Abstract
Gastric cancer is the sixth most common malignancy worldwide and is associated with substantial morbidity and mortality.
A 32-year-old woman was diagnosed with stage IV signet-ring cell gastric adenocarcinoma with peritoneal carcinomatosis, a Sister Mary Joseph nodule, and rare diffuse colonic metastases.
The patient received CAPOX-therapy, followed by maintenance ramucirumab plus paclitaxel, and subsequently irinotecan monotherapy; due to feeding difficulties, a nasojejunal feeding tube was placed.
Over 20 months of follow-up, only minimal clinical progression was observed, and the patient’s condition remained stable. This case underscores the importance of early detection, multidisciplinary care, and individualized therapeutic strategies in aggressive gastric cancer.
Keywords
Gastric cancer, Signet-ring cell adenocarcinoma, Sister Mary Joseph nodule, Peritoneal carcinomatosis, Colonic metastasis, CAPOX, Ramucirumab, Irinotecan
Introduction
Gastric cancer is the sixth most common malignancy worldwide, with approximately 1.2 million new cases diagnosed annually [1]. Although the overall incidence of gastric cancer has steadily declined in recent years, the incidence of a particularly aggressive subtype -signet ring cell adenocarcinoma—has been increasing [2]. Signet ring cell gastric adenocarcinoma accounts for approximately 25–30% of all malignant gastric tumors and is associated with a substantially poorer prognosis than other histologic subtypes [3].
The risk factors for this subtype mirror those of other histologic types of gastric cancer, including Helicobacter pylori infection, obesity, dietary habits, smoking, and alcohol consumption [4–6]. Notably, although these malignancies are most often sporadic, a mutation in the CDH1 tumor suppressor gene has been identified that confers a predisposition to this subtype [7,8].
From a therapeutic perspective, management of signet-ring cell gastric adenocarcinoma is multidisciplinary; both surgical resection and systemic chemotherapy are recommended, although recent studies indicate that chemotherapy alone may be sufficient in selected patient groups [9]. It is also noteworthy that ongoing investigations suggest that, in early-stage of this disease, endoscopic submucosal dissection (ESD) may become a standard treatment modality [10].
Case Presentation
A 32-year-old previously healthy woman in good general condition presented to our hospital’s emergency department during on-call hours with persistent, progressively worsening abdominal pain since giving birth to her child. She reported regular bowel movements and urination without abnormalities and denied any change in stool habit. Her socioeconomic background was favorable; she comes from an educated family and works as a teacher. Her height (approximately 160 cm) and weight (approximately 60 kg) were unremarkable, with a normal BMI index of approximately 23.5. On further inquiry, she stated that her symptoms had begun one year earlier, during pregnancy, but she had attributed them to her pregnancy. There was no family history of malignancy; she is a non-smoker and does not consume alcohol regularly.
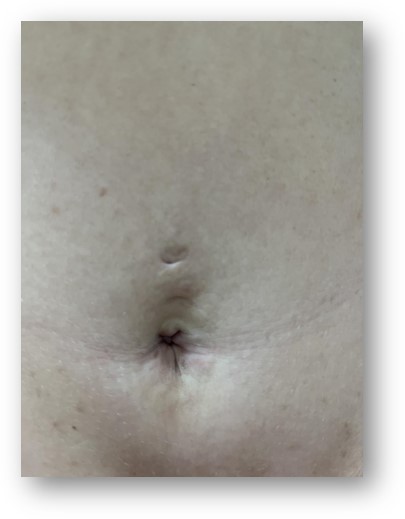
The initial attending physician in the emergency department performed a standard emergency and internal medical examination, which revealed no abnormalities of the neck and chest, and the neurological status was unremarkable as well. Two findings were noteworthy on the physical examination: a tender, well-palpable intra-abdominal mass in the periumbilical region—approximately the size of a clenched fist, and a small -roughly 1 cm in diameter- erythematosus nodule within the umbilical fossa (Figure 1). A palpable abdominal mass by itself does not indicate malignant diseases; additional imaging and specialist evaluation are required to establish a definitive diagnosis [11]. In light of the subsequent diagnosis, the erythematous cutaneous lesion is a Sister Mary Joseph nodule, which occurs in abdominopelvic adenocarcinomas in approximately 1–3% of cases and predicts a very poor prognosis, with an average survival of 11 months [12]. The nodule can be interpreted as a paraneoplastic manifestation—namely, a cutaneous metastasis of gastrointestinal malignancies—which may often be among the earliest signs of an underlying abdominal cancer [13].
Figure 1. Umbilical erythematous skin nodule (Sister Mary Joseph nodule).
