Abstract
Introduction: Peptic ulcer disease (PUD) is the most common disease of the stomach and duodenum, which affects daily life and is associated with Helicobacter pylori and drug-induced problems. PHD occurs due to increased aggressive factors (like HCl, gastrin, pepsin, etc.) and decreased protective factors (like mucosa secretion, and prostaglandin activity). According to recent research, up to 10% of the global population suffers with PUD. Proton pump inhibitors, H2 receptor blockers, prostaglandin analogues, and many other medications are frequently used to treat PUD, but because of their harmful effects and potential for drug interactions, they should not be taken in conjunction with normal bodily conditions. The goal of this review paper was to promote Chinese herbal medicinal plants, which have potent effects and less toxic effects in daily uses.
Methodology: In this review, 72 plants were identified and noted with their plant name, Chinese name, family, etc. All the information was obtained from Google Scholar, PubMed, Sci Finder, and Cochrane by using scientific terms like ‘peptic ulcer’, ’stomach ulcer’, and ‘duodenum ulcer’ and we took a lot of data from review and research articles.
Results: Our list of Chinese herbal plants consists of mostly the relevant data regarding Chinese herbs and all the listed drugs have a high potential effect against PUD.
Discussion: According to the reported research data, all the listed Chinese herbal plants have a high potential against PUD.
Keywords
GPreapphitciacl A ubsltcraectr , Chinese medicine, Helicobacter pylori, Stomach ulcer, Duodenum
Introduction
Peptic ulcer disease (PUD) is a digestive tract-related problem associated with acid-induced lesions in the stomach or duodenum [1]. Risk factors like H. pylori infection, alcohol, tobacco consumption, and drug-induced like NSAIDs and Zollinger-Ellison syndrome. Recent data claimed that 5-10% of the population suffers from PUD [2] and the day-by-day rate of hospital cases, and mortality associated with PUD decreases [3,4]. Mucosal disruption, hypersecretory acid with dietary factors, and stress are major factors causing PUD [5]. Recent data suggested that NSAIDs, aspirin use, and H. pylori infection increase the risk factor prevalence in developing countries like Africa, Central America, Asia, and Europe [6].
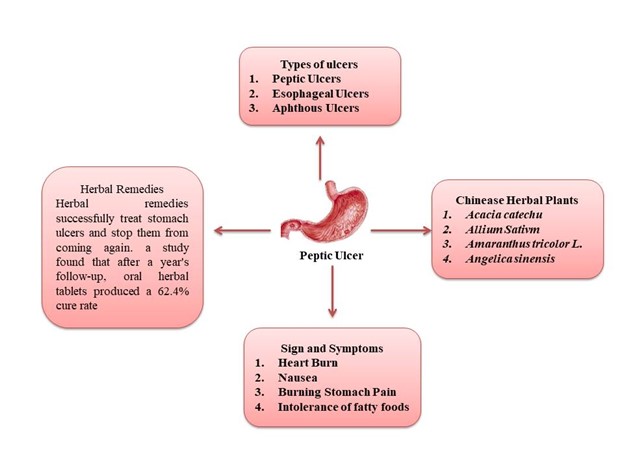
Sign and symptoms
They are many signs and symptoms such as [7,8]:
- Burning stomach pain
- Feeling of fullness, bloating or belching
- Intolerance to fatty foods
- Heartburn
- Nausea
Treatments of peptic ulcer
Chronic PUD is treated differently depending on the ulcer's etiology (HP or NSAID), whether it is an initial or recurring ulcer, and whether complications have developed. Treatments as a whole focused on minimizing ulcer-related problems, treating the ulcer, and preventing its recurrence. In HP positive patients with an active ulcer, a history of an ulcer-related complication, or both, the aim of therapy is to remove HP, heal the ulcer, and cure the condition [9-11].
Pathophysiology
Majority of PUD is caused by either H. pylori or NSAIDs. Some studies like the Danish study reported that stress is also a physiological incidence of peptic ulcer [12].
It is not entirely clear how H. pylori cause the development of various diseases in the gastroduodenal mucosa. The type of peptic ulcer can be determined by the H. pylori infection, which can cause either hyperchlorhydria or hypochlorhydria. Though H. pylori can directly alter the H+/K+ ATPase-subunit, activate calcitonin gene-related peptide (CGRP) sensory neurons linked to somatostatin, or suppress the formation of gastrin, cytokines that restrict parietal cell secretion are the principal mediators of H. pylori infection [13]. While hyposecretion is linked to the development of stomach ulcers, 10-15% of patients with H. pylori infection have lower antral somatostatin levels and enhanced gastric secretion due to hypergastrinemia [14]. Increased histamine production results from this, which in turn causes increased stomach and parietal secretion of acid or pepsin [15].
The primary mechanism of NSAID-induced damage to the gastroduodenal mucosa is the systemic inhibition of constitutively expressed cyclooxygenase-1 (COX-1), which is linked to reduced mucosal blood flow, low mucus and bicarbonate secretion, and inhibition of cell proliferation. COX-1 is responsible for prostaglandin synthesis. The enzyme is reversibly and concentration-dependently inhibited by NSAIDs. Co-administration of exogenous prostaglandins and cyclooxygenase-2 (COX-2) selective nonsteroidal anti-inflammatory drugs (NSAIDs) minimizes mucosal damage and ulcer risk [16].
NSAID toxicity varies, nevertheless, due to their various physicochemical characteristics. NSAIDs cause the uncoupling of mitochondrial oxidative phosphorylation and disturb mucusal phospholipids, which starts the damage to mucosa. NSAIDs become protonated when they come into contact with acidic stomach juice (pH 2), which allows them to pass lipid membranes and enter epithelial cells (pH 7.4) without ionizing [17].
