Abstract
Cardiovascular diseases (CVDs) remain a leading cause of morbidity and mortality worldwide, and early diagnosis, treatment, and management are essential for reducing the burden of these diseases. This systematic review provides an update on recent developments in the field of CVDs, including the use of artificial intelligence (AI) in diagnosis and management, advances in genetic testing and precision medicine, the role of telemedicine and remote monitoring technologies, novel therapies such as gene therapy and cell therapy, and the importance of addressing mental health in the prevention and management of CVDs. Advances in genetic testing and precision medicine have enabled personalized treatment of CVDs, with pharmacogenomic approaches helping to identify the most effective medications for individual patients. Telemedicine and remote monitoring technologies have shown promise in improving the management of CVDs, allowing for real-time data collection and early detection of complications. Novel therapies such as gene therapy and cell therapy are being explored for the treatment of CVDs, with early studies showing promising results. Overall, the recent developments in the diagnosis, treatment, and management of CVDs highlight the ongoing efforts to improve patient outcomes and reduce the burden of these diseases.
Keywords
Novel therapies, Biomarkers, Personalized medicine, Cardiovascular, Artificial intelligence
Background
Cardiovascular diseases (CVDs) are a group of disorders that affect the heart and blood vessels, including coronary artery disease, heart failure, and stroke. CVDs are a leading cause of death worldwide, accounting for more than 17 million deaths each year, and are projected to continue to rise in prevalence due to aging populations and unhealthy lifestyles [1]. The risk factors for CVDs can be divided into modifiable and non-modifiable factors. Non-modifiable factors include age, sex, and genetics, while modifiable risk factors include smoking, high blood pressure, high cholesterol levels, diabetes, obesity, and physical inactivity. Lifestyle modifications such as healthy eating habits, regular exercise, and smoking cessation can help to reduce the risk of CVDs, while medications and surgical interventions can also be used to manage and treat these diseases [2].
Early detection and management of CVDs are crucial for preventing complications and improving outcomes. Biomarkers, which are measurable indicators of biological processes or disease states, are increasingly being used in the diagnosis, risk stratification, and management of CVDs. Novel biomarkers such as microRNAs and extracellular vesicles show promise for improving the accuracy of CVD diagnosis and predicting disease progression [3]. In addition to traditional risk factors and biomarkers, advances in imaging technologies such as computed tomography (CT) and magnetic resonance imaging (MRI) have also revolutionized the diagnosis and management of CVDs. These imaging modalities can provide detailed information about the structure and function of the heart and blood vessels, allowing for early detection of CVDs and guiding treatment decisions [4]. Despite the progress made in the prevention, diagnosis, and management of CVDs, challenges remain. Limited access to healthcare and disparities in healthcare delivery can impact the early detection and management of CVDs in certain populations. Additionally, the complex and multifactorial nature of CVDs requires a comprehensive and personalized approach to treatment and management [5].
Research in the field of cardiovascular medicine continues to advance, with ongoing efforts to identify novel biomarkers, develop targeted therapies, and improve healthcare delivery [6]. The integration of multidisciplinary approaches, including genetics, genomics, and data science, holds promise for improving our understanding of the pathophysiology of CVDs and developing more effective prevention and treatment strategies [7]. Ultimately, the goal is to reduce the global burden of CVDs and improve the quality of life for those affected by these diseases. Artificial intelligence (AI) has the potential to revolutionize the field of cardiovascular medicine by improving the accuracy of diagnosis, predicting disease progression, and individualizing treatment plans. AI encompasses a range of techniques, including machine learning, deep learning, and natural language processing, which can be used to analyze large datasets and extract meaningful insights [8]. One area where AI has shown promise in cardiovascular medicine is in the analysis of medical images [9]. AI algorithms can be trained to accurately identify and classify abnormalities in images such as CT scans, MRI, and echocardiography, allowing for earlier and more accurate diagnosis of cardiovascular diseases [10-12]. AI can also be used to analyze other types of data, such as electrocardiograms (ECGs) and genetic data, to predict the risk of developing CVDs and guide treatment decisions. In addition to improving diagnosis and risk prediction, AI can also be used to develop personalized treatment plans for patients with CVDs [13]. By analyzing patient data, including medical history, imaging, and genetic data, AI algorithms can identify the most effective treatment options for individual patients, taking into account their unique characteristics and needs [14].
This review provides a comprehensive update on recent advances across the spectrum of cardiovascular disease care, with a focus on novel diagnostic modalities, emerging therapies, and innovative approaches like AI and precision medicine. Elucidating these latest developments is crucial to improving patient outcomes, advancing treatment strategies, and reducing the global burden of cardiovascular diseases.
Novel Biomarkers for Early Diagnosis of CVDs
Novel biomarkers have been identified as potential tools for early detection of CVDs.
High-sensitivity C-reactive protein (hs-CRP) has been identified as a potential biomarker for early detection of CVDs. Several studies have shown that hs-CRP is associated with an increased risk of cardiovascular events [15]. Another potential biomarker is N-terminal pro b-type natriuretic peptide (NT-proBNP), which has been found to be a useful marker for the diagnosis of heart failure [16]. Troponins are another biomarkers that have been widely studied for the diagnosis of acute coronary syndrome (ACS) [17]. Other novel biomarkers that have shown promise for early detection of CVDs include microRNAs and circulating endothelial cells (CECs). MicroRNAs are small non-coding RNAs that regulate gene expression and have been shown to be dysregulated in various CVDs. CECs are endothelial cells that have detached from blood vessels and can be easily detected in the peripheral blood. Several studies have demonstrated that CECs are elevated in patients with CVDs, including coronary artery disease (CAD) and heart failure [18].
The current state of research on blood biomarkers in CVDs, focusing on biomarkers related to inflammation, oxidative stress, and endothelial dysfunction. The article highlights the potential of novel biomarkers such as galectin-3, microRNAs, and extracellular vesicles for improving the accuracy of CVD diagnosis and predicting disease progression. The potential of novel biomarkers such as glycated hemoglobin, high-sensitivity troponin, and lipoprotein (a) for early detection and risk prediction of CVDs [19]. Dai et al. discusses the potential of microRNAs as biomarkers for CVDs, highlighting the advantages of their stability in circulation and ease of measurement [18]. microRNAs as therapeutic targets for CVDs, providing examples of preclinical studies that have shown promising results. The identification and validation of novel biomarkers for early prediction of CVDs, includes genetic, proteomic, and metabolomic biomarkers [21]. Novel biomarkers such as circulating microRNAs, lipids, and proteins have the potential of for improving risk prediction and guiding personalized treatment. The potential of extracellular vesicles as novel biomarkers for CVDs, emphasizing their role in intercellular communication and their potential as targets for therapeutic intervention [22]. Some examples of extracellular vesicle-based biomarkers, such as miR-126 and miR-92a, have the potential for predicting CVD risk and guiding treatment decisions. GDF-15 could be used in combination with other biomarkers and clinical parameters to improve risk stratification and treatment decisions in patients with CHF. For example, GDF-15 could be used alongside other biomarkers, such as brain natriuretic peptide (BNP) and troponin, to provide a more comprehensive picture of a patient's condition [23]. Lipid biomarkers for CVD risk assessment include traditional lipid biomarkers such as cholesterol and triglycerides, as well as novel biomarkers such as oxidized phospholipids and lysophosphatidylcholine. The potential of these biomarkers for improving risk prediction and guiding personalized treatment has been established [24,25].
However, biomarkers can be affected by various factors, including age, sex, and comorbidities, which can limit their specificity and sensitivity. Additionally, the cost and availability of biomarker testing can be a barrier to their widespread use in clinical practice. In conclusion, recent developments in novel biomarkers for early detection of CVDs highlight the ongoing efforts to improve patient outcomes and reduce the burden of these diseases [23].
Imaging Tools for Early Detection of CVDs
Imaging tools for early detection of CVDs include their clinical utility and limitations. Cardiac magnetic resonance imaging (MRI) has emerged as an accurate and safe tool for diagnosing various cardiovascular conditions, including myocarditis, hypertrophic cardiomyopathy, and stress-induced cardiomyopathy, among others [26]. MRI can provide detailed images of the heart and blood vessels, enabling identification of structural abnormalities and functional changes associated with CVDs. Computed tomography (CT) angiography has also shown promise in the early diagnosis of CVDs, particularly in the diagnosis of coronary artery disease (CAD) [27]. CT angiography can provide detailed images of the coronary arteries, allowing for identification of stenosis and plaque formation. Positron emission tomography (PET) imaging has been used to detect early-stage CVDs by identifying areas of inflammation and metabolic activity within the heart [28-36].
PET imaging can provide information on myocardial perfusion, metabolism, and function, which can aid in the early detection of CVDs.