Initial laboratory testing demonstrated mild leukocytosis (13.5 G/L) with moderate neutrophilia (72%), with no other clinically relevant aberrations observed. It is well documented that, in certain malignancies, tumor-induced immune responses in surrounding tissues can lead to leukocytosis in the peripheral blood counts; this finding is typically associated with poorer survival outcomes [14]. A surgical consultation was requested in the emergency department, and the on-call surgeon recommended contrast-enhanced abdominal CT to further characterize the well-palpable abdominal mass, as abdominal ultrasonography is not available during on-call hours at our institution. Following surgical examination, an acute abdominal process was not suspected based on the clinical presentation and history; the patient remained cardiopulmonary stable throughout the evaluation, and her long-standing symptoms were unchanged [15]. Given the patient’s age and overall condition, the abdominal CT was delayed, and an urgent abdominal ultrasound was scheduled for the following day [16]. The ultrasonography revealed conglomerate-like small-bowel loops with a small volume of free intraperitoneal fluid; however, the origin and nature of these findings could not be definitively determined with this imaging modality.
For further diagnostic clarification—consistent with the prior surgical recommendation—contrast-enhanced computed tomography (CT) was performed with a little delay, which identified peritoneal carcinomatosis and bilaterally enlarged ovaries. Peritoneal carcinomatosis is commonly present in various gastrointestinal and gynecologic malignancies, and when present it is typically associated with inferior survival metrics [17]. The carcinomatosis also involved the upper part of the urinary bladder and caused partial small- and large-bowel obstruction, raising the possibility of a primary ovarian tumor. Based on the radiological appearance, the consulting radiologist recommended magnetic resonance imaging (MRI). Recent research indicates that MRI allows a more accurate assessment of gynecological neoplastic lesions [18]. Subsequent abdominal and pelvic MRI did not demonstrate intrahepatic or nodal metastases but described several benign, asymptomatic findings, including left renal cysts and uterine fibroids. These abnormalities do not account for the patient’s symptoms; they are typically benign, relatively common, and infrequently require medical intervention. However, on the MRI, numerous peritoneal metastases were also described, along with a small volume of free intraperitoneal fluid and multiple tiny nodular lesions that likely represent pathologic lymph nodes or consistent with the CT findings—pelvic carcinomatosis. The MRI did not reveal any tumor-suggestive abnormalities in the ovaries.
Although imaging studies excluded the possibility of a primary gynecological tumor, the patient’s CA-125 level was markedly elevated. This tumor marker is frequently elevated in various gynecological malignancies [19]. Nevertheless, although CA-125 is informative for monitoring treatment efficacy and prognosis in these conditions, it is not specific to any particular cancer type and is therefore not diagnostic on its own [20]. In view of the elevated CA-125, the patient was also evaluated by a gynecologist, who saw no indication for gynecological intervention and considered the bilaterally enlarged ovaries to be benign incidental findings.
Accordingly, given the high likelihood of a primary gastrointestinal malignancy, the patient was admitted to our hospital’s Gastroenterology Department for further evaluation. After brief preparation, gastroscopy and colonoscopy were planned. Gastroscopy revealed a large antral lesion measuring approximately 4–5 cm (Figure 2), which was clinically highly suggestive of malignancy. While histopathologic sampling is required to establish malignancy, based on the endoscopic appearance the experienced gastroenterologist considered the lesion highly likely to be malignant [21]. Morphologically, the lesion resembled a doughnut with a central, fissure-like deep depression. Biopsies were obtained from this area; notably, the lesion felt distinctly firm and rigid during sampling. Histopathologic examination of the biopsy specimens confirmed grade IV signet-ring cell adenocarcinoma (Figure 3), which a gastric tumor subtype with extremely poor prognosis that typically affects young women. It accounts for approximately 25–30% of all gastric cancers, with a markedly low average survival [22].
Figure 2. Endoscopic image of an antral 4–5 cm lesion.
Figure 3. Grade IV signet ring cell adenocarcinoma (histology).
On the same day following gastroscopy, colonoscopy was performed, which demonstrated multiple erythematous masses covered by eroded mucosa throughout the entire colon (Figure 4). Histopathological examination confirmed these pathological lesions to be metastatic adenocarcinoma. Metastatic involvement of the colon is exceedingly rare for any primary malignancy; approximately 1–3% of all colonic tumors are metastatic in origin [23]. Notably, a 14-year investigation at Chonnam National University in South Korea identified only 13 cases in which a primary gastric tumor metastasized to the colon. This presentation is therefore distinctly uncommon, and the precise pathomechanism, and clinical characteristics remain poorly defined [24]. In addition, colonoscopy revealed unequivocally extraluminal lesions producing impressions on the mucosal surface. In the judgment of the experienced gastroenterologist performing the examination, these lesions are most likely represented peritoneal metastases —in accordance with the prior imaging examinations— or, alternatively, pathologically enlarged lymph nodes.