Ecological data
The most prevalent upper gastrointestinal tract condition is gastric ulceration. In the Western population, the prevalence of stomach ulcers is 2.4%, with annual incidence rates ranging from 0.10% to 0.19% [18,19]. Up to 6.07% of the general population in some areas of Mainland China suffers from gastric ulcers because of H. pylori bacteria, poor eating habits, smoking, history of gastrointestinal disorders, and family history of stomach cancer. Gastric ulcers affect 22.5% of patients who have gastrointestinal symptoms [20,21]. Those who use alcohol, smoke, or take nonsteroidal anti-inflammatory medicines (NSAIDs) typically have higher incidences [22]. The rate of recurrence can reach 60% [23]. The economic burden of gastric ulcers is substantial. In the US, the average yearly medical expense for a stomach ulcer is $23,819 [24]. The annual medical expenses in South Korea for stomach ulcers vary from $959.6 to $2553 [25].
Herbal remedies
Herbal remedies are effective in curing stomach ulcers in a variety of animal models, including those caused by ethanol, NSAIDs, cold-restraint stress, pylorus ligation, and erosive agents. The way herbal medicines are prepared and used determines how effective they are as a treatment in each model [26].
Herbal remedies successfully treat stomach ulcers and reduce the recurrence rate. For instance, a study found that after a year's follow-up, oral herbal tablets produced a 62.4% cure rate and a 17.7% recurrence rate. By comparison, the cure rate for ranitidine treatment was only 50.7%, and the recurrence rate was 54.1% [27].
Chinese communities have been using Traditional Chinese Medicine (TCM) for over 2,500 years [15] and since ancient times. The introduction of acupuncture to Western nations in the 1600s was a major contribution to TCM [28]. A significant addition to TCM's knowledge of general health concerns is the variolation vaccine, which was created in China around the 16th century to protect against smallpox [29]. TCM has grown to be an essential component of Chinese healthcare; in 2006, the industry treated over 200 million outpatients and 7 million inpatients, making up 10%–20% of all medical care in China [30].
The majority of recent studies on Chinese herbal medicine (CHM) use scientific methods to assess the safety and effectiveness of the herbs. However, the treatments of CHM are mostly linked to the traditional tales because of how important its cultural and religious core is. Chinese scientists studied CHM with modern technologies and approaches after World War II, and they were extremely successful in discovering artemisinin [31].
This led to the innovation of CHM through scientific drug development. This is not a simple trip, though, as we must battle the antiquated beliefs that are rooted in its history. The comprehensive ideas of CHM also spark a lot of discussion. We maintained the paradigm by resolving the two crucial issues in the connection between CHM and science [32].
A more comprehensive analysis revealed that there was no discernible cost difference per subject between eradication therapy and placebo, despite some studies showing that H. pylori eradication therapy is cost-effective [33]. Conventional regimens are effective, but their therapeutic utility is sometimes limited by their inevitable adverse effects [34]. Nonetheless, research in both clinical and experimental settings have shown that herbal remedies are more effective in treating stomach ulcers while posing fewer adverse effects. Furthermore, the expense of using herbal medication to treat stomach ulcers is just roughly one-sixth that of using Western treatment [35]. This study reviews the safety, effectiveness, and mechanisms of action of herbal remedies for the treatment of stomach ulcers. The Chinese herbal plants that are responsible for peptic ulcer treatment are listed in Table 1.
|
No. |
Plant Name |
Chinese Name |
Family |
Part Used |
Chemical Constituents |
Experimental Animals |
Refs. |
|
1 |
Acacia catechu |
Khair or Kaat |
Mimosaceae |
Heartwood |
Catechin |
Albino rats |
36 |
|
2 |
Acacia ferruginea DC. |
Rusty Acacia |
Mimosaceae |
Stem bark |
Quercetin |
Wistar rats |
37 |
|
3 |
Acacia nilotica L. |
Gum arabic tree |
Mimosaceae |
Young seedless pods |
Tannins, flavonoids, alkaloids, and saponins |
Albino Wistar rats |
37 |
|
4 |
Acer tegmento- sum Maxim. |
Manchustripe Maple |
Sapindaceae |
Heartwood |
Salidroside |
ICR mice |
38 |
|
5 |
Achillea mille- folium L. |
Yarrow |
Asteraceae |
Aerial parts |
Achilleine |
Wistar rats |
39 |
|
6 |
Allium Sativm |
Garlic |
Liliaceae |
Bulb and garlic cloves |
Alliin |
Wistar rats |
40 |
|
7 |
Allophylus serratus Kurz |
Tippani |
Sapindaceae |
Leaves |
Quercetin |
Sprague-Dawley rats |
41 |
|
8 |
aloe vera (L.) Burm. |
Aloe |
Liliaceae |
Leaves juice or gel |
Aloin |
Mice |
42 |
|
9 |
Alpinia calcarata Roscoe |
