Abstract
Augmentation cystoplasty (AC) is a surgical procedure for patients with refractory neurogenic bladder (NB). This study aimed to evaluate urinary incontinence (UI), vesicoureteral reflux (VUR), and urodynamic parameters in patients’ post-AC and compare these results to preoperative data.
Methods: In this retrospective cohort study, 42 consecutive patients with refractory NB underwent AC between February 2009 and March 2023, with a minimum follow-up of one year. Evaluated parameters included UI, VUR, maximum cystometric capacity (MCC), expected bladder capacity (EBC), bladder compliance, and end-filling detrusor pressure (EFP).
Results: Among 22 eligible patients (50% male, median age 12 years), 77.3% underwent ileocystoplasty with Mitrofanoff. Preoperatively, UI was diagnosed in 81.8% of patients, with complete resolution observed in 83.3% post-AC (p<0.001). Preoperative VUR was present in 31.8% of patients, and post-AC, high-grade VUR resolved in 90.9–100% (p=0.001). Median MCC increased from 120 mL to 405 mL (p<0.001), with EBC normalizing in 85.7% of cases (p<0.001). Bladder compliance improved from 6.4 to 38.3 mL/cmH?O (p<0.001), while median end-filling detrusor pressure decreased from 20.0 to 11.0 cmH?O (p<0.001).
Conclusions: After AC, children and adolescents with refractory NB showed significant improvement in UI, VUR, MCC/EBC, bladder compliance, and EFP.
Keywords
Augmentation cystoplasty, Neurogenic bladder, Urinary incontinence, Vesicoureteral reflux, Urodynamics
Abbreviations
AC: Augmentation Cystoplasty; CIC: Clean Intermittent Catheterization; EFP: End-filling Detrusor Pressure; ICCS: International Children's Continence Society (ICCS); MCC: Maximum Cystometric Capacity; NB: Neurogenic Bladder; Pdet: Detrusor Pressure; RBUS: Renal and Bladder Ultrasound; UI: Urinary Incontinence; UTIs: Urinary Tract Infections; UDS: Urodynamic Study; VUR: Vesicoureteral Reflux; VCUG: Voiding Cystourethrography
Introduction
Neurological disorders can impact the function of the bladder and/or sphincter, leading to neurogenic bladder (NB). Neural tube defects, especially spina bifida, are the primary cause of NB in children and adolescents [1-3]. NB is characterized by the inability to fill under low pressure and periodic emptying, which can lead to complications such as urinary incontinence, recurrent urinary tract infections (UTIs), secondary vesicoureteral reflux (VUR), reduced bladder capacity and compliance, significantly high detrusor pressure, and increased risk of kidney damage [1-5]. The initial approach involves clean intermittent catheterization (CIC) and anticholinergic medication. If the first-line therapy fails, botulinum toxin A may be used, before considering augmentation cystoplasty (AC) [1-4].
AC is a surgical procedure used in adults and children with refractory NB. The main goal of AC is to create a bladder reservoir with low pressure and high capacity to preserve kidney function and improve urinary incontinence and quality of life for patients and families [1,2,5,6]. The standard procedures for bladder augmentation are ileocystoplasty and colocystoplasty [5,7]. The ileum and colon are preferred due to their long segments and adequate distensibility [7]. In ileocystoplasty, a 20 to 30 cm segment of the terminal ileum, located at least 15 cm from the ileocecal valve, is removed. In colocystoplasty, the sigmoid colon is often used because of its vascularization and mobility, and a 15 to 20-cm segment of it is removed. These segments are reshaped into a patch based on the surgeon's preference after being detubularized. The bladder is then opened either sagittally or transversely. The detubularized ileal or colonic patch is then connected to the bladder with absorbable sutures [6]. Furthermore, AC may encompass the development of a catheterizable conduit utilizing either the vermiform appendix or the small intestine [8].
A recent systematic review conducted by our group showed improvements in urinary incontinence (UI), end-filling detrusor pressure (EFP), maximum cystometric capacity (MCC), expected bladder capacity (EBC), bladder compliance, and end-filling detrusor pressure (EFP), in children and adolescents with refractory NB undergoing AC [9]. However, the review included only a small number of studies, and the data were heterogeneous. Therefore, this study aimed to evaluate UI, VUR, and urodynamic parameters in patients after AC and compare these results to the preoperative data.