Other imaging modalities that have shown promise for early detection of CVDs include ultrasound and optical coherence tomography (OCT). Ultrasound can be used to assess blood flow and detect structural abnormalities within the heart and blood vessels [37]. OCT imaging can provide high-resolution images of the coronary artery wall, allowing for identification of early-stage plaque formation [38]. FT-CMR (feature tracking cardiac magnetic resonance) is able to detect significant changes in radial and circumferential strain after 18 weeks of treatment, which was shorter than the period required for echocardiography to detect structural and functional changes. The study also found that the changes in FT-CMR parameters were in agreement with the reduction in left ventricular end-diastolic volume (LVEDV) and left ventricular hypertrophy (LVH) as a result of reduced LV radial thickness. The article also notes that global longitudinal strain (GLS) and lateral mitral annular plane systolic excursion (MAPSE) obtained through CMR are independent predictors of mortality in hypertensive patients [39].
Despite the potential clinical utility of these imaging tools, there are limitations to their use. Imaging tools can be expensive and may not be available in all healthcare settings [40]. Additionally, some imaging modalities involve exposure to ionizing radiation, which can limit their use in certain patient populations. Overall, recent developments in imaging tools for early detection of CVDs highlight the ongoing efforts to improve patient outcomes and reduce the burden of these diseases [41].
Treatment of CVDs, Including Current Guidelines and Emerging Therapies
Current guidelines for the treatment of CVDs emphasize the importance of lifestyle modifications, such as smoking cessation, regular physical activity, and a healthy diet, in addition to pharmacologic therapies. Pharmacologic therapies for CVDs include antiplatelet agents, lipid-lowering agents, and antihypertensive agents. Antiplatelet agents, such as aspirin and clopidogrel, are commonly used in the treatment of acute coronary syndrome (ACS) and for secondary prevention of cardiovascular events [42]. Lipid-lowering agents, such as statins, have been shown to reduce the risk of cardiovascular events in patients with hyperlipidemia. Antihypertensive agents, such as angiotensin-converting enzyme (ACE) inhibitors and angiotensin receptor blockers (ARBs), are commonly used in the treatment of hypertension and heart failure [43].
Emerging therapies for the treatment of CVDs include novel anticoagulants and gene therapies. Novel anticoagulants, such as direct oral anticoagulants (DOACs), have been shown to be effective in the prevention of stroke in patients with atrial fibrillation. Gene therapies, such as RNA interference therapy, have shown promise in the treatment of hypercholesterolemia by reducing levels of low-density lipoprotein cholesterol (LDL-C) [44]. In addition to pharmacologic therapies, invasive procedures, such as percutaneous coronary intervention (PCI) and coronary artery bypass graft (CABG) surgery, are commonly used in the treatment of CVDs. PCI is a minimally invasive procedure that involves the placement of a stent in a narrowed coronary artery to improve blood flow, while CABG surgery involves the use of grafts to bypass narrowed or obstructed coronary arteries [45]. Overall, recent developments in the treatment of CVDs highlight the ongoing efforts to improve patient outcomes and reduce the burden of these diseases.
Advances in Novel Therapies for CVD and Their Effects on Patient Outcomes
Gene therapy has emerged as a promising approach for the treatment of CVD, with the potential to treat a range of diseases, including heart failure, inherited lipid disorders, and inherited arrhythmias. Gene therapy involves introducing therapeutic genes into cells to correct or replace defective or missing genes. Recent studies have shown that gene therapy can improve cardiac function and reduce symptoms in patients with heart failure. For example, gene therapy by using a viral vector was effective in treating patients with heart failure [46]. Stem cell therapy is another promising approach for the treatment of CVD, with the potential to regenerate damaged heart tissue and improve cardiac function. Stem cells can be derived from various sources, such as bone marrow, adipose tissue, and cardiac tissue. Recent studies have shown that stem cell therapy can improve cardiac function and reduce symptoms in patients with heart failure. For example, studies on stem cell therapy were effective in improving left ventricular ejection fraction and reducing hospitalization rates in patients with heart failure [47].
Pharmacogenomics involves using genetic information to tailor medication regimens to individual patients, while personalized medicine involves tailoring treatment to an individual patient's unique characteristics, such as age, sex, and comorbidities [48]. Recent studies have shown that pharmacogenomic approaches can improve medication efficacy and reduce adverse drug reactions in patients with CVD [49].
In conclusion, novel therapies for CVD, including gene therapy, stem cell therapy, and precision medicine approaches, offer promising opportunities for improving patient outcomes and reducing the burden of these complex diseases [50]. Ongoing research and collaboration between clinicians and researchers will be essential to realizing the potential of these novel therapies in CVD care [51].
Pharmacogenomic and Cardiovascular Diseases
Pharmacogenomic approaches have emerged as a promising tool for the treatment of cardiovascular diseases (CVD), with the potential to improve medication efficacy and reduce adverse drug reactions in patients with CVD. Pharmacogenomics uses genetic information to tailor medication regimens to individual patients, based on their genetic makeup. In this review, we will discuss the latest advances in pharmacogenomic approaches for CVD treatment and their effects on patient outcomes [52].
Recent studies have shown that pharmacogenomic approaches can improve medication efficacy and reduce adverse drug reactions in patients with CVD. For example, using a pharmacogenomic approach to tailor antiplatelet therapy was associated with a reduced risk of major adverse cardiovascular events in patients undergoing percutaneous coronary intervention [53]. Another study showed that a pharmacogenomic approach to warfarin dosing was associated with a reduced risk of bleeding events in patients with atrial fibrillation [54]. In both in vivo and in vitro models of ischemic stroke, MLIF facilitated the transformation of microglia into the M2 phenotype. The therapeutic effects of MLIF were mediated by targeting eEF1A1 through the involvement of the NF-κB signaling pathway [55].
Pharmacogenomic approaches have also been used to identify genetic variants associated with CVD risk and drug response. For example, a study genetic variant associated with increased risk of coronary artery disease and improved response to statin therapy [56]. Another study identified genetic variants associated with increased risk of heart failure and improved response to beta-blocker therapy [57]. Some studies suggested that to implement precision nutrition for CVD prevention [58], three essential elements need to be established: (1) development of data validation standards and clinical practice guidelines, (2) establishment of a certified training program, and (3) integration into the healthcare system. The first element involves creating validated databases to establish clinical practice guidelines for different gene-diet interactions. The second element involves developing a certified training program in precision nutrition for nutritionists and physicians. Finally, precision nutrition for the prevention of CVD must be integrated into the healthcare system to make it accessible to all individuals [59].
The implementation of pharmacogenomics (PGx)-testing in healthcare systems is often met with concerns about its practicality and impact on system performance [60]. However, a pilot analysis showed that PGx-testing is feasible and revealed high frequencies of patients receiving suboptimal drug regimens who may benefit from PGx testing. These findings highlight the importance of conducting explorative analysis, optimization, and troubleshooting exercises before introducing PGx-implementation initiatives. Such exercises could help alleviate concerns and provide favorable evidence for practitioners who may be hesitant to adopt PGx-testing [61].
Limitations of pharmacogenomics in cardiovascular diseases
Pharmacogenomics is a promising field that aims to tailor drug therapy to an individual's genetic makeup. However, there are several limitations to its application in cardiovascular diseases. One of the main challenges is the complexity of the genetic basis of these diseases. Cardiovascular diseases are multifactorial, meaning that they result from a combination of genetic and environmental factors. Identifying the genetic variants that contribute to disease susceptibility and drug response is therefore a difficult task [62].
Another limitation is the lack of validated biomarkers. Biomarkers are measurable indicators of disease or drug response, and they play a critical role in identifying patients who are likely to benefit from pharmacogenomic testing. However, there are currently few validated biomarkers for cardiovascular diseases, which limits the clinical utility of pharmacogenomics [63].
Finally, the limited availability of pharmacogenomic testing is a major barrier to its widespread adoption in clinical practice. While the cost of genetic testing has decreased over the years, it is still relatively expensive, and many healthcare providers lack the expertise to interpret genetic test results [64].
Challenges in translating pharmacogenomics into clinical practice for cardiovascular diseases
One of the main challenges in translating pharmacogenomics into clinical practice for cardiovascular diseases is the lack of evidence-based guidelines for using genetic information to guide drug therapy. While there are some pharmacogenomic tests that have been approved by regulatory agencies such as the FDA, there are still many unanswered questions about how best to use genetic information to tailor drug therapy [65].
Another challenge is the need for improved education and training of healthcare providers. Many physicians lack the knowledge and skills needed to interpret genetic test results and incorporate them into clinical decision-making. This highlights the need for increased education and training programs to ensure that healthcare providers are equipped with the necessary skills to use pharmacogenomics in clinical practice [66].