Snap Ginger or Cardamom |
Zingiberaceae |
Rhizome |
Calcaratarins A |
Albino rats |
43 |
|
10 |
Alpinia galanga L. |
Galangal |
Zingiberaceae |
Rhizome |
1'S-1'- acetoxychavicol acetate |
Sprague-Dawley rats |
36 |
|
11 |
Alstonia scholaris L. |
Blackboard tree |
Apocynaceae |
Leaves |
Scholaricine |
Swiss albino mice |
44 |
|
12
|
Amaranthus spinosus L. |
Prickly amaranthus |
Amaranthaceae |
Root, stem, and leaves |
Flavonoids, saponins, and tannins |
Wistar strain albino rats |
45 |
|
13 |
Amaranthus tricolor L. |
Edible amaranth |
Amaranthaceae |
Leaves |
Flavonoids, saponins, and steroidal glycosides |
Rats |
46 |
|
14 |
Angelica poly- morpha Maxim |
Zijingsha |
Apiaceae |
Root |
Bisabolangelone |
Sprague-Dawley rats |
47 |
|
15 |
Angelica sinensis |
Dong quai (female ginseng) |
Apiaceae |
Root |
Polysaccharides |
Sprague-Dawley rats |
48 |
|
16 |
Aralia elata (Miq.) Seem. |
Japanese angelica-tree |
Araliaceae |
Root bark |
Araloside |
Sprague-Dawley rats |
49 |
|
17 |
Arctium lappa L. |
Burdock |
Asteraceae |
Leaves |
Cynarine |
Wistar rats |
50 |
|
18 |
Azadirachta indica |
Neem |
Meliaceae |
Seed |
Azadiradione |
Rats |
51 |
|
19 |
Basella alba var. alba. |
Indian Spinach
|
Basellaceae |
Leaves |
Flavonoids, proteins, mucilage and saponins |
Albino Wistar rats |
52 |
|
20 |
Bauhinia Variegata L. |
Orchid tree |
Caesalpiniaceae |
Root |
Flavonoids |
Rats |
53 |
|
21 |
Beta vulgaris |
Beetroot |
Chenopodiaceousae |
Root |
Betaine |
Wistar rats |
54 |
|
22 |
Bidens pilosa L. |
Black-jack |
Asteraceae |
Leaves |
Flavonoids and polyacetylenes |
Swiss mice |
55 |
|
23 |
Brassica rapa L. |
Field mustard |
Brassicaceae |
Turnip root |
Sulforaphane |
mice |
56 |
|
24 |
Bryophyllum pinnatum (Lam.) Kurz |
Cathedral bells |
Crassulaceae |
Leaves |
Flavonoids |
Swiss mice |
57 |
|
25 |
Butea frondosa Roxb. |
Flame |
Fabaceae |
Leaves |
Butrin |
Albino mice |
58 |
|
26 |
Caesalpinia crista L. |
Crested fever nut |
Caesalpiniaceae |
Seed |
Tannin, flavonoids, glycosides, and alkaloids |
Wister rats |
59 |
|
27 |
Calotropis gigantea |
Crown flower |
Asclepiadaceae |
Leaves |
Calotropin |
Wister rats |
60 |
|
28 |
Camellia sinensis (L.) Kuntze |
Green tea |
Theaceae |
Leaves |
Catechin |
Rats |
61 |
|
29 |
Capparis zeylanica L. |
Asadhua or ardanda |
Capparidaceae |
Leaves |
Saponin, p- hydroxybenzoic, vanillic, ferrulic and p-coumanic acid |
Albino rats |
62 |
|
30 |
Centella asiatica (L.) Urb. |
Gotu kola |
Apiaceae |
Leaves |
Castillicetin |
Sprague Dawley rats |
63 |
|
31 |
Cinnamomum cassia |
Cinnamon |
Lauraceae |
Dry bark |
Eugenol |
Albino rats |
64 |
|
32 |
Citrus aurantium L. |
Bitter orange or Marmalade orange |
Rutaceae |
Fruit |
β-myrcene, |
Rats |
65 |
|
33 |
Citrus lemon |
Lemon |
Rutaceae |
Fruit bark |
Limonene |
In vitro anti- H. pylori activity |
66 |
|
34 |
Cocculus hirsutus L. |
Broom creeper or Patalgarudi |
Menisperma- ceae |
Leaves |
Alkaloids, flavonoids, and phenolic compounds, |
In vitro anti-Helicobacter pylori activity |
67
|
|
35 |
Coriandrum sativum L. |
Coriander |
Apiaceae |
Seed |
Linalool |
Wistar rats |
68 |
|
36 |
Cuphea aequipetala Cav. |
Mexican Loose- strife |
Lythraceae |
Aerial parts |
Polyphenols and flavonoid |
In vitro anti-Helicobacter pylori activity and mice |
69 |
|
37 |
Curcuma longa L. |
Turmeric |
Zingiberaceae |
Root and rhizome |
Curcumin (diferu- loylmethan) |
Rats |
70 |
|
38 |
Curcuma xant- horrhiza Roxb. |
Temulawak |
Zingiberaceae |
Leaves |
Curcuminoids |
Sprague–Dawley rats |
71 |
|
39 |
Desmostachia bipinnata (L.) Stapf |
Saved gram |
Poaceae |
Aerial parts |
Kaempferol |
Wister albino rats |
72 |
|
40 |
Emblica officinalis
|
Amla |
Phyllanthaceae |
Fruit |
Phenolic, flavonoid and carotenoid
|
In vitro anti-Helicobacter pylori activity |
73 |
|
41 |
Excoecaria agallocha L. |
Milky mangrove |
Euphorbiaceae |
Bark |
Excolabdona, excolabdoneb and excolabdone C |
Albino rats |
74 |
|
42 |
Ficus religiosa |
Sacred fig |
Moraceae |
Bark |
Naringenin |
Wistar rats |
75 |
|
43 |
Geranium wil- fordii Maxim |
Edibility Rating or Medicinal Rating |
Geraniaceae |
Aerial parts |
Flavonoids |
In vitro anti-Helicobacter pylori activity |
76 |
|
44 |
Ginkgo biloba |
Ginkgo or maidenhair tree |
Ginkgoaceae |
Leaves |
Ginkgolides |
Wistar albino rats |
77 |
|
45 |
Gynura procum- bens |
Longevity spinach |
Asteraceae |
Leaves |
Flavonoids |
Sprague-Dawley rats |
78 |
|
46 |
Hippophae rhamnoides l. |
Sea buckthorn |
Elaeagnaceae |
Aerial parts |
Carotenoid (α, β,γ), riboflavin, folic acid and tannin |
Wistar albino rats |
79 |
|
47 |
Indigofera tincto- ria |
True indigo or Neelum |
Papilionaceae |
Leaves |
Indican (a glucoside) |
Albino rats |
75 |
|
48 |
Lycium chinense Mill |
Goji berry or wolfberry |
Solanaceae |
Aerial parts |
Apigenin |
ICR mice |
0 |
|
49 |
Malus domestica |
Apple |
Rosaceae |
Fruit |
Polyphenol |
Rats |
78 |
|
50 |
Morus alba L.
