Abstract
Raynaud's phenomenon represents a harbinger of polyautoimmunity and although it is usually associated with systemic sclerosis, the discussion surrounding its classification in rheumatology is interesting given the current understanding of capillaroscopic findings in rheumatic diseases and improvements in immunological diagnostics. We present the case of a young woman who presented with biphasic Raynaud's phenomenon (pallor, cyanosis) associated with polyarthralgia affecting small joints of hands and feet in the context of immunological profile suggestive of undifferentiated connective tissue disease (ANA -anti-nuclear antibodies- 1:1280, mottled pattern and anti-RNP -anti-U1 ribonucleoprotein- at high titers: >200). Considering personal history of cohesive silicone buttock implants in 2012, a probable case of ASIA (Autoimmune/inflammatory Syndrome Induced by Adjuvants) syndrome was considered, given the presence of one major and two minor criteria. However, a debate arises regarding the clinical spectrum of mixed connective tissue disease and the controversial ASIA syndrome.
Keywords
Raynaud's phenomenon, Autoimmunity, Biopolymers, Silicone implants, Autoimmune/inflammatory syndrome induced by adjuvants
Introduction
Raynaud's phenomenon (RP) corresponds to an exaggerated, paroxysmal, and sometimes recurrent vasospastic response of digital arteries and subcutaneous arteriovenous shunts triggered by cold or emotional stress, characterized by the presence of reversible changes in the coloration of the fingers with or without increased sensitivity to cold. Described by Maurice Raynaud in 1862, it affects about 3-5% of the general population. Its prevalence and incidence are estimated at 4.8% and 0.25%, respectively [1].
Most patients with primary RP are young women without previous pathology or comorbidities, with a family history in about half of the cases and early debut (usually between 15-30 years). There is also a group of patients (10-20%) in whom there is an associated condition, which is called secondary RP. It has been described that up to 14% of cases of primary RP can progress to secondary RP [1,2]. Once RP is confirmed, establishing the previously described difference implies taking an adequate clinical history together with a complete autoimmunity profile, in addition to the analysis by capillaroscopy.
At the same time, it is necessary to distinguish RP from other vascular acrosyndromes, defined in turn as any vasospastic condition that induces alterations in the circulating microcirculation of the extremities following temperature changes, such as acrocyanosis, erythromelalgia and livedo [3]. On the other hand, the autoimmune/inflammatory syndrome induced by adjuvants (ASIA) has gained relevance, referring to a group of manifestations associated with exposure to exogenous substances (squalene, aluminum hydroxide and silicone, among others) which have in common the generation of non-specific autoantibodies after the loss of immune tolerance [4]. The onset of RP can be a manifestation of Autoimmune/inflammatory Syndrome Induced by Adjuvants (ASIA), an encompassing term that comprises clinical manifestations precipitated by the immune response to adjuvants like medical implants, metals, or vaccines.
Raynaud's phenomenon resulting from molecular mechanisms activated in response to environmental stressors [3,4]. Classically, three phases have been described: pallor (vasoconstriction), cyanosis (presence of deoxyhemoglobin) and hyperemia (reflex vasodilatation), which occur in order as changes in blood flow occur. However, sometimes only the first two phases are present, being considered a biphasic pattern. Each attack can last from several minutes to hours, with a clear demarcation of color changes both in the volar and dorsal aspect of extremities (it can affect nose, ears and even tongue) associated with other vasospastic phenomena such as migraine crisis, irritable bowel syndrome and chest pain of microvascular origin [1-3].