Finally, there are also challenges related to data privacy and security. Genetic information is highly sensitive, and there are concerns about how it will be used and protected. It is important to establish clear policies and regulations around the use of genetic information to ensure that patient privacy is protected [67].
Role of pharmacogenomics in identifying patients at risk for adverse drug reactions in cardiovascular diseases:
Pharmacogenomics can play a critical role in identifying patients who are at risk for adverse drug reactions in cardiovascular diseases [68]. Adverse drug reactions are a major cause of morbidity and mortality, and they often result from genetic factors that influence drug metabolism or response. By identifying genetic variants that affect drug metabolism or response, pharmacogenomic testing can help clinicians identify patients who are at increased risk for adverse drug reactions. This can enable clinicians to adjust drug doses or switch to alternative therapies that are less likely to cause adverse reactions [69].
Ethical considerations surrounding the use of pharmacogenomics in cardiovascular diseases
There are several ethical considerations surrounding the use of pharmacogenomics in cardiovascular diseases. One of the main concerns is the potential for genetic discrimination. Genetic information can be used to discriminate against individuals in employment, insurance, and other areas of life [70]. It is important to establish clear policies and regulations around the use of genetic information to prevent discrimination [71].
Another ethical concern is related to informed consent. Patients have the right to know how their genetic information will be used and who will have access to it. It is important to ensure that patients are fully informed about the risks and benefits of pharmacogenomic testing and that they provide informed consent before undergoing testing [72].
Finally, there are also concerns about access and equity. Pharmacogenomic testing is expensive, and it may not be accessible to all patients. This raises questions about whether access to pharmacogenomic testing should be considered a basic right, and how we can ensure that all patients have access to this technology regardless of their socioeconomic status [73].
Prevention and Management of Cardiovascular Diseases
Cardiovascular diseases (CVDs) are a leading cause of morbidity and mortality worldwide. Fortunately, many CVDs can be prevented or managed through lifestyle modifications and medical interventions. Here are some strategies for preventing and managing CVDs:
- Healthy lifestyle habits: Adopting healthy lifestyle habits such as regular exercise, a balanced diet, and avoiding smoking can help prevent CVDs. Exercise can improve cardiovascular health by reducing blood pressure and cholesterol levels, while a balanced diet can help maintain a healthy weight and reduce the risk of developing diabetes and other metabolic disorders [74].
- Medical interventions: Several medical interventions can help prevent or manage CVDs. These include medications such as statins to lower cholesterol levels, anti-hypertensive drugs to control blood pressure, and antiplatelet agents to prevent blood clots [75]. In some cases, surgical interventions such as coronary artery bypass surgery or angioplasty may be necessary to restore blood flow to the heart [76].
- Screening and early detection: Regular screening for CVD risk factors such as high blood pressure, high cholesterol levels, and diabetes can help identify individuals who are at risk for CVDs [77]. Early detection of CVDs can allow for earlier intervention and better outcomes [78].
- Patient education and self-management: Educating patients about their risk factors for CVDs and how to manage them can help prevent or manage CVDs. This includes teaching patients about healthy lifestyle habits, medication adherence, and recognizing symptoms of CVDs such as chest pain or shortness of breath [79].
- Multidisciplinary care: CVDs often require a multidisciplinary approach to care, involving healthcare providers from multiple disciplines such as cardiology, endocrinology, and nutrition. Collaboration between healthcare providers can help ensure that patients receive comprehensive care that addresses all aspects of their condition [80].
Overall, preventing and managing CVDs requires a comprehensive approach that involves lifestyle modifications, medical interventions, screening and early detection, patient education and self-management, and multidisciplinary care. By implementing these strategies, we can reduce the burden of CVDs and improve the health outcomes of individuals with these conditions.
Telemedicine and Cardiovascular Diseases
Telemedicine refers to the use of technology to deliver healthcare services remotely. Telemedicine has shown great promise in the field of CVD, particularly in improving access to care, reducing healthcare costs, and improving patient outcomes [81]. Some of the ways telemedicine is being used in the field of CVD research and clinical practice include:
- Remote monitoring: Telemedicine can be used to remotely monitor patients with CVD, such as those with heart failure or arrhythmias. This can help clinicians detect changes in a patient's condition early and adjust treatment plans accordingly [82].
- Teleconsultations: Teleconsultations can be used to provide remote consultations with CVD specialists, particularly for patients in remote or underserved areas. This can help improve access to care and reduce the burden on healthcare systems [83].
- Tele-rehabilitation: Tele-rehabilitation can be used to provide remote rehabilitation services to patients with CVD, such as those recovering from a heart attack or stroke. This can help improve patient outcomes and reduce healthcare costs [84].
- Patient education: Telemedicine can be used to provide patient education and support for self-management of CVD, such as through virtual coaching or online resources [85].
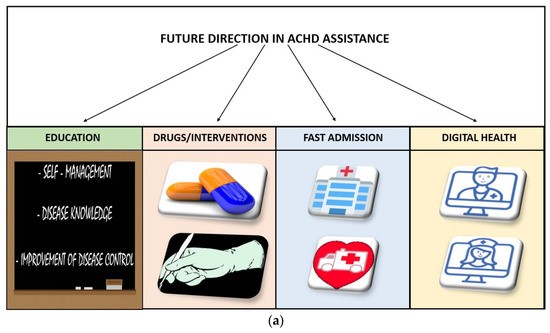
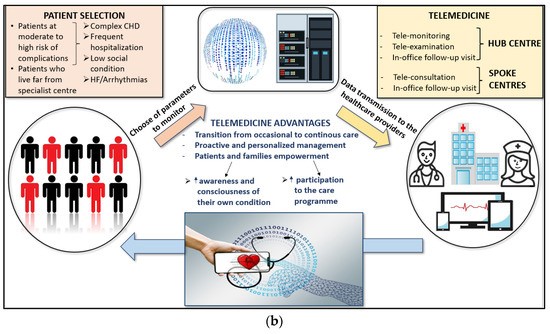
Telemedicine has the potential to provide a new care model for adults with congenital heart disease (ACHD) who are at high risk of repeated hospital admissions. The essential elements of this model include personalized ACHD care with patient and family education, quick access to ACHD databases with automatic notifications for specific actions, close monitoring of hemodynamic parameters on a daily basis, continuous phone and video communication, the ability to go to the ACHD hub directly from home in case of instability, and timely catheter interventions and surgery. This care model can provide highly specialized care for ACHD patients while also taking into account their quality of life. There is a need to improve the care of these complex patients, and telemedicine may represent a way to achieve this goal [86].
Telemedicine has the potential to revolutionize the field of cardiovascular disease by improving patient outcomes and increasing access to care. Recent studies have demonstrated its effectiveness in reducing hospitalizations and improving outcomes in heart failure patients, as well as reducing cardiovascular risk factors in patients with CVD. However, there are challenges that need to be addressed, including ensuring the quality and safety of telemedicine services, protecting patient privacy, and integrating telemedicine into clinical workflows. Nevertheless, telemedicine is an exciting and rapidly evolving field in CVD research and clinical practice. Integrated assistance, including the use of AI for monitoring vital signs, could enable continuous care and a patient-tailored care model. AI could also assist with diagnosis, personalized therapies, appointment reminders, and improving adherence to medications (Figure 1) [87].
Figure 1. (a) Future direction in Adult Congenital Heart Disease assistance. (b) Central illustration [87].
Herbal and Traditional Medicine for Cardiovascular Diseases
Herbal and traditional medicine have been used for centuries to treat and prevent various diseases, including cardiovascular diseases [88]. These remedies are often derived from plants, minerals, or animal products and are considered to have fewer side effects than conventional medications [89-92]. One example of an herbal remedy for cardiovascular disease is garlic. Garlic has been shown to have cholesterol-lowering effects and can help reduce blood pressure. Another example is hawthorn, which has been used to treat heart failure and angina [93].
Traditional Chinese medicine also offers remedies for cardiovascular diseases, such as ginseng, which has been shown to improve heart function and reduce inflammation. Acupuncture is another traditional Chinese medicine practice that has been used to treat hypertension and other cardiovascular conditions [94].
While herbal and traditional medicine can be beneficial, it is important to consult with a healthcare professional before using any new remedies, especially if you are taking prescription medications or have underlying health conditions [95].
Furthermore, some herbs and supplements can interact with prescription medications, which can cause adverse effects. For instance, Ginkgo biloba, which is commonly used to improve memory and cognitive function, can interfere with blood-thinning medications, leading to bleeding problems [96].
It is worth noting that herbal and traditional remedies are not regulated by the FDA in the same way that prescription medications are [97]. Therefore, the quality and purity of these products may vary widely. Some products may contain harmful substances or contaminants, which can be dangerous to health. In conclusion, herbal and traditional medicine can be valuable in preventing and treating cardiovascular diseases [98].