|
White mulberry |
Moraceae |
Leaves |
Flavonoids and phenolic acid |
Rats |
63 |
|
51 |
Murraya koenigii |
Curry tree |
Rataceae |
---------- |
Alkaloids, Gycozoline, Xanthotoxin and Sesquiterpene |
Albino rats |
82 |
|
52 |
Nyctanthes arbor-tristis L. |
Night jasmine |
Oleaceae |
Seed |
Arbortristoside-A |
Rats |
83 |
|
53 |
Oroxylum indi- cum (L.) Kurz |
Broken bones plant |
Bignoniaceae |
Root bark |
Baicalein |
Wistar albino rats |
84 |
|
54 |
Paeonia lactiflo- ra |
Chinese peony |
Paeoniaceae |
Root |
Paeonol |
In vitro anti-Helicobacter pylori activity |
85 |
|
55 |
Paeonia suffruti- cosa Andrews |
Mokdanpi |
Paeoniaceae |
Root Cortex |
Paeonol |
In vitro anti-Helicobacter pylori activity and Sprague-- Dawley rats |
86 |
|
56 |
Panax ginseng
|
Ginseng |
Araliaceae |
Root |
Ginsenoside R |
Rats |
87 |
|
57 |
Phyllanthus urinaria L. |
Chamber bitter or gripe weed
|
Phyllanthaceae |
Aerial parts |
Flavonoids |
In vitro anti-Helicobacter pylori activity |
88 |
|
58 |
Physalis alkekengi L. var. franchetii (Mast.) Makino |
Groundcherries |
Solanaceae |
Aerial parts |
Flavonoids |
In vitro anti-Helicobacter pylori activity and rats |
89 |
|
59
|
Plumbago indica
|
Indian leadwort |
Plumbagina- ceae |
Root |
Flavonoids |
In vitro anti-Helicobacter pylori activity |
90 |
|
60 |
Polygonum chinense L. |
Creeping smartweed |
Polygonaceae |
Leaves |
Flavonoids |
Male & Female Sprague - Dawley rats |
91 |
|
61 |
Prunus mume Siebold et Zucc
|
Chinese plum or Japanese apricot |
Rosaceae |
Fruit-juice |
Flavonoids |
In vitro anti-Helicobacter pylori activity and Mongolian gerbils |
92 |
|
62 |
Punica granatum L. |
------ |
Punicaceae |
Leaves |
Flavonoids |
Rats |
93 |
|
63 |
Rubus coreanus |
Korean Blackberry |
Rosaceae |
Fruit |
Anthocyanins |
Rats |
94 |
|
64 |
Tephrosia calophylla Bedd.
|
-------- |
Fabaceae |
Root |
Kaempferol-3-O- D-glucoside |
In vitro anti-Helicobacter pylori activity and Albino Swiss mice |
94 |
|
65 |
Tephrosia maxima L. |
----- |
Fabaceae |
Root |
Isoflavone |
In vitro anti-Helicobacter pylori activity and Albino Swiss mice |
94 |
|
66 |
Tephrosia purpurea Pers. |
Wild indigo |
Fabaceae |
Root |
Rotenoids, fla- vanones, isofla- vanones and coumarins |
In vitro anti-Helicobacter pylori activity and Albino Swiss mice |
94 |
|
67 |
Terminalia bellerica |
Behada |
Combretaceae |
Fruit |
Gallic acid |
Rats |
95 |
|
68 |
Terminalia chebula Retz. |
Chebulic myrobalan or black myrobalan |
Combretaceae |
Fruit |
Chebulinic acid |
Sprague Dawley rats |
96 |
|
69 |
Tinospora sagittata var. craveniana |
Vernacular |
Menisperma- ceae |
Aerial parts |
Palmatine |
In vitro anti-Helicobacter pylori activity |
97 |
|
70 |
Toona ciliata Roemer |
Red Cedar |
Meliaceae |
Heartwood |
Phenolic acids, flavonoids, coumarin, stilbenes and tannins |
Rats |
98 |
|
71 |
Wedelia calendulacea Less. |
Pitabringi or pila bhangra |
Asteraceae |
Whole plant |
Wedeloloactone |
Albino rats and albino mice |
75 |
|
72 |
Zingiber officinalis Roscoe |
Ginger |
Zingiberaceae |
Rhizome |
Gingerol |
Wistar rats |
99 |
|
73 |
Ziziphus jujube
|
Jujube |
Rhamnaceae |
Stem bark |
Quercetin |
Albino Wistar rats |
100 |
Discussion
Numerous botanicals with anti-ulcer properties have been shown (mostly through ethno-pharmacological investigations) (Table 1). Nonetheless, the majority of the published research has mostly concentrated on the pharmacological effects in test animals. Numerous studies have demonstrated that traditional Chinese medicine plants use a wide variety of herbs to treat gastrointestinal issues. Numerous findings have surfaced on natural products with anti-ulcer properties, including flavonoids, alkaloids, lactones from sesquiterpenes, diterpenes, and saponins. However, due to their anti-ulcer properties, flavonoids—the most prevalent secondary metabolites in the majority of plants—are especially noteworthy. The most prevalent plant groups that were shown to be anti-PUD were Asteraceae, Fabaceae, Apiaceae, Cucurbitaceae, and Lamiacea. In order to confirm the effectiveness and safety of these items in the clinical environment, proof-of-concept randomized controlled trials are advised, given the encouraging results on the anti-PUD action of a number of Chinese medicinal herbs and phytochemicals derived from plants.
Conclusion
Many Chinese herbal plants were identified, and out of them 72 herbal plants consist of anti-ulcer activity (Table 1). Many researchers reported that all these plants consist of potent pharmacological action for the treatment of PUD. In the end, we concluded that Chinese herbal plants may replace traditional drugs with fewer adverse effects. Given the promising findings on the anti-PUD activity of several medicinal plants and plant-derived phytochemicals, proof-of-concept randomized controlled trials are recommended to be carried out to verify the efficacy and safety of these products in the clinical setting. The Chinese plants have important characteristics that serve as the foundation of numerous research fields; thus it requires careful social research. They may be able to treat ailments and provide new research opportunities in the future.