Methods
Ethical approval
The institution's Ethics Committee approved the study under protocol CAAE 48678721600005149 (position statement: 4,874,121). Legal guardians and participants aged 10 to 17 signed the informed consent and assent forms. After obtaining consent from the institution and signing the data use commitment form, the medical records were selected through an active search in the medical service and archives.
Study design and patients
This retrospective cohort study involved 42 consecutive patients with refractory NB who underwent AC by the same surgeon at the same institution between February 2009 and March 2023. Of these 42 patients, seven with non-neurogenic lower urinary tract anomalies and 13 who had previously undergone vesicostomy were excluded from the study. Thus, 22 patients aged between seven and 19 years with NB caused by neurogenic urinary tract anomalies, followed for at least one year after the procedure, were eligible for the study.
Study protocol
The parameters evaluated in this study, pre- and post-AC, with follow-up for at least one year, included UI, VUR, and urodynamic data, such as MCC, EBC, bladder compliance, and EFP.
The pre-AC assessment followed a systematic protocol, which included a detailed anamnesis, clinical presentation, serial laboratory tests (blood gas, serum levels of creatinine, urea, electrolytes, urinalysis, urine culture), and imaging and radiologic tests performed at our institution. These tests included renal and bladder ultrasound (RBUS), voiding cystourethrography (VCUG), and a urodynamic study (UDS).
Any loss of urine through the urethra was considered UI. A bladder diary assessed daytime UI between CIC intervals when applicable, urinary loss during sleep, and UI improvement post-AC. The improvement of UI was evaluated using the International Children's Continence Society (ICCS) criteria [10]. Initial success was defined as no response (<50% reduction in symptoms), partial response (50% to 99% reduction in symptoms), and complete response (absent UI). For long-term success, relapse was defined as more than one recurrence of UI per month, and continued success was defined as no relapses within the 6th, and 12th months post-AC.
VUR was assessed using VCUG and categorized into grades I-V based on the system proposed by the Reflux Study Committee [11]. Reflux was further categorized as low-grade (I-II) and high-grade (III-V) based on the reflux grade [12]. An improvement was defined as any reduction in the grade of VUR or its absence.
In the analysis of urodynamic data, MCC was measured in ml. EBC was calculated using the Koff formula: 30 × (age in years + 1) for children and adolescents aged four to 12 years, while it is set at 390 mL for older patients [13]. The EBC was expressed as a percentage. Low bladder capacity is defined as less than 65% of the EBC, while average capacity ranges from 65% to 150%. Bladder compliance was calculated as the change in volume (ΔV) divided by the change in detrusor pressure (ΔPdet), expressed in ml/cmH2O. Compliance is considered altered if below 15 ml/cmH2O [10]. The EFP measures Pdet at maximum cystometric capacity, ideally increasing by up to 10 cmH2O [14]. Thus, the criteria for improving urodynamic parameters include EBC > 65%, bladder compliance > 15 ml/cmH2O, and EFP < 10 cmH2O.
Patients were evaluated daily post-AC during hospitalization and had follow-up appointments in the 1st, 3rd, 6th, and 12th months after discharge. The bladder diary containing UI data was reviewed during all post-AC follow-up appointments. RBUS and VCUG were performed post-AC in the 3rd month and UDS in the 6th month.
Statistical analysis
A descriptive analysis of numerical variables was conducted to characterize the sample using central tendency and variability measures. The mean and standard deviation were calculated for normally distributed variables, while the median and quartiles were used for asymmetric ones. Frequency distribution tables displayed absolute and relative frequencies for categorical variables. Normality was tested with the Shapiro-Wilk test.
When comparing pre- and post-AC results, the paired t-test and the Wilcoxon test were used for numerical variables with normal distribution (bladder compliance) and non-normal distribution (MCC/EBC and EFP), respectively. The McNemar test was used for nominal categorical variables (altered bladder compliance, altered EBC, and UI). The Marginal Homogeneity test was used for the ordinal categorical variable VUR.