AI and Cardiovascular Diseases
AI has continued to gain momentum in the field of cardiovascular medicine and is being applied in various ways to improve CVD diagnosis, prevention, and management [99]. AI has the potential to revolutionize cardiovascular medicine by enabling more precise risk prediction, earlier detection of disease, and personalized treatment strategies [100-104]. Some of the ways AI is being used in the field of CVD research and clinical practice include:
- Risk prediction: AI algorithms can analyze large amounts of patient data, including medical records, imaging studies, and genetic information, to identify patterns and predict a patient's risk of developing CVD [105]. This can help clinicians tailor prevention and treatment strategies to individual patients [88].
- Image analysis: AI can analyze medical images, such as cardiac ultrasound or MRI scans, to detect subtle changes that may be indicative of CVD. This can help clinicians make more accurate diagnoses and monitor disease progression [106].
- Drug discovery: AI can be used to identify new drug targets and develop more effective treatments for CVD [107]. By analyzing large datasets of genetic and molecular information, AI algorithms can help researchers identify novel pathways and drug candidates that may not have been discovered through traditional methods [108].
- Virtual assistants: AI-powered virtual assistants can help patients manage their CVD by providing personalized advice and support. For example, a virtual assistant may remind patients to take their medication, track their physical activity, or provide dietary recommendations [109].
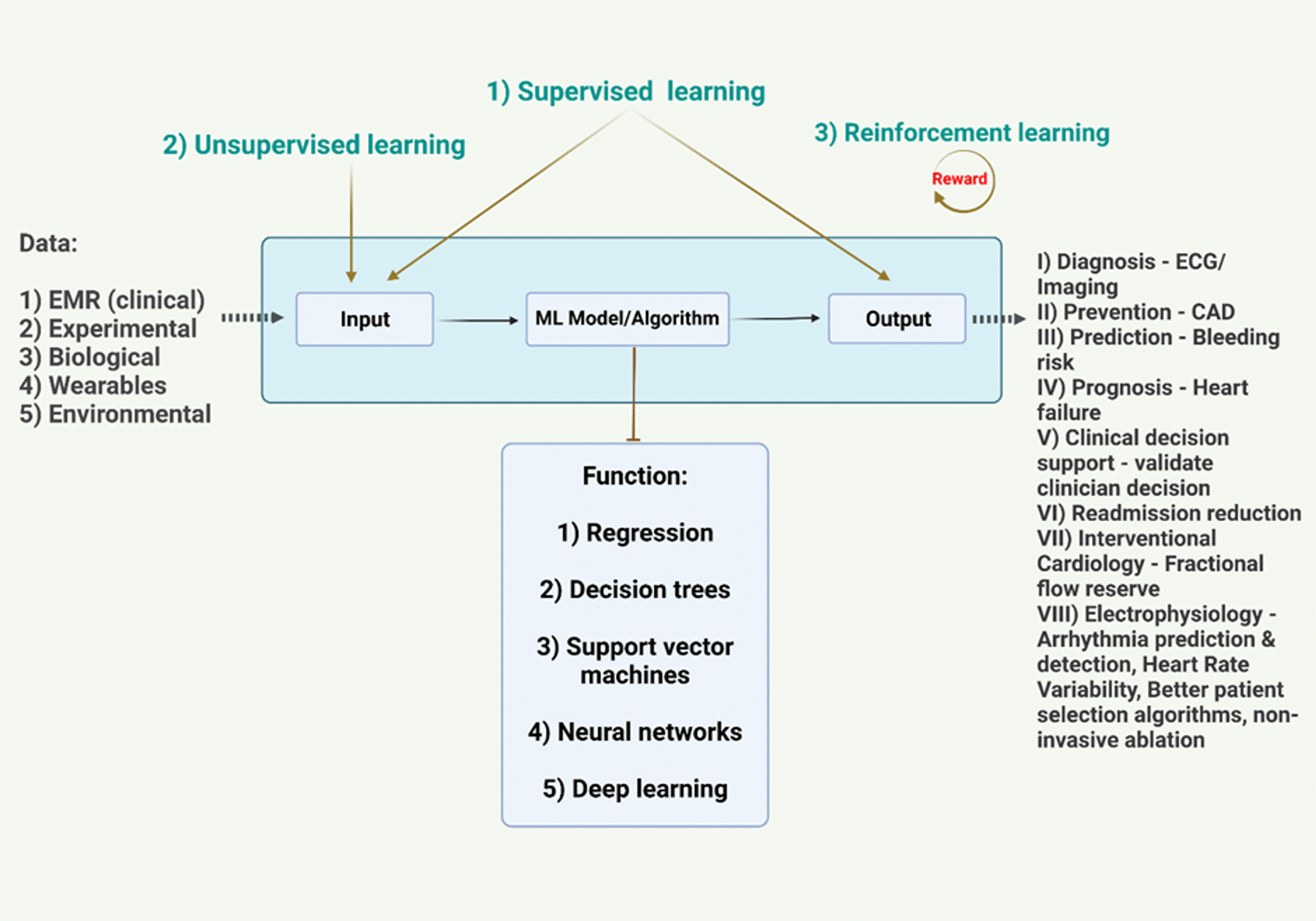
Recent studies have shown promising results for the use of AI in CVD. For example, an AI algorithm could predict the risk of heart attack or stroke in patients with no history of CVD with greater accuracy than traditional risk factors [93]. Additionally, it could predict the risk of heart failure hospitalization in patients with heart failure with preserved ejection fraction (Figure 2) [110].
Figure 2. Outline of the training process of an AI system [110].
The existing literature showed that several developments were underway in applying AI and other innovative approaches in diagnosing, treating, and managing cardiovascular diseases. These recent developments come with some advantages and disadvantages. The advantages are that AI can quickly analyze vast amounts of medical data, aiding in the early detection of cardiovascular issues before symptoms manifest. AI allows personalized treatment plans by considering individual patient data, genetics, and other factors, leading to more targeted and effective interventions. AI-enhanced imaging technologies provide more accurate and detailed diagnostic information, enabling better-informed decision-making by healthcare professionals. Predictive AI modeling helps identify patients at a higher risk of developing cardiovascular diseases, allowing for proactive and preventative measures.
The disadvantages are that AI involves handling sensitive patient data, raising concerns about data security, privacy breaches, and the potential misuse of health information. The regulatory framework for AI applications in healthcare is still evolving, leading to uncertainties about standardization, compliance, and ethical considerations. Integrating AI technologies into existing healthcare systems can be challenging, requiring significant investments in infrastructure, training, and interoperability. AI algorithms may be biased, leading to disparities in diagnosis and treatment. Generalization of AI models across diverse populations can be a challenge. Overreliance on AI may lead to reduced clinical judgment and human intuition, potentially impacting the quality of patient care. As technology advances, ongoing research, and collaboration between healthcare professionals. Overall, the use of AI in CVD research and clinical practice is an exciting and rapidly evolving field that has the potential to improve patient outcomes and advance our understanding of these complex diseases. However, there are also challenges that need to be addressed, such as ensuring the accuracy and reliability of AI algorithms, protecting patient privacy, and integrating AI into clinical workflows in a meaningful way [111-115].
The limitations of AI
While AI has shown great promise in the field of cardiovascular disease (CVD) diagnosis, there are also several limitations to its use [116]. Some of these limitations include:
- Limited data: AI algorithms require large amounts of high-quality data to be trained effectively. However, in some cases, there may not be enough data available to develop accurate models, particularly for rare or complex diseases [117].
- Bias in data: AI algorithms can be biased if the data used to train them is not representative of the population being studied. For example, if the data used to train an algorithm is predominantly from one ethnicity or gender, the algorithm may not perform well on patients from other ethnicities or genders [118].
- Lack of interpretability: Some AI models may be difficult to interpret, making it challenging to understand how they arrived at a particular diagnosis or prediction. This can make it difficult for clinicians to trust and use AI in their practice [119].
- Limited clinical validation: Many AI models have been developed and tested in research settings, but they have not been sufficiently validated in real-world clinical practice. This means that their performance in clinical settings may be significantly different from what was observed in the research setting [120].
- Cost and infrastructure: Implementing AI in clinical practice can be expensive, requiring significant resources and infrastructure. This can be a barrier to its widespread adoption, particularly in lower-resource settings [121].
Overall, while AI has great potential to improve CVD diagnosis, there are several limitations that need to be addressed before it can be widely adopted in clinical practice. Clinicians and researchers need to work together to ensure that AI is used in a responsible and effective way that benefits patients.
Conclusion
Novel biomarkers and imaging modalities have improved early detection and risk stratification. Advances in pharmacogenomics, gene therapy, cell therapy, and AI offer promising opportunities to develop personalized therapies and improve patient outcomes. However, challenges remain in validating biomarkers, making advanced technologies accessible, and integrating new approaches into clinical practice. Addressing these challenges through multidisciplinary collaboration and additional research will be key to reducing the global burden of cardiovascular diseases.