Declaration of Competing Interest
Authors have no competing interests to declare regarding the publication of this paper.
Funding Support
None.
References
2. Lanas A, García-Rodríguez LA, Polo-Tomás M, Ponce M, Quintero E, Perez-Aisa MA, et al. The changing face of hospitalisation due to gastrointestinal bleeding and perforation. Aliment Pharmacol Ther. 2011 Mar;33(5):585-91.
3. Sonnenberg A. Review article: historic changes of Helicobacter pylori-associated diseases. Aliment Pharmacol Ther. 2013 Aug;38(4):329-42.
4. Zhang BB, Li Y, Liu XQ, Wang PJ, Yang B, Bian DL. Association between vacA genotypes and the risk of duodenal ulcer: a meta-analysis. Mol Biol Rep. 2014 Nov;41(11):7241-54.
5. Siddique O, Ovalle A, Siddique AS, Moss SF. Helicobacter pylori Infection: An Update for the Internist in the Age of Increasing Global Antibiotic Resistance. Am J Med. 2018 May;131(5):473-79.
6. Hooi JKY, Lai WY, Ng WK, Suen MMY, Underwood FE, Tanyingoh D, et al. Global Prevalence of Helicobacter pylori Infection: Systematic Review and Meta-Analysis. Gastroenterology. 2017 Aug;153(2):420-29.
7. Youb F, Khullar V, Banerjee D, Stoner P, Lambrou T, Westerveld DR, et al. Once Versus Twice-Daily Oral Proton Pump Inhibitor Therapy for Prevention of Peptic Ulcer Rebleeding: A Propensity Score-Matched Analysis. Gastroenterology Res. 2018 Jun;11(3):200-206.
8. Gnanapandithan K, Sharma A. Mesenteric Vasculitis. StatPearls [Internet]. StatPearls Publishing; Treasure Island (FL): Jul 2, 2021.
9. Rainsford KD. The effects of 5-lipoxygenase inhibitors and leukotriene antagonists on the development of gastric lesions induced by nonsteroidal antiinflammatory drugs in mice. Agents Actions. 1987 Aug;21(3-4):316-9.
10. Singh S. Evaluation of gastric anti-ulcer activity of fixed oil of Ocimum basilicum Linn. and its possible mechanism of action. Indian J Exp Biol. 1999 Mar;37(3):253-7.
11. Kumar V, Abbas AK, Fausto N. Robbins and Cotran Pathologic basis of disease. 7th edition. New Delhi: Saunders; 2003. pp. 787-802.
12. Levenstein S, Rosenstock S, Jacobsen RK, Jorgensen T. Psychological stress increases risk for peptic ulcer, regardless of Helicobacter pylori infection or use of nonsteroidal anti-inflammatory drugs. Clin Gastroenterol Hepatol. 2015 Mar;13(3):498-506.e1.
13. Zaki M, Coudron PE, McCuen RW, Harrington L, Chu S, Schubert ML. H. pylori acutely inhibits gastric secretion by activating CGRP sensory neurons coupled to stimulation of somatostatin and inhibition of histamine secretion. Am J Physiol Gastrointest Liver Physiol. 2013 Apr 15;304(8):G715-22.
14. El-Omar EM, Oien K, El-Nujumi A, Gillen D, Wirz A, Dahill S, et al. Helicobacter pylori infection and chronic gastric acid hyposecretion. Gastroenterology. 1997 Jul;113(1):15-24.
15. Moss SF, Legon S, Bishop AE, Polak JM, Calam J. Effect of Helicobacter pylori on gastric somatostatin in duodenal ulcer disease. Lancet. 1992 Oct 17;340(8825):930-2.
16. Coxib and traditional NSAID Trialists' (CNT) Collaboration; Bhala N, Emberson J, Merhi A, Abramson S, Arber N, Baron JA, et al. Vascular and upper gastrointestinal effects of non-steroidal anti-inflammatory drugs: meta-analyses of individual participant data from randomised trials. Lancet. 2013 Aug 31;382(9894):769-79.
17. Bjarnason I, Scarpignato C, Takeuchi K, Rainsford KD. Determinants of the short-term gastric damage caused by NSAIDs in man. Aliment Pharmacol Ther. 2007 Jul 1;26(1):95-106.
18. Groenen MJ, Kuipers EJ, Hansen BE, Ouwendijk RJ. Incidence of duodenal ulcers and gastric ulcers in a Western population: back to where it started. Can J Gastroenterol. 2009 Sep;23(9):604-8.
19. Sung JJ, Kuipers EJ, El-Serag HB. Systematic review: the global incidence and prevalence of peptic ulcer disease. Aliment Pharmacol Ther. 2009 May 1;29(9):938-46.
20. Dong WG, Cheng CS, Liu SP, Yu JP. Epidemiology of peptic ulcer disease in Wuhan area of China from 1997 to 2002. World J Gastroenterol. 2004 Nov 15;10(22):3377-9.
21. Li Z, Zou D, Ma X, Chen J, Shi X, Gong Y, et al. Epidemiology of peptic ulcer disease: endoscopic results of the systematic investigation of gastrointestinal disease in China. Am J Gastroenterol. 2010 Dec;105(12):2570-7.
22. Maity P, Biswas K, Roy S, Banerjee RK, Bandyopadhyay U. Smoking and the pathogenesis of gastroduodenal ulcer--recent mechanistic update. Mol Cell Biochem. 2003 Nov;253(1-2):329-38.
23. Fujino S, Suzuki Y, Tanaka T. Cost-benefit analysis of medicinal treatment for gastric ulcers. Long-term model including healing and recurrence. Health Policy. 1985;5(1):45-72.
24. Joish VN, Donaldson G, Stockdale W, Oderda GM, Crawley J, Sasane R, et al. The economic impact of GERD and PUD: examination of direct and indirect costs using a large integrated employer claims database. Curr Med Res Opin. 2005 Apr;21(4):535-44.