Figure 4. Colonoscopic changes: diffuse, eroded, mass-like lesions.
With the histopathologic results available, the case was reviewed by the multidisciplinary oncological team. Given that both surgical and systemic oncologic management are applicable for this tumor subtype, the patient received combination chemotherapy with capecitabine and oxaliplatin (CAPOX). CAPOX is one of the most often used first-line chemotherapy worldwide in metastatic gastric cancer [25]. The chemotherapeutic management of signet-ring cell gastric cancer remains incompletely defined. In general, these tumors respond less favorably to neoadjuvant therapy than non–signet-ring cell counterparts, whereas the uncommon chemosensitive cases are typically associated with improved survival. Clinical experience suggests that taxane-based regimens combined with platinum derivatives provide the most favorable survival outcomes. To these days, no universally established targeted therapy exists; however some studies indicate that this disease tends to respond well to the anti-VEGFR2 monoclonal antibody—ramucirumab—whereas responses to anti-HER2 agents are generally uncommon. Nevertheless, individualized case-by-case assessment is warranted [26].
Six months after diagnosis, following CAPOX treatment, the case was referred to a surgeon experienced in CRS–HIPEC surgeries, who recommended diagnostic laparoscopy as the initial step. During this procedure, extensive peritoneal carcinomatosis was identified, and radical surgery was therefore deferred. Intraoperative histopathologic sampling demonstrated that the disease had responded well to the previously administered oxaliplatin.
Subsequently, the therapy was transitioned to ramucirumab plus paclitaxel (RAM + TAX), which is a standard second-line treatment option [27]. The treatment plan was changed because the tumor showed no regression, but also no progression. The patient tolerated this regimen well, and a CT performed 18 months after diagnosis demonstrated minimal progression.
In June 2025, systemic therapy was modified to irinotecan, administered every three weeks as monotherapy. This change was necessary because of minimal progression after 18 months of RAM + TAX therapy. Irinotecan monotherapy can be a later-line treatment for patients with advanced gastric tumors and according to studies it does not need to be combined [28]. At that time—approximately 20 months after the initial tumor diagnosis—clinical symptoms were minimal to barely perceptible.
In July 2025, owing to feeding difficulties, the patient was re-admitted to our hospital. The gastric tumor had caused a stricture, resulting in repeated vomiting of poorly ingested food at home. She declined the offered surgical option; therefore, a nasojejunal feeding tube was placed under endoscopic guidance, and enteral nutrition was initiated via the tube. Endoscopy-guided nasojejunal feeding tube placement is a common gastroenterological procedure with excellent success rates [29].
In August 2025, she presented to the treating oncology institute for the third cycle of irinotecan therapy. Her overall condition was poor; physical examination revealed tense ascites, for which a peritoneal drainage catheter was inserted to permit fluid evacuation over 1–2 weeks. At the time of placement, approximately 3 liters of clear fluid were drained, and 1,000 mL of intravenous fluid replacement was administered. The correct management of ascites-paracentesis, peritoneal catheters, shunts—has a great positive impact on the patients’ quality of life [30,31]. Her general condition was poor, but she remained cardiopulmonary stable.
During the observation period, the treatments were well tolerated; however, from August 2025 her overall condition declined, psychological support was provided, and she continued to tolerate therapy well both physically and mentally.
Discussion and Conclusions
Gastric cancer is the sixth most common malignancy worldwide. As noted in the introduction, signet-ring cell gastric adenocarcinomas accompanied by a Sister Mary Joseph nodule are typically associated with poor average survival; however, owing to early detection and appropriate multidisciplinary oncologic and gastroenterologic management, this patient achieved a survival duration several-fold greater than the expected average, underscoring the importance of accurately recognizing cutaneous manifestations that may herald underlying visceral malignancy. Our case is remarkable in the international context, as colorectal metastases in gastric cancer are extremely rare, with only a few scattered case reports available in the literature. It is also a sign of a very aggressive tumor [32].
Signet ring cell carcinoma component is known to significantly worsen outcomes for patients with colorectal cancer; however the overall survival depends on the primary tumor location [33,34]. The 5-year survival of patients with stage IV signet ring cell carcinoma is approximately 4-5% [35]. The patient’s unusually long 20-month survival further underscores the significance of this report, as it clearly exceeds the average survival reported for patients in similar stages.