Recommendations
Further research is needed to validate emerging biomarkers and imaging techniques, determine their cost-effectiveness, and promote their integration into clinical guidelines and practice. More high-quality clinical trials should evaluate the real-world efficacy of novel therapies like gene therapy, cell therapy, and AI. Health policies and interventions must focus on making advanced technologies more equitably accessible. Medical education should incorporate training in new modalities and precision medicine approaches. Multidisciplinary teams including geneticists, data scientists, and clinicians should collaborate to elucidate the complex pathophysiology of cardiovascular diseases and translate research innovations into patient care. A patient-centered approach considering socioeconomic status, mental health, and quality of life should guide efforts to prevent and manage cardiovascular diseases.
List of Abbreviations
CVD: Cardiovascular Disease; AI: Artificial Intelligence; ECG: Electrocardiogram; NT-proBNP: N-terminal pro B-type Natriuretic Peptide; hs-CRP: High-sensitivity C-Reactive Protein; PET: Positron Emission Tomography; MRI: Magnetic Resonance Imaging; CT: Computed Tomography; PCI: Percutaneous Coronary Intervention; CABG: Coronary Artery Bypass Grafting; ACS: Acute Coronary Syndrome; HF: Heart Failure; AF: Atrial Fibrillation; LVAD: Left Ventricular Assist Device; ECMO: Extracorporeal Membrane Oxygenation; VAD: Ventricular Assist Device; LVEF: Left Ventricular Ejection Fraction; CRISPR: Clustered Regularly Interspaced Short Palindromic Repeats; CAR-T: Chimeric Antigen Receptor T-cell Therapy; LDL-C: Low-Density Lipoprotein Cholesterol; HDL-C: High-Density Lipoprotein Cholesterol; TG: Triglycerides; ACE: Angiotensin-Converting Enzyme; ARB: Angiotensin Receptor Blocker; NOAC: Non-vitamin K Antagonist Oral Anticoagulant; TAVR: transcatheter aortic valve replacement; PFO: Patent Foramen Ovale; Omega-3: Omega-3 Fatty Acids; BMI: Body Mass Index; DAPT: Dual Antiplatelet Therapy; CPR: Cardiopulmonary Resuscitation; CAD: Coronary Artery Disease; SCAI: Society for Cardiovascular Angiography and Interventions; ESC: European Society of Cardiology; AHA: American Heart Association; ACC: American College of Cardiology; HFrEF: Heart Failure with Reduced Ejection Fraction; HFpEF: Heart Failure with Preserved Ejection Fraction; LAA: Left Atrial Appendage; MI: Myocardial Infarction.
Declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable
Availability of data and materials
All data is available, and sharing is available as well as publication.
Competing interests
The authors hereby declare that they have no competing interests.
Funding
The corresponding author supplied all study materials. There was no further funding for this study.
Authors' contributions
The authors completed the study protocol and were the primary organizers of data collection and the manuscript's draft and revision process. Tamer A. Addissouky wrote the article and ensured its accuracy. All authors contributed to the discussion, assisted in designing the study and protocol and engaged in critical discussions of the draft manuscript. Lastly, the authors reviewed and confirmed the final version of the manuscript.
Acknowledgements
The authors thank all the researchers, editors, reviewers, and the supporting universities that have made great efforts on their studies. Moreover, we are grateful to the editors, reviewers, and readers of this journal.
References
2. Patel JC, Gupta A, Kumar P, Waidha KM, Deep A, Kumar A, et al. Cardiovascular diseases display etiological and seasonal trend in human population: Evidence from seasonal cardiovascular comorbid diseases (SCCD) index. American Journal of Human Biology. 2023 Jan 18:e23867.
3. Grudzińska A, Dudzińska P, Milanowska M, Jarosz D, Tsitko H. Sexual activity in terms of cardiac rehabilitation after cardiovascular incidents. Journal of Education, Health and Sport. 2023 Jan 20;13(3):98-104.
4. Tkachenko AL, Mazin AV, Tyurina MM. Analysis and modeling of the probability of developing cardiovascular diseases in humans to identify a risk group. In2nd International Conference on Computer Applications for Management and Sustainable Development of Production and Industry (CMSD-II-2022) 2023 Jan 5 (Vol. 12564, pp. 158-163). SPIE.
5. Herrmann J, López-Fernández T, Lyon AR. The year in cardiovascular medicine 2022: the top 10 papers in cardio-oncology. European Heart Journal. 2023 Feb 1;44(5):348-50.
6. Sanusi A, Elsey H, Golder S, Sanusi O, Oluyase A. Cardiovascular health promotion: A systematic review involving effectiveness of faith-based institutions in facilitating maintenance of normal blood pressure. PLOS Global Public Health. 2023 Jan 20;3(1):e0001496.
7. Aminova OS, Tyatenkova NN, Melentev AV. Assessment of the risks of cardiovascular system functional disorders developing in young people aged 18–25 years. Kazan Medical Journal. 2023 Mar 26;104(2):176-82.
8. Chrysant SG, Chrysant GS. Association of physical activity and trajectories of physical activity with cardiovascular disease. Expert Review of Cardiovascular Therapy. 2023 Feb 1;21(2):87-96.
9. Dibben GO, Faulkner J, Oldridge N, Rees K, Thompson DR, Zwisler AD, et al. Exercise-based cardiac rehabilitation for coronary heart disease: a meta-analysis. European Heart Journal. 2023 Feb 7;44(6):452-69.
10. Ski CF, Cartledge S, Foldager D, Thompson DR, Fredericks S, Ekman I, et al. Integrated care in cardiovascular disease: a statement of the Association of Cardiovascular Nursing and Allied Professions of the European Society of Cardiology. European Journal of Cardiovascular Nursing. 2023 Jan 6:zvad009.
11. Addissouky TA, Khalil AA, El Agroudy AE. Assessment of potential biomarkers for early detection and management of Glomerulonephritis Patients with Diabetic Diseases. American Journal of Clinical Pathology. 2023;160(Supplement_1):S18–S19.
12. Addissouky T, Ali M, El Sayed IE, Wang Y. Revolutionary innovations in diabetes research: from biomarkers to genomic medicine. Iranian Journal of Diabetes and Obesity. 2023 Dec 28; 15(4) :228-42.
13. Chen L, Li X, Lv Y, Tan X, Zhong VW, Rong S, et al. Physical frailty, adherence to ideal cardiovascular health and risk of cardiovascular disease: a prospective cohort study. Age and Ageing. 2023 Jan 1;52(1):afac311.
14. Ávila-Gandía V, Ramos-Campo DJ, García-Sánchez E, Luque-Rubia AJ, López A, López-Román FJ. Training, detraining and retraining effects of moderate vs. high intensity exercise training programme on cardiovascular risk factors. Journal of Hypertension. 2023 Mar;41(3):411-9.
15. Mohebi R, McCarthy CP, Magaret CA, Barnes G, Rhyne RF, Peters C, et al. Performance of a protein biomarker panel for prediction of cardiovascular events in patients with diabetes mellitus. European Journal of Preventive Cardiology. 2022 May 1;29(8):e270-1.
16. Piani F, Tossetta G, Cara-Fuentes G, Agnoletti D, Marzioni D, Borghi C. Diagnostic and Prognostic Role of CD93 in Cardiovascular Disease: A Systematic Review. Biomolecules. 2023 May 30;13(6):910.
17. Carmona-Maurici J, Rosa A, Azcona-Granada N, Peña E, Ricart-Jané D, Viñas A, et al. Irisin as a Novel Biomarker of Subclinical Atherosclerosis in Severe Obesity. International Journal of Molecular Sciences. 2023 May 3;24(9):8171.
18. Dai M, Li K, Sacirovic M, Zemmrich C, Buschmann E, Ritter O, et al. Autophagy-related genes analysis reveals potential biomarkers for prediction of the impaired walking capacity of peripheral arterial disease. BMC Medicine. 2023 May 18;21(1):186.
19. Rong J, He T, Zhang J, Bai Z, Shi B. Serum lipidomics reveals phosphatidylethanolamine and phosphatidylcholine disorders in patients with myocardial infarction and post-myocardial infarction-heart failure. Lipids in Health and Disease. 2023 Dec;22(1):66.
20. Cham Y, Fong AYY, Chung JBK, Ling HS, Pang IX, Thien LK, et al. The Novel Biomarker Growth Differentiation Factor-15 in Patients with Chronic Heart Failure at a Heart Failure Clinic in the Asia-Pacific Region: A Prospective Observational Study. Journal of Asian Pacific Society of Cardiology. 2023;2:e14.