25. Song HJ, Kwon JW, Kim N, Park YS. Cost Effectiveness Associated with Helicobacter pylori Screening and Eradication in Patients Taking Nonsteroidal Anti-Inflammatory Drugs and/or Aspirin. Gut Liver. 2013 Mar;7(2):182-9.
26. Jainu M, Devi CS. Antiulcerogenic and ulcer healing effects of Solanum nigrum (L.) on experimental ulcer models: possible mechanism for the inhibition of acid formation. J Ethnopharmacol. 2006 Mar 8;104(1-2):156-63.
27. Li JB, Jin YQ. [Treatment of peptic ulcer with jian-wei yu-wang tablets]. Zhong Xi Yi Jie He Za Zhi. 1991 Mar;11(3):141-3, 131-2.
28. ang JL, Zhan SY, Ernst E. Review of randomised controlled trials of traditional Chinese medicine. BMJ. 1999 Jul 17;319(7203):160-1.
29. Wang G, Mao B, Xiong ZY, Fan T, Chen XD, Wang L, et al; CONSORT Group for Traditional Chinese Medicine. The quality of reporting of randomized controlled trials of traditional Chinese medicine: a survey of 13 randomly selected journals from mainland China. Clin Ther. 2007 Jul;29(7):1456-67.
30. Zhang L, Yan J, Liu X, Ye Z, Yang X, Meyboom R, et al. Pharmacovigilance practice and risk control of Traditional Chinese Medicine drugs in China: current status and future perspective. J Ethnopharmacol. 2012 Apr 10;140(3):519-25.
31. Wang M, Lamers RJ, Korthout HA, van Nesselrooij JH, Witkamp RF, van der Heijden R, et al. Metabolomics in the context of systems biology: bridging traditional Chinese medicine and molecular pharmacology. Phytother Res. 2005 Mar;19(3):173-82.
32. Chan K. Chinese medicinal materials and their interface with Western medical concepts. J Ethnopharmacol. 2005 Jan 4;96(1-2):1-18.
33. Ford AC, Delaney BC, Forman D, Moayyedi P. Eradication therapy in Helicobacter pylori positive peptic ulcer disease: systematic review and economic analysis. Am J Gastroenterol. 2004 Sep;99(9):1833-55.
34. Schunack W. Pharmacology of H2-receptor antagonists: an overview. J Int Med Res. 1989;17 Suppl 1:9A-16A.
35. Xiao YL, Nie YQ, Hou XH, Xie PY, Fang JY, Yuan YZ, et al. The efficacy, safety and cost-effectiveness of hydrotalcite versus esomeprazole in on-demand therapy of NERD: A multicenter, randomized, open-label study in China. J Dig Dis. 2013 Sep;14(9):463-8.
36. Patankar R, Devale T, Pophale R, Gawande V, Raghav N. Antiulcer activity of acacia catechu willd in rats. Int J Res Ayurveda Pharm. 2011:2.
37. Sowndhararajan K, Kang SC. Protective effect of ethyl acetate fraction of Acacia ferruginea DC. against ethanol-induced gastric ulcer in rats. J Ethnopharmacol. 2013 Jun 21;148(1):175-81.
38. Yoo YM, Nam JH, Kim MY, Choi J, Lee KT, Park HJ. Analgesic and anti-gastropathic effects of salidroside isolated from Acer tegmentosum heartwood. Open Bioactive Compd J 2009 Jan 26;2(1):1-7.
39. Cavalcanti AM, Baggio CH, Freitas CS, Rieck L, de Sousa RS, Da Silva-Santos JE, et al. Safety and antiulcer efficacy studies of Achillea millefolium L. after chronic treatment in Wistar rats. J Ethnopharmacol. 2006 Sep 19;107(2):277-84.
40. Matsuda H, Pongpiriyadacha Y, Morikawa T, Ochi M, Yoshikawa M. Gastroprotective effects of phenylpropanoids from the rhizomes of Alpinia galanga in rats: structural requirements and mode of action. Eur J Pharmacol. 2003 Jun 13;471(1):59-67.
41. Dharmani P, Mishra PK, Maurya R, Singh Chauhan V, Palit G. Allophylus serratus: a plant with potential anti-ulcerogenic activity. J Ethnopharmacol. 2005 Jul 14;99(3):361-6.
42. Park CH, Son HU, Yoo CY, Lee SH. Low molecular-weight gel fraction of Aloe vera exhibits gastroprotection by inducing matrix metalloproteinase-9 inhibitory activity in alcohol-induced acute gastric lesion tissues. Pharm Biol. 2017 Dec;55(1):2110-2115.
43. Arambewela L, Arawwawala L, Ratnasooriya W. Effect of Alpinia calcarata rhizomes on ethanol-induced gastric ulcers in rats.Pharmacogn Mag 2009;5(19):226.
44. Arulmozhi S, Papiya Mitra Mazumder LS, Prasad A. Analgesic, Anti-inflammatory and Anti-ulcerogenic Activities of Fractions from Alstonia scholaris. Pharmacologia.2012; 3: 132-7.
45. Mitra PK. Comparative evaluation of anti-gastric ulcer activity of root, stem and leaves of Amaranthus spinosus Linn. in rats. Int Jou Herbal Med. 2013;1:22-9.
46. Devaraj VC, Krishna BG. Gastric antisecretory and cytoprotective effects of leaf extracts of Amaranthus tricolor Linn. in rats. Zhong Xi Yi Jie He Xue Bao. 2011 Sep;9(9):1031-8.
47. Wang J, Zhu L, Zou K, Cheng F, Dan F, Guo Z, et al. The anti-ulcer activities of bisabolangelone from Angelica polymorpha. J Ethnopharmacol. 2009 Jun 22;123(2):343-6.
48. Ye YN, So HL, Liu ES, Shin VY, Cho CH. Effect of polysaccharides from Angelica sinensis on gastric ulcer healing. Life Sci. 2003 Jan 10;72(8):925-32.