Case Report
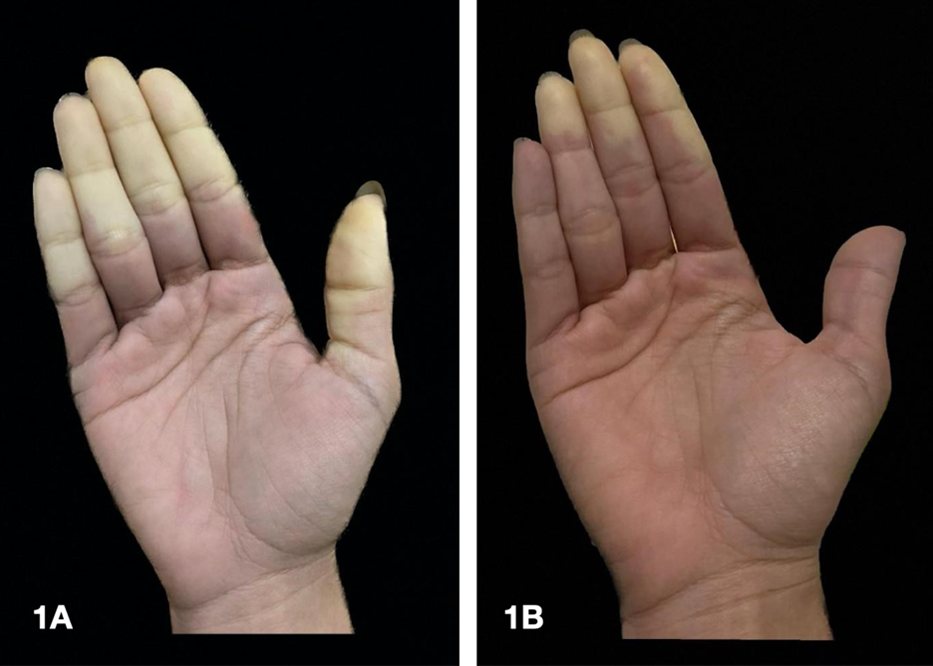
We present the case of a 26-year-old woman with a past medical history of smoking and silicone buttock implants in 2012, who consulted for three months of color changes in fingers of both hands, compatible with biphasic RP (pallor, cyanosis) (Figure 1). Concomitantly, acral dysesthesias during the episodes and arthralgias worsened with rest in small joints of hands and feet. On review by systems, she did not report dysphagia, dyspnea or lesions or changes in skin texture. Autoimmunity studies showed positivity for antinuclear antibodies (ANA) 1:1280 speckled pattern and anti-RNP at high titers (>200); the rest of specific antibodies (anti-Sm 3.8, anti-Ro 3.4, anti-La 0.1) and antiphospholipid antibodies were negative (lupus anticoagulant, anticardiolipin IgG/IgM and beta 2-glycoprotein IgG/IgM). Capillaroscopy was normal. Considering personal history of cohesive silicone buttock implants in 2012, a probable case of ASIA syndrome was considered, given the presence of one major and two minor criteria [4] (Table 1).
|
Major criteria |
- Myalgia, myositis or muscle weakness - Arthralgia and/or arthritis - Chronic fatigue, non-restorative sleep or sleep disturbances - Neurological manifestations (especially associated with demyelination) - Cognitive impairment, memory loss - Fever, xerostomia
|
|
Minor criteria |
|
Figure 1. First phase of RP or "white attack", manifested as pallor secondary to vasoconstriction and ischemia (A); this first phase can last between 15 and 20 minutes. It usually starts in the second or fourth finger and then occurs in the others. Then comes the phase of tissue hypoxia (cyanotic phase or "blue attack") and finally, with warming, a third phase (of variable occurrence), characterized by tissue reperfusion (red coloration) (B). Source: Authors' personal file.
The treatment started with nifedipine 30 mg/day, acetylsalicylic acid 100 mg/day and use of gloves. Other studies (transthoracic echocardiogram, high resolution chest tomography and upper gastrointestinal endoscopy) showed no relevant findings. Rheumatology started hydroxychloroquine: 200 mg/day due to the suspicion of ASIA syndrome in the spectrum of undifferentiated connective tissue disease. The removal of gluteal prostheses was suggested as part of the comprehensive medical management; however, the patient expressed disagreement. At one-year follow-up, the patient showed no clinical manifestations indicative of a specific autoimmune disease. The intensity of the RP diminished, yet it persisted.