21. Sæther JC, Vesterbekkmo EK, Taraldsen MD, Gigante B, Follestad T, Røsjø HR, et al. Associations between circulating microRNAs and lipid-rich coronary plaques measured with near-infrared spectroscopy. Scientific Reports. 2023 May 10;13(1):7580.
22. Huang Z, Klaric L, Krasauskaite J, Khalid W, Strachan MW, Wilson JF, et al. Combining serum metabolomic profiles with traditional risk factors improves 10-year cardiovascular risk prediction in people with type 2 diabetes. European Journal of Preventive Cardiology. 2023 May 12:zwad160.
23. Drăgan A, Sinescu I. The Role of the Cardiac Biomarkers in the Renal Cell Carcinoma Multidisciplinary Management. Diagnostics. 2023 May 30;13(11):1912.
24. Dutta A, Saha S, Bahl A, Mittal A, Basak T. A comprehensive review of acute cardio-renal syndrome: need for novel biomarkers. Frontiers in Pharmacology. 2023 May 23;14:1313.
25. Addissouky TA, El Sayed IE, Ali MM, Wang Y, El Baz A, Elarabany N, et al. Shaping the future of cardiac wellness: exploring revolutionary approaches in disease management and prevention. Journal of Clinical Cardiology. 2024 Jan 5;5(1):6-29.
26. Jafari M, Shoeibi A, Khodatars M, Ghassemi N, Moridian P, Alizadehsani R, et al. Automated diagnosis of cardiovascular diseases from cardiac magnetic resonance imaging using deep learning models: A review. Computers in Biology and Medicine. 2023 May 6:106998.
27. Mikail N, Rossi A, Bengs S, Haider A, Stähli BE, Portmann A, et al. Imaging of heart disease in women: review and case presentation. European Journal of Nuclear Medicine and Molecular Imaging. 2022 Dec;50(1):130-59.
28. Kwan JM, Arbune A, Henry ML, Hu R, Wei W, Nguyen V, et al. Quantitative cardiovascular magnetic resonance findings and clinical risk factors predict cardiovascular outcomes in breast cancer patients. Plos One. 2023 May 30;18(5):e0286364.
29. Addissouky TA, Sayed IE, Ali MM, Wang Y, Baz AE, Khalil AA, et al. Latest advances in hepatocellular carcinoma management and prevention through advanced technologies. Egyptian Liver Journal. 2024 Jan 2;14(1):2.
30. Addissouky TA, Ali MM, El Sayed IE, Wang Y, El Baz A, Elarabany N, et al. Preclinical promise and clinical challenges for innovative therapies targeting liver fibrogenesis. Archives of Gastroenterology Research. 2023 Nov 14;4(1):14-23.
31. Addissouky TA, Wang Y, Megahed FA, El Agroudy AE, El Sayed IE, El-Torgoman AM. Novel biomarkers assist in detection of liver fibrosis in HCV patients. Egyptian Liver Journal. 2021 Dec;11(1):86.
32. Addissouky TA, El-Agroudy AE, El-Torgoman AMAK, E. El-Sayed IE. Efficacy of Biomarkers in Detecting Fibrosis Levels of Liver Diseases. World Journal of Medical Sciences. 2019;16(1):11-8.
33. Addissouky TA, El Agroudy AE, El-Torgoman AM, El Sayed IE, Ibrahim EM. Efficiency of alternative markers to assess liver fibrosis levels in viral hepatitis B patients. Biomedical Research. 2019 Jan 15;30(2):351-6.
34. Addissouky T. Detecting Liver Fibrosis by Recent Reliable Biomarkers in Viral Hepatitis Patients. American Journal of Clinical Pathology. 2019 Oct 1;152:S85.
35. El Agroudy AE, Elghareb MS, Addissouky TA, Elshahat EH, Hafez EH. Serum hyaluronic acid as non invasive biomarker to predict liver fibrosis in viral hepatitis patients. Journal of Bioscience and Applied Research. 2016 May 24;2(5):326-33.
36. Addissouky TA, Ali M, Sayed IE, Wang Y. Emerging advanced approaches for diagnosis and inhibition of liver fibrogenesis. The Egyptian Journal of Internal Medicine. 2024 Dec;36(1):19.
37. Mobini N, Codari M, Riva F, Ienco MG, Capra D, Cozzi A, et al. Detection and quantification of breast arterial calcifications on mammograms: a deep learning approach. European Radiology. 2023 May 9:1-0.
38. Thupakula S, Nimmala SS, Ravula H, Chekuri S, Padiya R. Emerging biomarkers for the detection of cardiovascular diseases. The Egyptian Heart Journal. 2022 Oct 20;74(1):77.
39. Pezeshki PS, Ghorashi SM, Houshmand G, Ganjparvar M, Pouraliakbar H, Rezaei-Kalantari K, et al. Feature tracking cardiac magnetic resonance imaging to assess cardiac manifestations of systemic diseases. Heart Failure Reviews. 2023 May 16:1-11.
40. Al-Absi HR, Islam MT, Refaee MA, Chowdhury ME, Alam T. Cardiovascular disease diagnosis from DXA scan and retinal images using deep learning. Sensors. 2022 Jun 7;22(12):4310.
41. Raisi-Estabragh Z, Cooper J, McCracken C, Crosbie EJ, Walter FM, Manisty CH, et al. Incident cardiovascular events and imaging phenotypes in UK Biobank participants with past cancer. Heart. 2023 Jul 1;109(13):1007-15.
42. Mszar R, Bart S, Sakers A, Soffer D, Karalis DG. Current and Emerging Therapies for Atherosclerotic Cardiovascular Disease Risk Reduction in Hypertriglyceridemia. Journal of Clinical Medicine. 2023 Feb 9;12(4):1382.
43. Kumric M, Urlic H, Bozic J, Vilovic M, Ticinovic Kurir T, Glavas D, et al. Emerging Therapies for the Treatment of Atherosclerotic Cardiovascular Disease: From Bench to Bedside. International Journal of Molecular Sciences. 2023 Apr 29;24(9):8062.
44. Reed SC, Dhir N, Widmer RJ. Optimal cardiovascular medical therapy: current guidelines and new developments. Proceedings (Baylor University. Medical Center). 2022;35(5):636-42.
45. Butel-Simoes LE, Haw TJ, Williams T, Sritharan S, Gadre P, Herrmann SM, et al. Established and Emerging Cancer Therapies and Cardiovascular System: Focus on Hypertension—Mechanisms and Mitigation. Hypertension. 2023 Apr;80(4):685-710.
46. Yang Y, Gao J, Qin Z, Lu Y, Xu Y, Guo J, et al. The Present Clinical Treatment and Future Emerging Interdisciplinary for Heart Failure: Where We Are and What We Can Do. Intensive Care Research. 2023 Mar;3(1):3-11.
47. Herrera D, Sanz M, Shapira L, Brotons C, Chapple I, Frese T, et al. Association between periodontal diseases and cardiovascular diseases, diabetes and respiratory diseases: Consensus report of the Joint Workshop by the European Federation of Periodontology (EFP) and the European arm of the World Organization of Family Doctors (WONCA Europe). Journal of Clinical Periodontology. 2023 Jun;50(6):819-41.
48. Kronenberg F, Mora S, Stroes ES, Ference BA, Arsenault BJ, Berglund L, et al. Lipoprotein (a) in atherosclerotic cardiovascular disease and aortic stenosis: a European Atherosclerosis Society consensus statement. European Heart Journal. 2022 Oct 14;43(39):3925-46.
49. Kittleson MM, Panjrath GS, Amancherla K, Davis LL, Deswal A, Dixon DL, et al. 2023 ACC Expert Consensus Decision Pathway on Management of Heart Failure With Preserved Ejection Fraction: A Report of the American College of Cardiology Solution Set Oversight Committee. Journal of the American College of Cardiology. 2023 May 9;81(18):1835-78.
50. Fleenor BS, Carlini NA, Martens CR. Nutraceuticals in the Prevention and Therapeutic Treatment of Cardiovascular and Cerebrovascular Disease. Journal of Cardiopulmonary Rehabilitation and Prevention. 2023 May 1;43(3):162-9.
51. Delbaere Q, Chapet N, Huet F, Delmas C, Mewton N, Prunier F, et al. Anti-Inflammatory Drug Candidates for Prevention and Treatment of Cardiovascular Diseases. Pharmaceuticals. 2023 Jan 4;16(1):78.
52. Dziemitko S, Harasim-Symbor E, Chabowski A. How do phytocannabinoids affect cardiovascular health? An update on the most common cardiovascular diseases. Therapeutic Advances in Chronic Disease. 2023 Jan;14:20406223221143239.