Thus, this case is noteworthy not only for its rarity but also for offering hope and a new perspective for clinical practice. Although this is a single case, the reported clinical course provides lessons that are generalizable to everyday practice. Diagnostic accuracy, multidisciplinary management, timely therapeutic decision-making, and emphasis on palliative support are all aspects that extend beyond this report and may guide the broader medical community. In this way, the case contributes to the collective knowledge base and facilitates the international exchange of experience in managing rare and complex malignancies.
Patient Consent
Written informed consent was obtained from the patient for publication of this case report; no identifying information has been included.
Ethics
Written informed consent was obtained from the patient for publication of this case report and accompanying images.
Funding
The authors received no fundings.
Conflicts of Interest
There is no conflict of interests.
References
2. Fung BM, Patel M, Patel N, Brown AF, Ostrzega NL, Tabibian JH. Signet Ring Cell Gastric Carcinoma: Clinical Epidemiology and Outcomes in a Predominantly Latino County Hospital Population. Dig Dis Sci. 2021 Apr;66(4):1240–8.
3. Pernot S, Voron T, Perkins G, Lagorce-Pages C, Berger A, Taieb J. Signet-ring cell carcinoma of the stomach: Impact on prognosis and specific therapeutic challenge. World J Gastroenterol. 2015 Oct 28;21(40):11428–38.
4. Mamun TI, Younus S, Rahman MH. Gastric cancer-Epidemiology, modifiable and non-modifiable risk factors, challenges and opportunities: An updated review. Cancer Treat Res Commun. 2024;41:100845.
5. Thrift AP, Wenker TN, El-Serag HB. Global burden of gastric cancer: epidemiological trends, risk factors, screening and prevention. Nat Rev Clin Oncol. 2023 May;20(5):338–49.
6. Duan Y, Xu Y, Dou Y, Xu D. Helicobacter pylori and gastric cancer: mechanisms and new perspectives. J Hematol Oncol. 2025 Jan 23;18(1):10.
7. Kole C, Charalampakis N, Sakellariou S, Papaxoinis G, Apostolou KG, Machairas N, et al. Hereditary Diffuse Gastric Cancer: A 2022 Update. J Pers Med. 2022 Dec 8;12(12):2032.
8. Shah D, Bentrem D. Environmental and Genetic Risk Factors for Gastric Cancer. Cancer Treat Res. 2024;192:1–17.
9. Yu C, Yang J, Li H, Wang J, Jin K, Li Y, et al. Prognostic prediction and treatment options for gastric signet ring cell carcinoma: a SEER database analysis. Front Oncol. 2024 Oct 21;14:1473798.
10. Tang YH, Ren LL, Mao T. Update on diagnosis and treatment of early signet-ring cell gastric carcinoma: A literature review. World J Gastrointest Endosc. 2023 Apr 16;15(4):240–7.
11. Expert Panel on Gastrointestinal Imaging; Fowler KJ, Garcia EM, Kim DH, Cash BD, Chang KJ, et al. ACR Appropriateness Criteria® Palpable Abdominal Mass-Suspected Neoplasm. J Am Coll Radiol. 2019 Nov;16(11S):S384–91.
12. Bellahyane I, Moukhlissi M, Bouabid M, Berhili S, Mezouar L. Sister Mary Joseph Syndrome: A Report of a Rare Case. Cureus. 2024 Jul 17;16(7):e64707.
13. Schadt CR. The cutaneous manifestations of gastrointestinal malignancy. Semin Oncol. 2016 Jun;43(3):341–6.
14. Kim KH, Sim NS, Chang JS, Kim YB. Tumor immune microenvironment in cancer patients with leukocytosis. Cancer Immunol Immunother. 2020 Jul;69(7):1265–77.
15. Börner N, Kappenberger AS, Weber S, Scholz F, Kazmierczak P, Werner J. The Acute Abdomen: Structured Diagnosis and Treatment. Dtsch Arztebl Int. 2025 Mar 7;122(5):137–44.
16. Poosiri S, Krisanachinda A, Khamwan K. Evaluation of patient radiation dose and risk of cancer from CT examinations. Radiol Phys Technol. 2024 Mar;17(1):176–85.
17. Coccolini F, Gheza F, Lotti M, Virzì S, Iusco D, Ghermandi C, et al. Peritoneal carcinomatosis. World J Gastroenterol. 2013 Nov 7;19(41):6979–94.
18. Engbersen MP, Van Driel W, Lambregts D, Lahaye M. The role of CT, PET-CT, and MRI in ovarian cancer. Br J Radiol. 2021 Sep 1;94(1125):20210117.