49. Lee EB, Kim OJ, Kang SS, Jeong C. Araloside A, an antiulcer constituent from the root bark of Aralia elata. Biol Pharm Bull. 2005 Mar;28(3):523-6.
50. Carlotto J, da Silva LM, Dartora N, Maria-Ferreira D, Sabry Dde A, Filho AP, et al. Identification of a dicaffeoylquinic acid isomer from Arctium lappa with a potent anti-ulcer activity. Talanta. 2015 Apr;135:50-7.
51. Singh R, Mishra V, Pandeti S, Palit G, Barthwal MK, Pandey HP, et al. Cytoprotective and Anti-secretory Effects of Azadiradione Isolated from the Seeds of Azadirachta indica (neem) on Gastric Ulcers in Rat Models. Phytother Res. 2015 Jun;29(6):910-6.
52. Kumar V, Bhat ZA, Kumar D, Khan NA, Chashoo IA, Ara I. Gastroprotective effect of leaf extracts of Basella alba var. alba against experimental gastric ulcers in rats.Rev. Bras. Farmacogn. 2012;22:657-62.
53. Kumar YR, Rajani G. Extracts of Root of Bauhinia variegata Linn. Int J Pharmacol. 2011;7:616-22.
54. Arora R, Trivedi N. Evaluation of synergistic antiulcer potential of ethanolic extract of beta vulgaris (taproot) & ficus religiosa (bark) in wistar rats. European Journal of Pharmacy and Medical Research. 2017;4(7):698-703.
55. Kouitcheu Mabeku LB, Eyoum Bille B, Nguepi E. In Vitro and In Vivo Anti-Helicobacter Activities of Eryngium foetidum (Apiaceae), Bidens pilosa (Asteraceae), and Galinsoga ciliata (Asteraceae) against Helicobacter pylori. Biomed Res Int. 2016;2016:2171032.
56. Kim AY, Ki M-R, Park S-H, Ahn Y-T, Lee E-M, Lee E-J, et al. Anti-Helicobacter pylori activity of phytochemicals from Brassica rapa L. Biomedical Research. 2016;27(4):1123-9.
57. Kouitcheu Mabeku LB, Eyoum Bille B, Tchouangueu TF, Nguepi E, Leundji H. Treatment of Helicobacter pylori infected mice with Bryophyllum pinnatum, a medicinal plant with antioxidant and antimicrobial properties, reduces bacterial load. Pharm Biol. 2017 Dec;55(1):603-10.
58. Londonkar R, Ranirukmini R. Antiulcerogenic study of different extracts of Butea frondosa Roxb in albino mice. J. Pharmacol. 2010;1:6-9.
59. Patel K, Patel B, Patel A, Shah S. Pharmacological Evaluation of Anti-ulcer Activity of Caesalpinia crista in Rats. International Journal of Pharmaceutical Sciences and Nanotechnology (IJPSN). 2017 Jul 31;10(4):3772-9.
60. Swapna P, Robertson S, Elumalai A, Eswaraiah MC, Nirmala K. Evaluation of antiulcer activity of Calotropis gigantea R. Br leaves. Int J Pharm Sci Res. 2011 Nov 1;2(11):2938.
61. Borato DG, Scoparo CT, Maria-Ferreira D, da Silva LM, de Souza LM, Iacomini M, et al. Healing mechanisms of the hydroalcoholic extract and ethyl acetate fraction of green tea (Camellia sinensis (L.) Kuntze) on chronic gastric ulcers. Naunyn Schmiedebergs Arch Pharmacol. 2016 Mar;389(3):259-68.
62. Sini KR, Sinha BN, Rajasekaran A. Protective Effects of Capparis zeylanica Linn. Leaf Extract on Gastric Lesions in Experimental Animals. Avicenna J Med Biotechnol. 2011 Jan;3(1):31-5.
63. Abdulla MA, Al-Bayaty FH, Younis LT, Abu Hassan MI. Anti-ulcer activity of Centella asiatica leaf extract against ethanol-induced gastric mucosal injury in rats.J. Med. Plants Res.2010 Jul 4;4(13):1253-9.
64. Amr AR, Maysa ME. Anti-ulcer effect of cinnamon and chamomile aqueous extracts in rat models.. J. Am. Sci.2010;6(12):209-16.
65. Bonamin F, Moraes TM, Dos Santos RC, Kushima H, Faria FM, Silva MA, et al. The effect of a minor constituent of essential oil from Citrus aurantium: the role of β-myrcene in preventing peptic ulcer disease. Chem Biol Interact. 2014 Apr 5;212:11-9.
66. Rozza AL, Moraes Tde M, Kushima H, Tanimoto A, Marques MO, Bauab TM, et al. Gastroprotective mechanisms of Citrus lemon (Rutaceae) essential oil and its majority compounds limonene and β-pinene: involvement of heat-shock protein-70, vasoactive intestinal peptide, glutathione, sulfhydryl compounds, nitric oxide and prostaglandin E₂. Chem Biol Interact. 2011 Jan 15;189(1-2):82-9.
67. Poovendran P, Kalaigandhi V, Poongunran E. Antimicrobial activity of the leaves of Cocculus hirsutus against gastric ulcer pro ducing Helicobacter pylori. J Pharm Res. 2011;4:4294-5.
68. Jahan N, Nikhat S, Ahmad G. Efficacy of Tukhm Kishneez (Coriandrum sativum Linn.) in stress induced gastric ulcer.Unani Research.2011;1(1):17-22.
69. Palacios-Espinosa JF, Arroyo-García O, García-Valencia G, Linares E, Bye R, Romero I. Evidence of the anti-Helicobacter pylori, gastroprotective and anti-inflammatory activities of Cuphea aequipetala infusion. J Ethnopharmacol. 2014 Feb 3;151(2):990-8.
70. Czekaj R, Majka J, Magierowska K, Sliwowski Z, Magierowski M, Pajdo R, et al. Mechanisms of curcumin-induced gastroprotection against ethanol-induced gastric mucosal lesions. J Gastroenterol. 2018 May;53(5):618-630.