Discussion
Among 80-90% of cases correspond to primary RP, scenarios in which the cold or emotional stimulus induces catecholamine-dependent vasoconstriction through alpha 2C adrenergic receptors in muscle smooth cells [2,5]. The remaining percentage is due to secondary causes, involving underlying diseases in addition to exposure to some drugs mediated by molecular changes that translate into vascular phenomena manifested at three different levels: vasoconstriction (increase in endothelin 1, decrease in nitric oxide with respective decrease in cyclic guanosine monophosphate -cGMP-, responsible for reducing intracellular calcium levels in smooth muscle cells and therefore, vascular tone), vascular proliferation through a migration process of apoptosis and attenuation of apoptosis mechanisms (mediated by vascular endothelial growth factor -VEGF- and its receptor VEGF-R2) and increased platelet aggregation (increase in availability of von Willebrand factor and decreased adenosine reuptake] [6].
In the secondary RP scenario, persistent digital ischemia can lead to ulcer formation and digital necrosis [6,7]. Aspects such as symmetry, age of onset and others such as the absence of pathological findings in capillaroscopy make the presence of a secondary cause less likely, with a negative predictive value that can reach 93% [5-7].
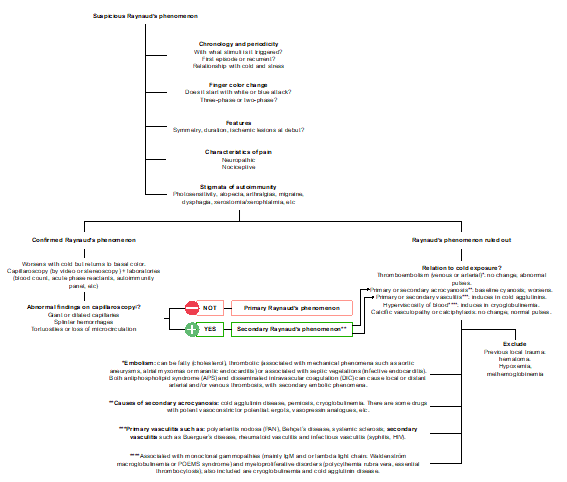
Diagnosis requires an affirmative answer to these three questions: are your fingers sensitive to cold, do they change color when exposed to cold, and do they turn white or blue? In the international consensus criteria for the diagnosis of RP published in 2014 [7] it was agreed that at least biphasic color change must be present to establish the diagnosis: white (pallor) and blue (cyanosis). Capillaroscopy allows the study of findings compatible with "sclerodermiform pattern": presence of dilated "giant" capillaries with an apical diameter > or equal to 50 µm, abnormal architecture, microhemorrhages and an extremely low number of capillaries; these microvascular structural alterations can be classified as early, active, and late changes [8]. Therefore, this study should guide the clinical approach to any case of Raynaud's phenomenon (Figure 2). The main secondary causes of RP are listed below (Table 2).
|
Secondary cause of RP |
Specific etiology |
|
Large vessel (often proximal and asymmetric involvement) |
Compressive (cervical rib, thoracic operculum) |
|
Inflammatory vascular disease (thromboangiitis obliterans or large vessel vasculitis: Takayasu, extracranial giant cell arteritis). |
|
|
Accelerated atherosclerosis (up to 60% of cases in those over 60 years of age; rule out embolism due to cholesterol crystals). |
|
|
Occupational |
Hand-arm vibration syndrome |
|
Hypothenar hammer syndrome |
|
|
Autoimmune disorders |
Systemic sclerosis (up to 90% of cases, especially in a limited variety). |
|
Systemic lupus erythematosus (10-45%) |
|
|
Sjogren’s syndrome (33%) |
|
|
Mixed connective tissue disease/overlapping syndromes (85%) |
|
|
Idiopathic inflammatory myopathies (20%) |
|
|
Systemic vasculitis (up to 10%) |
|
|
Drugs/Toxics |
Amphetamines, nonselective beta-blockers (timolol, nadololol), cocaine, bleomycin, cisplatin, clonidine, cyclosporine, interferon (α, ß), methysergide, errgotamine, polyvinyl chloride |
|
Vaso-occlusive disorders |
Cryoglobulinemia, cryofibrinogenemia, paraproteinemias (POEMS syndrome*), paraneoplastic phenomena (solid tumors: lung, kidney, prostate) |
|
Other causes |
Carpal tunnel syndrome, frostbite, severe hypothyroidism |
Figure 2. ‡Several approaches to nail fold capillaroscopy have been introduced, from low magnification techniques (dermatoscopy, stereomicroscopy and USB microscopy) to high magnification techniques (videocapillaroscopy); the normal shape of a capillary in a healthy individual is defined as "hairpin or tortuous". Any other shape observed is considered abnormal, pointing to secondary RP.