For all analyses, a p-value <0.05 was considered statistically significant. Statistical analysis was performed using IBM SPSS Statistics version 21.0 (IBM Corp, Armonk, NY, USA)
Results
Of the 22 eligible patients, 50% were male, and the median age was 12 years (range 9 to 16), at the time of AC. The NB etiology was spina bifida in 81.2% (18/22) of patients. In 77.3% (17/22) patients, the AC technique performed was ileocystoplasty with Mitrofanoff.
Pre-AC UI was diagnosed in 81.8% (18/22) of patients, with complete resolution in 83.3% (15/18) of these post-AC patients (p<0.001) (Table 1)
|
|
Pre-AC |
Post-AC |
p- value |
|
UI |
|||
|
Absent |
4 (18.2) |
19 (86.4) |
<0.001 a |
|
Present |
18 (81.8) |
3 (13.6) |
|
|
Right VUR |
|||
|
Absent |
19 (86.4) |
22 (100) |
<0.001 b |
|
Low-grade |
2 (9.1) |
0 (0) |
|
|
High-grade |
1 (4.5) |
0 (0) |
|
|
Left VUR |
|||
|
Absent |
15 (68.2) |
20 (90.9) |
0.012 b |
|
Low-grade |
1 (4.5) |
2 (9.1) |
|
|
High-grade |
6 (27.3) |
0 (0) |
|
|
UI: Urinary Incontinence; AC: Augmentation Cystoplasty; VUR: Vesicoureteral Reflux a McNemar test. b Marginal Homogeneity test |
|||
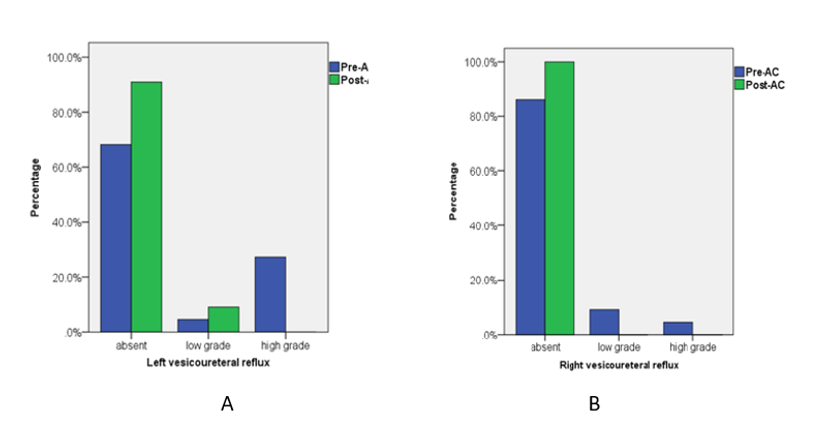
VUR was found in 31.8% (7/22) of patients (10 kidney units) pre-AC. None of them underwent ureteral reimplantation. In post-AC, left and right high-grade VUR (III-V) were resolved in 90.9% of cases (p=0.01) and 100% (p=0.001) of the patients, respectively (Table 1 and Figures 1A and 1B).
The urodynamic findings are detailed in Table 2. The median MCC increased from 120 ml pre-AC to 405 ml post-AC (p<0.001). The EBC normalized in 85.7% (18/21) of patients’ post-AC (p<0.001). There was a significant increase in bladder compliance from 6.4 ml/cmH2O pre-AC to 38.3 ml/cmH2O post-AC (p<0.001). The median values of the EFP decreased from 20.0 (13.8 -27.8) cmH2O pre-AC to 11 (9-12.6) cmH2O post-AC (p<0.001).