53. Dimosiari A, Patoulias D, Kitas GD, Dimitroulas T. Do Interleukin-1 and Interleukin-6 Antagonists Hold Any Place in the Treatment of Atherosclerotic Cardiovascular Disease and Related Co-Morbidities? An Overview of Available Clinical Evidence. Journal of Clinical Medicine. 2023 Feb 6;12(4):1302.
54. Al-Mahayri ZN, Khasawneh LQ, Alqasrawi MN, Altoum SM, Jamil G, Badawi S, et al. Pharmacogenomics implementation in cardiovascular disease in a highly diverse population: initial findings and lessons learned from a pilot study in United Arab Emirates. Human Genomics. 2022 Dec;16(1):42.
55. Liu Y, Deng S, Song Z, Zhang Q, Guo Y, Yu Y, et al. MLIF modulates microglia polarization in ischemic stroke by targeting eEF1A1. Frontiers in Pharmacology. 2021 Sep 7;12:725268.
56. Sethi Y, Patel N, Kaka N, Kaiwan O, Kar J, Moinuddin A, et al. Precision Medicine and the future of Cardiovascular Diseases: A Clinically Oriented Comprehensive Review. Journal of Clinical Medicine. 2023 Feb 23;12(5):1799.
57. Li MY, Peng LM, Chen XP. Pharmacogenomics in drug-induced cardiotoxicity: Current status and the future. Frontiers in Cardiovascular Medicine. 2022 Oct 13;9:966261.
58. Wang X, Zhang M, Wang X. Chronic inflammation and pharmacological interventions in cardiovascular diseases. Frontiers in Pharmacology. 2022 Aug 24;13:993569.
59. Desjardins LC, Vohl MC. Precision Nutrition for Cardiovascular Disease Prevention. Lifestyle Genomics. 2023 Jan 30;16(1):73-82.
60. Kiiskinen T, Ripatti S. Genetic prediction of medication use patterns in cardiometabolic disease. Nature Medicine. 2023 Jan;29(1):43-4.
61. Venkat V, Abdelhalim H, DeGroat W, Zeeshan S, Ahmed Z. Investigating genes associated with heart failure, atrial fibrillation, and other cardiovascular diseases, and predicting disease using machine learning techniques for translational research and precision medicine. Genomics. 2023 Mar 1;115(2):110584.
62. Shawky A, Sabit H, Nazih M, Baraka K, El-Zawahry M. CYP2C19 Polymorphism in Ischemic Heart Disease Patients Taking Clopidogrel After Percutaneous Coronary Intervention in Egypt. Journal of Epidemiology and Global Health. 2023 Jun;13(2):374-83.
63. Sitinjak BD, Murdaya N, Rachman TA, Zakiyah N, Barliana MI. The Potential of Single Nucleotide Polymorphisms (SNPs) as Biomarkers and Their Association with the Increased Risk of Coronary Heart Disease: A Systematic Review. Vascular Health and Risk Management. 2023 Dec 31:289-301.
64. Salas-Hernández A, Galleguillos M, Carrasco M, López-Cortés A, Redal MA, Fonseca-Mendoza D, et al. An updated examination of the perception of barriers for pharmacogenomics implementation and the usefulness of drug/gene pairs in Latin America and the Caribbean. Frontiers in Pharmacology. 2023 May 11;14:1175737.
65. Sagaro GG, Angeloni U, Marotta C, Nittari G, Rezza G, Silenzi A, et al. The Magnitude of Cardiovascular Disease Risk Factors in Seafarers from 1994 to 2021: A Systematic Review and Meta-Analysis. Journal of Personalized Medicine. 2023 May 20;13(5):861.
66. Ganopoulou M, Moysiadis T, Gounaris A, Mittas N, Chatzopoulou F, Chatzidimitriou D, et al. Single Nucleotide Polymorphisms’ Causal Structure Robustness within Coronary Artery Disease Patients. Biology. 2023 May 12;12(5):709.
67. Sukasem C, Biswas M, Lungchukiet P, Sangtian M. Clinical pharmacogenomics implementation in Thailand: a dream come true. Pharmacogenomics. 2023 Apr;24(6):297-301.
68. Li MY, Peng LM, Chen XP. Pharmacogenomics in drug-induced cardiotoxicity: Current status and the future. Frontiers in Cardiovascular Medicine. 2022 Oct 13;9:966261.
69. Polasek TM. Calculation of the pharmacogenomics benefit score for patients with medication-related problems. Frontiers in Genetics. 2023 May 5;14:1152585.
70. Asiimwe IG, Pirmohamed M. Drug–Drug–Gene Interactions in Cardiovascular Medicine. Pharmacogenomics and Personalized Medicine. 2022 Dec 31:879-911.
71. Tardif JC, Pfeffer MA, Kouz S, Koenig W, Maggioni AP, McMurray JJ, et al. Pharmacogenetics-guided dalcetrapib therapy after an acute coronary syndrome: the dal-GenE trial. European Heart Journal. 2022 Oct 14;43(39):3947-56.
72. Johnson D, Wilke MA, Lyle SM, Kowalec K, Jorgensen A, Wright GE, et al. A systematic review and analysis of the use of polygenic scores in pharmacogenomics. Clinical Pharmacology & Therapeutics. 2022 Apr;111(4):919-30.
73. Chang R, Zhou W, Ye Y, Zhang X, Liu Y, Wu J, et al. Relationship between CYP2C19 polymorphism and clopidogrel resistance in patients with coronary heart disease and ischemic stroke in China. Genetics Research. 2022 Jan;2022:1901256.
74. Aerts N, Anthierens S, Van Bogaert P, Peremans L, Bastiaens H. Prevention of cardiovascular diseases in community settings and primary health care: a pre-implementation contextual analysis using the consolidated framework for implementation research. International Journal of Environmental Research and Public Health. 2022 Jul 11;19(14):8467.
75. LaMonte MJ. Cardiorespiratory Fitness in the Prevention and Management of Cardiovascular Disease. Reviews in Cardiovascular Medicine. 2022 Nov 15;23(11):382.
76. Alavudeen SS, Easwaran V, Khan NA, Venkatesan K, Paulsamy P, Mohammed Hussein AT, et al. Cardiovascular Disease-Related Health Promotion and Prevention Services by Pharmacists in Saudi Arabia: How Well Are They Prepared?. Healthcare. 2023 May 31;11(11):1614.
77. Zheng W, Xu F, Bian Y, Zhang J, Tang MX, Li CB, et al. Enhance the management of cardiac arrest and improve the prognosis of the patients. Zhonghua yi xue za zhi. 2023 Jun 1;103(21):1585-90.
78. Gabulova R, Marza-Florensa A, Isayeva M, Rahimov U, Gahramanova S, Alaskarova SH, et al. Arterial hypertension management in patients with confirmed coronary heart disease: how far are we from meeting prevention goals?. European Journal of Preventive Cardiology. 2023 Jun;30(Supplement_1):zwad125-303.
79. Khan Z, Rao S, Bhatia R, Ray S, Dalal JJ. Recommendations for cardiovascular disease prevention in women: An Indian perspective. Journal of the Practice of Cardiovascular Sciences. 2023 Jan 1;9(1):11-7.
80. Wang Y, Cui H, Li L, Cao Y, Qu H, Ailina H, et al. Digitalization of prevention and treatment and the combination of western and Chinese medicine in management of acute heart failure. Frontiers in Cardiovascular Medicine. 2023 May 25;10:1146941.
81. Knaepen L, Falter M, Scherrenberg M, Dendale P, Desteghe L, Heidbuchel H. Assessment of functionalities and attitude toward telemedicine for patients with cardiovascular disease. Digital Health. 2023 May;9:20552076231176941.
82. Mahalwar G, Kumar A, Kalra A. Virtual Cardiology: Past, Present, Future Directions, and Considerations. Current Cardiovascular Risk Reports. 2023 May 29:1-6.
83. Knaepen L, Falter M, Scherrenberg M, Dendale P, Desteghe L, Heidbuchel H. Perspective of patients on the use of telemedicine in cardiovascular care. Europace. 2023 Jun;25(Supplement_1):euad122-549.
84. Alarabyat IA, Al-Nsair N, Alrimawi I, Al-Yateem N, Shudifat RM, Saifan AR. Perceived barriers to effective use of telehealth in managing the care of patients with cardiovascular diseases: a qualitative study exploring healthcare professionals’ views in Jordan. BMC Health Services Research. 2023 Dec;23(1):452.
85. Omboni S. Telemedicine for hypertension management: where we stand, where we are headed. Conn Health. 2022 Jun 30;1:85-97.
86. Nomura A. Digital health, digital medicine, and digital therapeutics in cardiology: current evidence and future perspective in Japan. Hypertension Research. 2023 May 31;46:2126-34.