Capillaroscopy allows the study of findings compatible with "sclerodermiform pattern": presence of dilated "giant" capillaries with an apical diameter > or equal to 50 µm, abnormal architecture, microhemorrhages and an extremely low number of capillaries; these microvascular structural alterations can be classified as early, active, and late changes [8,9].
The ASIA syndrome is a term that encompasses several autoimmune conditions and/or phenomena induced by substances with adjuvant activity, with a heterogeneous clinical presentation [9]. Adjuvants are compounds that enhance a specific immune reaction, giving rise to an increased number of antibodies against a specific antigen. In the last decade it has become clear that medical implants, including injectables such as silicones and polypropylene meshes, can also act as nonspecific adjuvants, influencing both adaptive and innate immunity [10].
Adjuvants are largely exogenous substances administered intentionally (e.g., aluminum hydroxide in vaccines) or unintentionally (e.g., plasticizers) that enhance the innate immune response by mimicking conserved molecules. in evolution, as bacterial walls or unmethylated CpG-DNA residues, binding to Toll-like receptors (TLR) in CPA16. The innate response also leads to the activation of NLRP3-type inflammasomes or directly of macrophages (or other cells with phagocytic capacity such as neutrophils), natural killer lymphocytes (NK cells) or innate lymphocytic cells, through various receptors (Toll-like receptors, type NOD and C-type lectin). Furthermore, adjuvants can improve the adaptive response by promoting the interaction of dendritic cells with T cells, increasing the uptake of antigens by antigen-presenting cells [10-12].
On the other hand, there seems to be a genetic predisposition that favors the development of ASIA syndrome: an area of ??the genome that encodes the major histocompatibility complexes (MHC)—the locus known as the human leukocyte antigen (HLA) system—is of special importance, since it encodes proteins that are involved in antigen presentation and is closely involved in pathogen recognition and autoimmunity. Polymorphisms in HLA, especially HLA-DRB1, explain both the rarity and the greater susceptibility of some individuals to develop autoimmune diseases and pathological responses to adjuvants. 3 HLA class I and II haplotypes (HLA DR2DQ6, DR4DQ8 and DR3DQ2) have been considered to explain the association with most autoimmune diseases. The classification of autoimmune diseases into discrete clusters is well recognized, but they share a common genetic and environmental background: it is necessary that external environmental factors (infectious agents, adjuvants, silicone, aluminum salts, etc.) come together to promote the appearance of the disease in genetically susceptible individuals [9,11].
This large spectrum of disease includes chronic fatigue, fever, autoimmune-related symptoms as myalgia, arthralgia, sicca symptoms, and RP. Additionally, it involves neurological (memory loss, sleep disturbances), dermatological (skin rashes, pruritus, alopecia) and gastrointestinal symptoms (abdominal pain and alterations in bowel movement patterns) [10,11]. However, these symptoms in themselves are quite vague, even more so considering that a follow-up of at least 5 years with periodic capillaroscopy is considered prudent before ruling out a specific differentiation towards a specific autoimmune entity, including mixed connective tissue disease, taking into account the high anti-RNP titers at the time of the debut of Raynaud's phenomenon in the illustrated case, and probable overlapping clinical features of systemic lupus erythematosus (SLE), systemic sclerosis, and inflammatory myopathies over time [11].