|
Urodynamic parameters |
Pre-AC |
Post-AC |
p-value |
|
MCC (ml) |
|||
|
Median (P25 e P75) |
120 (97.5 – 165) |
405 (375 – 427.5) |
<0.001c |
|
% EBC |
|||
|
Median (P25 - P75) |
35 (25 – 50) |
109.6 (99.4 – 127.4) |
<0.001 c |
|
Normal (66 a 149 EBC) n (%) |
1 (4.5) |
19 (86.4) |
<0.001 b |
|
Altered (≤ 65 ou ≥ 150 EBC) n (%) |
21 (95.5) |
3 (13.6) |
|
|
Compliance ml/cmH2O |
|||
|
Mean ± SD |
6.4 ± 2.1 |
38.3 ± 8.4 |
<0.001a |
|
Normal (≥15) n (%) |
0 (0) |
22 (100) |
<0.001b |
|
Altered (<15) n (%) |
22 (100) |
0 (0) |
|
|
EFP cmH2O |
|||
|
Median (P25 - P75) |
20 (13.8 – 27.8) |
11 (9 – 12.6) |
<0.001c |
|
AC: Augmentation Cystoplasty; MCC: Maximum Cystometric capacity; EBC: Expected Bladder Capacity; SD: Standard Deviation; EFP: End -Filling Detrusor Pressure a Paired t- test; b McNemar test; c Wilcoxon test |
|||
Discussion
This study compared the pre- and post-AC in 22 children and adolescents with refractory NB, primarily due to spina bifida. The parameters analyzed were UI, VUR, and urodynamic parameters (MCC/EBC, bladder compliance, and EFP) over at least one year post-AC. All patients underwent ileocystoplasty, associated with Mitrofanoff in 77.3% of cases, and there was an improvement in all evaluated parameters. These findings are consistent with a recent systematic review [9].
One of AC's goals is to achieve urinary continence [1,2,4-6]. The presence of UI in children and adolescents with NB is associated with reduced self-esteem, social interaction challenges, and vulnerability to bullying [15]. Non-surgical treatment may achieve urinary continence in 44% of cases [16]. In the analysis of this series, we observed a pre-AC UI rate of 81.8% (18/22) and complete resolution in 83.3% (15/18) of post-AC patients. Our findings were consistent with other studies that performed AC alone and observed improvement in UI in 76.5% to 100% of patients [9,15,17-19]. The systematic review by Johnston et al. [16] presented slightly lower results (64%) for UI complete resolution of post-AC in these patients. The treatment of VUR in NB patients, particularly those undergoing AC, remains debated. It is unclear which patients need ureteral reimplantation. Continued VUR in bacteriuric patients raises concerns about pyelonephritis [20], while complications from ureteral reimplantation [21] question the need for the procedure during AC. This study diagnosed VUR in 31.8% of patients, and resolution was achieved in 90.9% to 100% of high-grade VUR cases post-AC without ureteral reimplantation. Chiba et al. [22] and Zhang et al. [21] demonstrate results in agreement with our findings, showing resolution of VUR in 83 to 85% of pediatric patients with NB after AC, without ureteral reimplantation. Some authors suggest that NB patients with high-grade VUR and low-pressure VUR could benefit from AC concomitantly with ureteral reimplantation [20,23].
Our urodynamic findings showed improvements in all evaluated parameters. The increase in MCC/EBC was consistent with the findings of Chang et al. [24], who reported a rise in EBC from 53.2 ± 27.4% pre-AC to 110.6 ± 22.4% after AC. Likewise, Zaragoza et al. [25] observed an improvement in EBC from 52.8 ± 20.1% pre-AC to 95.9 ± 8.8% post-AC. Although the term for this surgical reconstruction of the bladder suggests an increase in the reservoir's anatomical capacity, this urodynamic parameter is not the most critical factor influencing surgical outcomes. Instead, bladder filling pressure and compliance are the vital urodynamic factors for assessing bladder storage [18]. McGuire et al. [26] showed that detrusor pressure values > 40 cm H2O can lead to upper urinary tract injuries.
Our series demonstrated a 100% recovery of urodynamic data regarding bladder compliance and achieved an 85.7% success rate for EFP outcomes. Similar findings regarding these urodynamic outcomes have been identified in other studies. Zaragoza et al. [25] found a variation in compliance pre- and post-AC from 4.6 ± 3.2 to 41.3 ± 4.3 ml/cmH2O and a variation in EFP from 40.8 ± 18.9 to 11 ± 8.9 cmH2O. Chang et al. [24] reported that the detrusor pressure measured during cystometric capacity, referred to as EFP, showed a median value change from 21 (10 –55) cmH2O pre-AC to 11 (8 – 20) cmH2O post-AC in this series. In one of the most extensive published series on AC with long-term evaluation, Wang et al. [23] showed a variation in EFP from 38.0 ± 28.6 pre-AC to 14 ± 9.2 cmH2O post-AC. Sun et al. [7] observed lower bladder pressure results, particularly for augmentations performed using bladder auto-augmentation and ureterocystoplasty techniques.
