud EM, Ichiyama RM, Havton LA, Chang HH. Spinal stimulation of the upper lumbar spinal cord modulates urethral sphincter activity in rats after spinal cord injury. American Journal of Physiology-Renal Physiology. 2015 May 1;308(9):F1032-40.
61. Marson L, Gravitt K. Spinal neurons activated with the urethrogenital reflex in the male rat. Brain Research. 2004 Nov 5;1026(1):108-15.
62. Kadekawa K, Majima T, Shimizu T, Wada N, de Groat WC, Kanai AJ, et al. The role of capsaicin-sensitive C-fiber afferent pathways in the control of micturition in spinal-intact and spinal cord-injured mice. American Journal of Physiology-Renal Physiology. 2017 Sep 1;313(3):F796-804.
63. Takahashi R, Yoshizawa T, Yunoki T, Tyagi P, Naito S, De Groat WC, et al. Hyperexcitability of bladder afferent neurons associated with reduction of Kv1. 4 α-subunit in rats with spinal cord injury. The Journal of Urology. 2013 Dec;190(6):2296-304.
64. Bareyre FM, Kerschensteiner M, Raineteau O, Mettenleiter TC, Weinmann O, Schwab ME. The injured spinal cord spontaneously forms a new intraspinal circuit in adult rats. Nature Neuroscience. 2004 Mar;7(3):269-77.
65. Tsujioka H, Yamashita T. Comparison of gene expression profile of the spinal cord of sprouting-capable neonatal and sprouting-incapable adult mice. BMC Genomics. 2019 Dec 1;20(1):619.
66. Liu J, Yang X, Jiang L, Wang C, Yang M. Neural plasticity after spinal cord injury. Neural Regeneration Research. 2012 Feb 15;7(5):386-391.
67. Flynn JR, Dunn LR, Galea MP, Callister R, Callister RJ, Rank MM. Exercise training after spinal cord injury selectively alters synaptic properties in neurons in adult mouse spinal cord. Journal of Neurotrauma. 2013 May 15;30(10):891-6.
68. Thor KB, de Groat WC. Neural control of the female urethral and anal rhabdosphincters and pelvic floor muscles. American Journal of Physiology-Regulatory, Integrative and Comparative Physiology. 2010 Aug;299(2):R416-38.
69. Sasaki M. Morphological analysis of external urethral and external anal sphincter motoneurones of cat. Journal of Comparative Neurology. 1994 Nov 8;349(2):269-87.