In cases of silicone implant incompatibility syndrome (also known as siliconosis, in the ASIA syndrome spectrum), it has been proposed that the measurement of IgG antibodies against G protein-coupled adrenergic and muscarinic receptors may serve as early markers of autoimmunity, whose positivity precedes the appearance of conventional autoantibodies (antinuclear antibodies [ANA], extractable nuclear antigen [ENA], antiDNA and anti-cardiolipin IgM and IgG). Furthermore, such antibodies appear to be directly involved in small nerve fiber damage related to chronic fatigue and muscle weakness, as well as nonspecific autonomic gastrointestinal symptoms [12].
We manually reviewed the results looking for case reports and case series and selected 11 articles (Table 3). In this literature review, the average age across studies was 44 years and the interval between implantation and RP onset varied widely (ranging from 4 months to 35 years). Most patients were presented with RP and were later diagnosed with a systemic autoimmune disease, with systemic sclerosis being the most prevalent. In some cases, the Raynaud’s phenomenon may improve or deteriorate after implants were removed, depending on particular situation of each case.
|
Author and year |
Age (years) |
Case description |
Interval between surgery and RP onset |
Diagnosis of systemic autoimmune disease |
|
Silva et al., 2023 [12] |
51 |
51-year-old female, with biphasic RP on both hand and feet of six months duration. One and a half years before, she got silicone breast implants. Antinuclear and extractable nuclear antigen antibodies were negative, anti-RNA-polymerase II and anti-Th/To were weak positive, capillaroscopy was normal. Imaging showed intraprosthetic rupture, so both implants were removed. The RP spontaneously resolved two weeks later without relapse. |
1.5 years |
No |
|
Pavlov et al., 2017 [13] |
28, 37, and 40 |
Three cases of ASIA syndrome in association with a silicone breast implant. Case number 1 was not associated with RP. Case number 2 was a 55-year-old female patient with a past medical history remarkable for bilateral silicone breast implants. After 10 years, she developed RP, puffy fingers and toes, pain in her wrists and shoulders, dyspnea, heartburn, and dysphagia. She was diagnosed with Systemic sclerosis with interstitial lung disease. Breast implants were removed without resolution of clinical manifestations. Case number 3 was a 37-year-old female, who presented with severe RP, leg pain and sleep disturbances four years after bilateral silicone breast implants, capillaroscopy showed nonspecific capillary changes, she decided not to remove the implants. |
10 and 4 years |
Systemic sclerosis (case 2) |
|
Psarras et al., 2014 [14] |
35 |
35-year-old female who presented with RP four months after silicone breast implantation surgery. Afterwards, she developed puffy fingers and gastroesophageal reflux disease, capillaroscopy was compatible with active scleroderma pattern. Laboratories showed high titles of ANA, other autoantibodies were negative. In HRCT there was no evidence of prosthetic rupture. She was diagnosed with systemic sclerosis. |
4 months |
Systemic sclerosis |
|
Mustafá et al., 2022 [15] |
33 |
33-year-old woman who developed RP 6 months after breast implant surgery, associated with fatigue, night sweats, emotional lability, urticaria and shortness of breath. During follow up she developed sacral inflammatory pain and peripheral arthritis, and anti- interleukin 17 treatment was started. The patient complained of breast tenderness, MRI and histology identified silicone gel bleed and migration of silicone. After explantation, most symptoms improved but the Raynaud’s phenomenon persisted. |
6 months |
Axial versus peripheral spondyloarthritis (diagnosis not specified) |
|
Mir et al., 2022 [16] |
37 |
37-year-old woman with history of silicone breast implants. Two years after surgery she presented with Raynaud’s phenomenon, arthralgia, myalgia, and sleep disturbances. Over time, her condition worsened leading to the development of arthritis, alopecia, oral ulcers, and skin bullous lesions. Laboratory tests revealed positive ANA and anti-dsDNA, leading to the diagnosis of silicone breast implant associated ASIA syndrome causing systemic lupus erythematosus. The removal of breast implants was not performed. The patient developed severe complications including myelopathy and nephrotic syndrome and died of a severe lupus flare with pulmonary embolism. |