87. Borrelli N, Grimaldi N, Papaccioli G, Fusco F, Palma M, Sarubbi B. Telemedicine in Adult Congenital Heart Disease: Usefulness of Digital Health Technology in the Assistance of Critical Patients. International Journal of Environmental Research and Public Health. 2023 May 10;20(10):5775.
88. Shaito A, Thuan DT, Phu HT, Nguyen TH, Hasan H, Halabi S, et al. Herbal medicine for cardiovascular diseases: efficacy, mechanisms, and safety. Frontiers in Pharmacology. 2020 Apr 7;11:422.
89. Kabbani D, Akika R, Wahid A, Daly AK, Cascorbi I, Zgheib NK. Pharmacogenomics in practice: a review and implementation guide. Frontiers in Pharmacology. 2023 May 18;14:1189976.
90. Addissouky TA, Ali MM, El Sayed IE, Wang Y. Recent advances in diagnosing and treating helicobacter pylori through botanical extracts and advanced technologies. Archives of Pharmacology and Therapeutics. 2023 Nov 3;5(1):53-66.
91. Addissouky TA, Megahed FA, Elagroudy AE, El Sayed IE. Efficiency of mixture of olives oil and figs as an antiviral agent: a review and perspective. International Journal of Medical Science and Health Research. 2020 Aug;4(4):107-11.
92. Addissouky TA, Khalil AA, El Agroudy AE. Assessing the efficacy of a modified triple drug regimen supplemented with mastic gum in the eradication of helicobacter pylori infection. American Journal of Clinical Pathology. 2023;160(Supplement_1):S19.
93. Tripathi P, Gupta G, Chauhan PS. Role of phytomedicine in diabetes and cardiovascular diseases. In: New Look to Phytomedicine. Academic Press; 2019. pp. 409-433.
94. Aggarwal K, Madan S, Sarwat M. Traditional nutritional and health practices to tackle the lifestyle diseases. In: Herbal Medicines. Academic Press; 2022. pp. 253-269.
95. Lin JG, Huang GJ, Su YC. Efficacy analysis and research progress of complementary and alternative medicines in the adjuvant treatment of COVID-19. J Biomed Sci. 2023;30:30.
96. Fan Y, Yang Z, Wang L, Liu Y, Song Y, Liu Y, et al. Traditional Chinese medicine for heart failure with preserved ejection fraction: clinical evidence and potential mechanisms. Frontiers in Pharmacology. 2023 May 10;14:1154167.
97. Lee B, Ha NY, Park HJ, Kim AR, Kwon OJ, Cho JH, et al. Herbal Medicine Yukgunja-Tang for Functional Dyspepsia: A Protocol for a Randomized, Controlled, Multicenter Clinical Trial. Healthcare. 2023 May 17;11(10):1456.
98. Wu T, Li S, Li Z, Long W, Liu Q, Tang H, et al. Efficacy and safety of Ginkgo biloba dropping pills in the treatment of coronary heart disease with stable angina pectoris and depression: study protocol for a randomised, placebo-controlled, parallel-group, double-blind and multicentre clinical trial. BMJ Open. 2023 May 1;13(5):e055263.
99. Kulkarni P, Mahadevappa M, Chilakamarri S. The emergence of artificial intelligence in cardiology: Current and future applications. Current Cardiology Reviews. 2022 May 5;18(3):e191121198124.
100. Ledziński Ł, Grześk G. Artificial Intelligence Technologies in Cardiology. Journal of Cardiovascular Development and Disease. 2023 May 6;10(5):202.
101. Addissouky TA, Wang Y, El Sayed IE, Baz AE, Ali MM, Khalil AA. Recent trends in Helicobacter pylori management: harnessing the power of AI and other advanced approaches. Beni-Suef University Journal of Basic and Applied Sciences. 2023 Sep 2;12(1):80.
102. Addissouky TA, El Agroudy AE, Khalil AA. Developing a novel non-invasive serum-based diagnostic test for early detection of colorectal cancer. American Journal of Clinical Pathology. 2023 Nov 1;160(Supplement_1):S17.
103. Addissouky TA, El Sayed IE, Ali MM, Wang Y, El Baz A, Khalil AA,et al. Can vaccines stop cancer before it starts? Assessing the promise of prophylactic immunization against high-risk preneoplastic lesions. Journal of Cellular Immunology. 2023 Nov 29;5(4):127-40.
104. Addissouky TA, Khalil AA. Detecting Lung Cancer Stages Earlier By Appropriate Markers Rather Than Biopsy And Other Techniques. American Journal of Clinical Pathology. 2020 Oct;154(Supplement_1):S146-7.
105. Schepart A, Burton A, Durkin L, Fuller A, Charap E, Bhambri R,et al. Artificial intelligence–enabled tools in cardiovascular medicine: A survey of current use, perceptions, and challenges. Cardiovascular Digital Health Journal. 2023 May 3;4(3):101-10.
106. Wang ZY, Guo ZH. Intelligent Chinese Medicine: A New Direction Approach for Integrative Medicine in Diagnosis and Treatment of Cardiovascular Diseases. Chinese Journal of Integrative Medicine. 2023 Jul;29(7):634-643.
107. Das S, Sultana M, Bhattacharya S, Sengupta D, De D. XAI–reduct: accuracy preservation despite dimensionality reduction for heart disease classification using explainable AI. The Journal of Supercomputing. 2023 May 12:1-31.
108. Park J, Yoon Y, Cho Y, Kim J. Feasibility of Artificial Intelligence–Based Electrocardiography Analysis for the Prediction of Obstructive Coronary Artery Disease in Patients With Stable Angina: Validation Study. JMIR cardio. 2023 May 2;7(1):e44791.
109. Garcha I, Phillips SP. Social bias in artificial intelligence algorithms designed to improve cardiovascular risk assessment relative to the Framingham Risk Score: a protocol for a systematic review. BMJ open. 2023 May 1;13(5):e067638.
110. Tan Y, Sun X. Ocular images-based artificial intelligence on systemic diseases. BioMedical Engineering OnLine. 2023 Dec;22(1):1-4.
111. Addissouky TA, Ali MMA, El Sayed IET, Wang Y, Khalil AA. Translational insights into molecular mechanisms of chemical hepatocarcinogenesis for improved human risk assessment. Advances in Clinical Toxicology. 2024;9(1):294.
112. Addissouky TA, Wang Y, El Tantawy El Sayed I, Majeed MAA, Khalil AA. Emerging technologies and advanced biomarkers for enhanced toxicity prediction and safety pharmacology. Advances in Clinical Toxicology. 2024;9(1):293.
113. Addissouky TA, Wang Y, El Tantawy El Sayed I, Majeed MAA, Khalil AA. Transforming toxicity assessment through microphysiology, bioprinting, and computational modeling. Advances in Clinical Toxicology. 2024;9(1):295.
114. Addissouky TA, El Sayed IE, Ali MM. Regenerating Damaged Joints: The Promise of Tissue Engineering and Nanomedicine in Lupus Arthritis. J Clinical Orthopaedics and Trauma Care. 2024;6(2):2694-0248.
115. Addissouky TA, El Sayed IE, Ali MM. Conservative and Emerging Rehabilitative Approaches for Knee Osteoarthritis Management. J Clinical Orthopaedics and Trauma Care. 2024;6(2):2694-0248.
116. Biswas N, Ali MM, Rahaman MA, Islam M, Mia MR, Azam S, et al. Machine Learning-Based Model to Predict Heart Disease in Early Stage Employing Different Feature Selection Techniques. BioMed Research International. 2023 May 2;2023:6864343.
117. Ganipineni VD, Gutlapalli SD, Paramsothy J, Okorie IJ, Ugwendum D, Farid MA, et al. Artificial Intelligence in Cardiovascular Medicine: A Comprehensive Clinical Review. Preprints.org 2023, 2023051061. https://doi.org/10.20944/preprints202305.1061.v1
118. Barriada RG, Masip D. An Overview of Deep-Learning-Based Methods for Cardiovascular Risk Assessment with Retinal Images. Diagnostics. 2022 Dec 26;13(1):68.
119. Arooj S, Rehman SU, Imran A, Almuhaimeed A, Alzahrani AK, Alzahrani A. A Deep Convolutional Neural Network for the Early Detection of Heart Disease. Biomedicines. 2022 Nov 3;10(11):2796.
120. Romiti S, Vinciguerra M, Saade W, Anso Cortajarena I, Greco E. Artificial intelligence (AI) and cardiovascular diseases: an unexpected alliance. Cardiology Research and Practice. 2020 Jun 27;2020:4972346.
121. Taylan O, Alkabaa AS, Alqabbaa HS, Pamukçu E, Leiva V. Early prediction in classification of cardiovascular diseases with machine learning, neuro-fuzzy and statistical methods. Biology. 2023 Jan 11;12(1):117.