This study has limitations that should be acknowledged. First, it employs a retrospective design, which may impact the reliability of the findings. Additionally, the small sample size restricts the generalizability of the findings. It’s worth noting that all surgeries included in this study were performed by the same surgeon at the same institution, and the same examiners conducted the follow-up exams. This consistency enhances the reliability of the data analysis.
Conclusions
AC effectively normalized urodynamic parameters in children and adolescents with refractory NB who did not respond to clinical treatments. Specifically, it improved MCC/EBC, bladder compliance, and EFP. These urodynamic enhancements positively impacted the resolution of UI and VUR. More extensive long-term prospective studies are needed to better evaluate outcomes after AC in these patients.
Acknowledgments
We would like to express our heartfelt gratitude to the patients and their guardians who have been cared for in our pediatric urology service. Your willingness to allow us access to your data has been essential for us to carry out this study.
Ethical Approval
The institution's Ethics Committee approved the study under protocol CAAE 48678721600005149 (position statement: 4,874,121). Legal guardians and participants aged 10 to 17 signed the informed consent and assent forms. After obtaining consent from the institution and signing the data use commitment form, the medical records were selected through an active search in the medical service and archives.
Funding Source
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Conflict of Interest Statement
The authors have no conflicts of interest relevant to this article to disclose.
Financial Disclosure Statement
The authors have no financial relationships relevant to this article to disclose.
Data Availability Statement
The data supporting this study's findings are available from the corresponding author and the first author. Data will be made available upon reliable request.
References
2. Brownrigg N, Lorenzo AJ, Rickard M, Dos Santos J. The urological evaluation and management of neurogenic bladder in children and adolescents—what every pediatric nephrologist needs to know. Pediatric Nephrology. 2024 Feb;39(2):409-21.
3. Sturm RM, Cheng EY. The management of the pediatric neurogenic bladder. Current bladder dysfunction reports. 2016 Sep;11:225-33.
4. Li Y, Stern N, Wang PZ, Braga LH, Dave S. Systematic review and meta-analysis to study the outcomes of proactive versus delayed management in children with a congenital neurogenic bladder. Journal of Pediatric Urology. 2023 Dec 1;19(6):730-41.
5. Mehmood S, Alhazmi H, Al-Shayie M, Althobity A, Alshammari A, Altaweel WM, et al. Long-term outcomes of augmentation cystoplasty in a pediatric population with refractory bladder dysfunction: a 12-year follow-up experience at single center. International Neurourology Journal. 2018 Dec 31;22(4):287-94.
6. Tran WT, Boxley PJ, Wilcox DT, Vemulakonda VM, Wood D, Rove KO. Retrospective analysis of bladder perforation risk in patients after augmentation cystoplasty using an extraperitoneal approach. Journal of Pediatric Urology. 2023 Apr 1;19(2):192-e1-8.
7. Sun XG, Wang RY, Xu JL, Li DG, Chen WX, Li JL, et al. Surgical outcomes of bladder augmentation: A comparison of three different augmentation procedures. World journal of clinical cases. 2020 Aug 6;8(15):3240.
8. Mitrofanoff P: Cystostomie continente trans-appendiculaire dans le traitement des vessies neurologiques [Trans-appendicular continent cystostomy in the management of the neurogenic bladder]. Chir Pediatr. 1980;21:297-30
9. Reis OAF, Ito HN, de Oliveira Otávio J, de Oliveira Filho DJ, Lima EM, de Bessa J Jr, et al. Clinical and urodynamic findings in children and adolescents with neurogenic bladder undergoing augmentation cystoplasty: a systematic review. Pediatr Nephrol. 2025 Feb;40(2):355-65.