2 years |
Systemic lupus erythematosus |
|
Muñoz et al., 2021 [17] |
45 |
45-year-old woman who was diagnosed with systemic sclerosis four years later after undergoing silicone breast implantation. Despite the right implant rupturing, explantation was not carried out. The patient developed chronic fatigue, polyarthralgia and RP. During follow up, she developed severe nephritic syndrome, alveolar hemorrhage and polyserositis, and was diagnosed with microscopic polyangiitis and responded positively to immunosuppression. |
4 years |
Systemic sclerosis and microscopic polyangiitis
|
|
Powell et al., 2019 [18] |
69 |
69-year-old woman with a remarkable history of silicone breast implant surgery at the age of 31, with posterior replacement of the left implant because of leakage, she developed RP at age 66. Three years later, was diagnosed with systemic sclerosis and a scleroderma renal crisis. ANA and anti-RNA polymerase III antibodies were positive. |
35 years |
Systemic sclerosis |
|
Wro?ski et al., 2019 [19] |
48 |
48-year-old-woman who presented with RP, puffy fingers, chronic cough, dyspnea, and dysphagia. Laboratory tests were positive for ANA, anti-Scl-70 and anti-RP155 antibodies. She had a history of mammoplasty eleven years prior to the first symptom, an implant had previously ruptured, but surgery was postponed. The patient got both implants removed, afterwards the cough and dysphagia resolved, but dyspnea persisted, and RP intensified. Autoantibodies persisted. |
11 years |
Systemic sclerosis |
|
De Backer et al., 2015 [20] |
47 |
47-year-old woman who had an active smoking history and a prior silicon breast implantation presented with dyspnea, decreased exercise tolerance and fatigue and RP. Imaging of the chest showed a crazy paving pattern and bronchoalveolar lavage confirmed pulmonary alveolar proteinosis. A silicone leakage was later confirmed. |
16 years |
Pulmonary alveolar proteinosis |
|
Al Aranji et al., 2014 [21] |
47 |
47-year-old woman who had a prior silicon breast implantation developed RP seven years later. Because of implant rupture se required reimplantation, afterwards the RP became more severe. Later, she developed puffy fingers, rapid skin thickening and acute renal failure. She was diagnosed with diffuse cutaneous systemic sclerosis and a scleroderma renal crisis. |
7 years |
Systemic sclerosis |
|
Del Giacco et al., 2012 [22] |
42 |
42-year-old female, life-long smoker, with history of Hashimoto's thyroiditis and vitiligo. At age 33 she underwent silicone gel breast implants. Nine months later, she developed episodes of triphasic RP. During follow up, RP episodes worsened, with progression to systemic sclerosis. After surgical removal of implants, an improvement in RP was observed. |
9 months |
Systemic sclerosis |
Regarding the treatment of ASIA syndrome, although there is a lack of well-designed studies and evidence-based management guidelines that demonstrate the positive effects of certain drugs in local inflammatory panniculitis-type disorders related to biological implants and even more so, in the control of systemic manifestations. Cessation of smoking is recommended, as well as correction of vitamin D deficiency, especially in patients with fatigue, arthralgias and myalgias. Systemic steroids are the most commonly used treatment for acute and delayed immune-mediated filler adverse reactions. To date, no refractory cases have been described when medium-high doses of prednisone (0.5 to 1 mg/kg/day) have been used [10-12]. Given the chronic nature of these pathological reactions, patients may sometimes develop corticosteroid dependence criteria, which is why steroid-sparing agents such as antimalarials, cyclosporine, tacrolimus and, anecdotally, azathioprine, methotrexate, mycophenolate mofetil and minocycline have been proposed [13-15].
The role of implant removal is debated. However, is recommended in systemic, severe, or refractory cases, and an objective clinical improvement is achieved in about 50% [14-16]. More cohort and even case-control studies are still required to elucidate the conditioning factors for greater control of symptoms after the removal of biopolymer grafts according to their nature, still measured in terms of temporality, from the moment from the implant to the onset of symptoms, for example.