19. Magalhães JS, Jammal MP, Crispim PCA, Murta EFC, Nomelini RS. Role of biomarkers CA-125, CA-15.3 and CA-19.9 in the distinction between endometriomas and ovarian neoplasms. Biomarkers. 2021 May;26(3):268–74.
20. Zhang M, Cheng S, Jin Y, Zhao Y, Wang Y. Roles of CA125 in diagnosis, prediction, and oncogenesis of ovarian cancer. Biochim Biophys Acta Rev Cancer. 2021 Apr;1875(2):188503.
21. Kim JH, Kim YJ, An J, Lee JJ, Cho JH, Kim KO, et al. Endoscopic features suggesting gastric cancer in biopsy-proven gastric adenoma with high-grade neoplasia. World J Gastroenterol. 2014 Sep 14;20(34):12233–40.
22. Altay SB, Akkurt G, Yılmaz N, Özdemir N. Clinicopathological Evaluation of Gastric Signet Ring Cell Carcinoma: Our Experience. Euroasian J Hepatogastroenterol. 2020 Jul-Dec;10(2):76–84.
23. Hanzel J, Ranković B, Ribnikar M. Metastatic Gastric Signet Ring Cell Adenocarcinoma Presenting With Colonic Stenosis. Cureus. 2020 Jun 3;12(6):e8420.
24. Jung JY, Lee J, Park HM, Lee SY, Jung MR, Kim CH, et al. Colorectal metastasis from gastric cancer: insights from a 14-year case series at a tertiary hospital. J Gastrointest Oncol. 2024 Dec 31;15(6):2437–46.
25. Peng Z, Wei J, Wang F, Ying J, Deng Y, Gu K, et al. Camrelizumab Combined with Chemotherapy Followed by Camrelizumab plus Apatinib as First-line Therapy for Advanced Gastric or Gastroesophageal Junction Adenocarcinoma. Clin Cancer Res. 2021 Jun 1;27(11):3069–78.
26. Pernot S, Voron T, Perkins G, Lagorce-Pages C, Berger A, Taieb J. Signet-ring cell carcinoma of the stomach: Impact on prognosis and specific therapeutic challenge. World J Gastroenterol. 2015 Oct 28;21(40):11428–38.
27. Shitara K, Van Cutsem E, Gümüş M, Lonardi S, de la Fouchardière C, Coutzac C, et al. Trastuzumab Deruxtecan or Ramucirumab plus Paclitaxel in Gastric Cancer. N Engl J Med. 2025 Jul 24;393(4):336–48.
28. Sakai D, Kadowaki S, Kawabata R, Hara H, Satake H, Takahashi M, et al. Randomized Phase III Trial of Ramucirumab Beyond Progression Plus Irinotecan in Patients With Ramucirumab-Refractory Advanced Gastric Cancer: RINDBeRG Trial. J Clin Oncol. 2025 Jul;43(19):2196–207.
29. Lu G, Xiang Q, Wang S, Pan M, Xiang X, Yang Y, et al. Endoscopic- versus x-ray-guidance for placement of nasojejunal tubes in critically ill patients: a systematic review and meta-analysis of randomized controlled trials. Am J Transl Res. 2022 Apr 15;14(4):2134–46.
30. Meyer L, Suidan R, Sun C, Westin S, Coleman RL, Mills GB. The management of malignant ascites and impact on quality of life outcomes in women with ovarian cancer. Expert Rev Qual Life Cancer Care. 2016;1(3):231–8.
31. Reshamwala PA. Management of ascites. Crit Care Nurs Clin North Am. 2010 Sep;22(3):309–14.
32. Mohamed M, Al Hillan A, Zurkovsky E, Zheng M, Asif A, Akhtar R, et al. A Rare Case of Signet Cell Carcinoma of Transverse Colon in a Young Patient With Ulcerative Colitis. J Med Cases. 2020 May;11(5):135–9.
33. Wang L, Hirano Y, Heng G, Ishii T, Kondo H, Hara K, et al. Does signet ring cell carcinoma component signify worse outcomes for patients with colorectal cancer? Asian J Surg. 2021 Jan;44(1):105–10.
34. Wu SG, Chen XT, Zhang WW, Sun JY, Li FY, He ZY, et al. Survival in signet ring cell carcinoma varies based on primary tumor location: a Surveillance, Epidemiology, and End Results database analysis. Expert Rev Gastroenterol Hepatol. 2018 Feb;12(2):209–14.
35. Yang LL, Wang M, He P. Clinicopathological characteristics and survival in colorectal signet ring cell carcinoma: a population-based study. Sci Rep. 2020 Jun 26;10(1):10460.