10. Austin PF, Bauer SB, Bower W, Chase J, Franco I, Hoebeke P, et al. The standardization of terminology of lower urinary tract function in children and adolescents: Update report from the standardization committee of the International Children's Continence Society. Neurourology and urodynamics. 2016 Apr;35(4):471-81.
11. Lebowitz RL, Olbing H, Parkkulainen KV, Smellie JM, Tamminen-Möbius TE. International system of radiographic grading of vesicoureteric reflux. Pediatric radiology. 1985 Feb;15:105-9.
12. Naseri M, Karimi M, Bakhtiari E, Tafazoli N, Alamdaran SA, Tafazoli N. Diagnostic values of kidney ultrasonography for vesicoureteral reflux (VUR) and high grade VUR. Iran J Kidney Dis. 2021 Sep 1;15(5):328-35.
13. Koff SA. Estimating bladder capacity in children. Urology. 1983 Mar 1;21(3):248.
14. Nevéus T, von Gontard A, Hoebeke P, Hjälmås K, Bauer S, Bower W, et al. The standardization of terminology of lower urinary tract function in children and adolescents: report from the Standardisation Committee of the International Children’s Continence Society. The Journal of urology. 2006 Jul;176(1):314-24.
15. Dave S, Gandhi K, Clark J, Davidson J, Welk B, Wang PZ. Results of a defined surgical protocol for treating pediatric neurogenic bladder incontinence in a single institution. Journal of Pediatric Urology. 2024 Jan 1;20:S74-80.
16. Johnston AW, Wiener JS, Purves JT. Pediatric neurogenic bladder and bowel dysfunction: will my child ever be out of diapers?. European urology focus. 2020 Sep 15;6(5):838-67.
17. Medel R, Ruarte AC, Herrera M, Castéra R, Podestá ML. Urinary continence outcome after augmentation ileocystoplasty as a single surgical procedure in patients with myelodysplasia. The Journal of urology. 2002 Oct;168(4 Part 2):1849-52.
18. Sinha S, Shah M. Augmentation cystoplasty in children with stages III and IV chronic kidney disease secondary to neurogenic bladder. Asian Journal of Urology. 2022 Jul 1;9(3):313-7.
19. Venn SN, Mundy AR. Long-term results of augmentation cystoplasty. European urology. 1998 Aug 1;34(Suppl. 1):40-2.
20. Soygur T, Burgu B, Zümrütbas A, Süer E. The need for ureteric re‐implantation during augmentation cystoplasty: video‐urodynamic evaluation. BJU international. 2010 Feb;105(4):530-2.
21. Zhang HC, Yang J, Ye X, Hu HF. Augmentation enterocystoplasty without reimplantation for patients with neurogenic bladder and vesicoureteral reflux. The Kaohsiung Journal of Medical Sciences. 2016 Jun 1;32(6):323-6.
22. Chiba H, Kitta T, Higuchi M, Kusakabe N, Kon M, Nakamura M, et al. Ureteral reimplantation during augmentation cystoplasty is not needed for vesicoureteral reflux in patients with neurogenic bladder: a long-term retrospective study. BMC urology. 2022 Mar 29;22(1):48.
23. Wang Z, Liao L. Effectiveness and complications of augmentation cystoplasty with or without nonrefluxing ureteral reimplantation in patients with bladder dysfunction: a single center 11-year experience. Journal of Urology. 2018 Jan 1;199(1):200-5.
24. Chang JW, Kuo FC, Lin TC, Chin TW, Yang LY, Chen HH, et al. Long-term complications and outcomes of augmentation cystoplasty in children with neurogenic bladder. Scientific reports. 2024 Feb 20;14(1):4214.
25. Zaragoza-Torres RI, Galarza-Flores ME, Gómez-Castellanos JC, Barrera-de León JC. Urodynamic changes after bladder augmentation surgery in paediatric patients with myelomeningocele due to neurogenic bladder. Cirugía y Cirujanos (English Edition). 2016 Mar 1;84(2):115-20.
26. McGuire EJ, Woodside JR, Borden TA, Weiss RM. Prognostic value of urodynamic testing in myelodysplastic patients. The Journal of urology. 1981 Aug 1;126(2):205-9.