Limitations
The authors recognize that to date the evidence linking the association between ASIA syndrome and Raynaud's phenomenon, as a secondary cause of the latter entity, is quite scarce and corresponds mostly to reports and some descriptive case series, which could represent a publication bias with its potential impact on the interpretation of findings and conclusions, taking into account that there is still marked controversy regarding profiles and autoimmunity that support mixed connective tissue disease and even systemic sclerosis in the context of ASIA syndrome. Due to the above, we do not consider it pertinent to assume association ratios from a statistical perspective between Raynaud's phenomenon, ASIA syndrome and a history of silicone implants. Further prospective studies are required.
Conclusions
Although RP is part of the minor criteria for adjuvant-induced autoimmune/inflammatory syndrome (ASIA), we propose to be very careful in scenarios of patients with a history of silicone implants or similar, without losing sight of a rigorous periodic clinical follow-up that may include specific imaging studies (chest tomography, endoscopy digestive, among others), as well as capillaroscopy, always pursuing the possibility of differentiation towards a particular clinical entity even in the context of polyautoimmunity, taking into special consideration the clinical spectrum of mixed connective tissue disease.
The presence of dilation of the “true” capillary diameters in primary RP could be observed. By definition, in patients with primary RP the capillaroscopic pattern is completely normal and there are no dilated capillaries present, which could be related to the duration and severity of the symptoms. It is possible that longer duration and greater severity are associated with the appearance of capillary dilations, but more research is needed to confirm it. Rarely, pathological capillaroscopic features of microangiopathy could be observed in “primary RP”. These cases should be defined as “suspected secondary RP” and require closer follow-up for the assessment of symptom evolution.
Finally, large-scale studies are required that have well-defined inclusion criteria, reproducible outcomes and long-term follow-up, which allow the identification of modifiable and non-modifiable epigenetic factors. New advances in pharmacogenomics will allow us to understand even better the immune response triggered by adjuvants, in order to anticipate their adverse effects over time and, if possible, extrapolate these findings to the field of plastic and reconstructive surgery, where it is done active use of silicone-based bioprostheses, so one of the recommendations would be to screen for autoimmunity (Hashimoto's thyroiditis, for example) patients profiled for aesthetic procedures that involve the application of biopolymers and similar, in order to determine the risk in the future of developing clinical manifestations linked to polyautoimmunity, where the so-called ASIA syndrome begins to stand out, despite having diagnostic criteria that are still controversial, as proposed in this case.
Acknowledgments
Funding
None.
Conflicts of interest
In compliance with the ICMJE uniform disclosure form, all authors declare the following: Payment/services info: All authors have declared that no financial support was received from any organization for the submitted work. Financial relationships: All authors have declared that they have no financial relationships at present or within the previous three years with any organizations that might have an interest in the submitted work. Other relationships: All authors have declared that there are no other relationships or activities that could appear to have influenced the submitted work.
Ethical Statement
The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. Written informed consent was obtained from the patient for publication of this case report and accompanying images.
References
2. Nawaz I, Nawaz Y, Nawaz E, Manan MR, Mahmood A. Raynaud’s Phenomenon: Reviewing the Pathophysiology and Management Strategies. Cureus. 2022 Jan;14(1):e21681.
3. Belch J, Carlizza A, Carpentier PH, Constans J, Khan F, Wautrecht JC, et al. ESVM guidelines - the diagnosis and management of Raynaud’s phenomenon. Vasa. 2017 Oct;46(6):413-23.
4. Cohen Tervaert JW, Martinez-Lavin M, Jara LJ, Halpert G, Watad A, Amital H, et al. Autoimmune/inflammatory syndrome induced by adjuvants (ASIA) in 2023. Autoimmun Rev. 2023 May;22(5):103287.
5. Rodríguez-Criollo JA, Jaramillo-Arroyave D. Fenómeno de Raynaud. Revisión. Revista de la Facultad de Medicina. 2014 Jul;62(3):1-24.
6. Pauling JD, Hughes M, Pope JE. Raynaud’s phenomenon-an update on diagnosis, classification and management. Clin Rheumatol. 2019 Dec;38(12):3317-30.
7. Maverakis E, Patel F, Kronenberg DG, Chung L, Fiorentino D, Allanore Y, et al. International consensus criteria for the diagnosis of Raynaud’s phenomenon. J Autoimmun. 2014;48-49:60-5.
8. Ocampo-Garza SS, Villarreal-Alarcón MA, Villarreal-Treviño AV, Ocampo-Candiani J. Capilaroscopia: una herramienta diagnóstica valiosa. Actas Dermosifiliogr. 2019 Jun 1;110(5):347-52.
9. Alijotas-Reig J, Esteve-Valverde E, Gil-Aliberas N, Garcia-Gimenez V. Autoimmune/inflammatory syndrome induced by adjuvants-ASIA-related to biomaterials: analysis of 45 cases and comprehensive review of the literature. Immunol Res. 2018 Feb;66(1):120-40.
10. Miro-Mur F, Hindié M, Kandhaya-Pillai R, Tobajas V, Schwartz S, Alijotas-Reig J. Medical-grade silicone induces release of proinflammatory cytokines in peripheral blood mononuclear cells without activating T cells. J Biomed Mater Res B Appl Biomater. 2009 Aug;90(2):510-20.
11. John KJ, Sadiq M, George T, Gunasekaran K, Francis N, Rajadurai E, et al. Clinical and Immunological Profile of Mixed Connective Tissue Disease and a Comparison of Four Diagnostic Criteria. Int J Rheumatol. 2020 Jan 29;2020:9692030.
12. Silva A, Vieira Sousa E, Cruz Machado AR. Raynaud phenomenon as an isolated manifestation of autoimmune/inflammatory syndrome induced by adjuvants (ASIA). Clinical and Experimental Rheumatology. 2024 Mar;42(3):768.
13. Pavlov-Dolijanovic S, Vujasinovic Stupar N. Women with silicone breast implants and autoimmune inflammatory syndrome induced by adjuvants: description of three patients and a critical review of the literature. Rheumatology International. 2017 Aug;37(8):1405-11.
14. Psarras A, Gkougkourelas I, Tselios K, Sarantopoulos A, Boura P. Systemic sclerosis and silicone breast implant: a case report and review of the literature. Case Reports in Rheumatology. 2014;2014:809629.
15. Mustafá JCR, Fleury E de FC, Dijkman HBPM. Case Report: Evidence of Migratory Silicone Particles Arising From Cohesive Silicone Breast Implants. Frontiers in Global Women’s Health. 2022;3:730276.
16. Mir T, Khan A, Omar Parvaiz M, Rath P, Sharma A. ASIA syndrome with severe lupus - A case report. Lupus. 2022 Oct;31(12):1532-5.
17. Muñoz CC, Rodríguez JG, Rivera AA, Estarán E, Cárcel JR, Medrano AS. Systemic sclerosis and microscopic polyangiitis after systemic exposure to silicone. Clinical Kidney Journal. 2021;14(7):1848-50.
18. Powell RO, Singh A, McCormick F, King J, Houlberg K, Connor A. Normotensive scleroderma renal crisis after silicone breast implant rupture. Kidney International. 2019;95(6):1520.
19. Wroński J, Bonek K, Stanisławska-Biernat E. Scleroderma-like syndrome in a woman with silicone breast implants – case report and critical review of the literature. Reumatologia. 2019;57(1):55-8.
20. De Backer H, Darquennes K, Dooms C, Yserbyt J, Coolen J, Verschakelen J, et al. The inner and outer of our thorax: silicone breast implants and pulmonary alveolar proteinosis. Acta Clinica Belgica. 2015 Sep 3;70(5):384-6.
21. Al Aranji G, White D, Solanki K. Scleroderma renal crisis following silicone breast implant rupture: A case report and review of the literature. Clinical and Experimental Rheumatology. 2014;32(2):262-6.
22. Del Giacco SR, Firinu D, Piludu G, Settembrini AM, Tulli M, Pirari P, et al. Raynaud’s phenomenon and scleroderma associated with silicone gel breast implants: An example of Asia syndrome. European Journal of Inflammation. 2012;10(2):233-8