71. Rahim NA, Hassandarvish P, Golbabapour S, Ismail S, Tayyab S, Abdulla MA. Gastroprotective effect of ethanolic extract of Curcuma xanthorrhiza leaf against ethanol-induced gastric mucosal lesions in Sprague-Dawley rats. Biomed Res Int. 2014;2014:416409.
72. Awaad AS, Mohamed NH, Maitland DJ, Soliman GA. Anti-ulcerogenic Activity of Extract and Some Isolated Flavonoids from Desmostachia bipinnata (L.) Stapf.Rec. Nat Prod. 2008 Jul 1;2(3).
73. Franklin A, Rao U, Vijayakumar R, Srikumar R. In-vitro Anti Helicobacter Pylori Activity of Emblica officinalis. Int J Microbiol Res. 2012;3:216-20.
74. Thirunavukkarasu P, Ramkumar L, Ramanathan T. Anti-ulcer activity of Excoecaria agallocha bark on NSAID-induced gastric ulcer in albino rats.Glob. J. Pharmacol.2009;3(3):123-6.
75. Umarani N, Ilango K, Valentina P, Sunitha G, Anandaraja Gopala K. Antiulcer activity of various extracts of leaves of In digofera tinctoria. International Journal of Chemical Sciences. 2008;6:281-4.
76. Zhang XQ, Gu HM, Li XZ, Xu ZN, Chen YS, Li Y. Anti-Helicobacter pylori compounds from the ethanol extracts of Geranium wilfordii. J Ethnopharmacol. 2013 May 2;147(1):204-7.
77. Chen SH, Liang YC, Chao JC, Tsai LH, Chang CC, Wang CC, et al. Protective effects of Ginkgo biloba extract on the ethanol-induced gastric ulcer in rats. World J Gastroenterol. 2005 Jun 28;11(24):3746-50.
78. Mahmood AA, Mariod AA, Al-Bayaty F, Abdel-Wahab SI. Anti-ulcerogenic activity of Gynura procumbens leaf extract against experimentally-induced gastric lesions in rats. . J. Med. Plants Res.2010 Apr 18;4(8):685-91.
79. Turan MI, Bilen H, Demiryilmaz I, Ozgeris FB, Baykal H, Turkoglu M, et al. Effects of Hypericum perforatum and Hippophae rhamnoides extracts on indomethacin-induced gastric oxidative stress in rats. Biomed Res. 2013 Jul 1;24(3):314-9.
80. Olatunji OJ, Chen H, Zhou Y. Anti-Ulcerogenic Properties of Lycium chinense Mill Extracts against Ethanol-Induced Acute Gastric Lesion in Animal Models and Its Active Constituents. Molecules. 2015 Dec 16;20(12):22553-64.
81. D'Argenio G, Mazzone G, Tuccillo C, Grandone I, Gravina AG, Graziani G, et al. Apple polyphenol extracts prevent aspirin-induced damage to the rat gastric mucosa. Br J Nutr. 2008 Dec;100(6):1228-36.
82. Patidar DK. Anti-ulcer activity of aqueous extract of Murraya koenigii in albino rats. Int J Pharma Bio Sci. 2011 Jan;2(1):524-9.
83. Mishra V, Shukla A, Pandeti S, Barthwal MK, Pandey HP, Palit G, et al. Arbortristoside-A and 7-O-trans-cinnamoyl-6β-hydroxyloganin isolated from Nyctanthes arbortristis possess anti-ulcerogenic and ulcer-healing properties. Phytomedicine. 2013 Sep 15;20(12):1055-63.
84. Zaveri M, Jain S. Gastroprotective effects of root bark of Oroxylum indicum, vent. J Nat Rem. 2007;7:269-77.
85. Ngan LT, Moon JK, Shibamoto T, Ahn YJ. Growth-inhibiting, bactericidal, and urease inhibitory effects of Paeonia lactiflora root constituents and related compounds on antibiotic-susceptible and -resistant strains of Helicobacter pylori. J Agric Food Chem. 2012 Sep 12;60(36):9062-73.
86. Jung J, Bae KH, Jeong CS. Anti-Helicobacter pylori and antiulcerogenic activities of the root cortex of Paeonia suffruticosa. Biol Pharm Bull. 2013;36(10):1535-9.
87. Jeong CS, Hyun JE, Kim YS. Ginsenoside Rb1: the anti-ulcer constituent from the head of Panax ginseng. Arch Pharm Res. 2003 Nov;26(11):906-11.
88. Lai CH, Fang SH, Rao YK, Geethangili M, Tang CH, Lin YJ, et al. Inhibition of Helicobacter pylori-induced inflammation in human gastric epithelial AGS cells by Phyllanthus urinaria extracts. J Ethnopharmacol. 2008 Aug 13;118(3):522-6.
89. Wang Y, Wang SL, Zhang JY, Song XN, Zhang ZY, Li JF, et al. Anti-ulcer and anti-Helicobacter pylori potentials of the ethyl acetate fraction of Physalis alkekengi L. var. franchetii (Solanaceae) in rodent. J Ethnopharmacol. 2018 Jan 30;211:197-206.
90. Paul AS, Islam A, Yuvaraj P. Anti-Helicobacter pylori and cytotoxic activity of detoxified root of Plumbago auriculata, Plumbago indica and Plumbago zeylanica.Journal of Phytopharmaco logy.2013;2(3):4-8.
91. Ismail IF, Golbabapour S, Hassandarvish P, Hajrezaie M, Abdul Majid N, Kadir FA, et al. Gastroprotective Activity of Polygonum chinense Aqueous Leaf Extract on Ethanol-Induced Hemorrhagic Mucosal Lesions in Rats. Evid Based Complement Alternat Med. 2012;2012:404012.
92. Enomoto S, Yanaoka K, Utsunomiya H, Niwa T, Inada K, Deguchi H, et al. Inhibitory effects of Japanese apricot (Prunus mume Siebold et Zucc.; Ume) on Helicobacter pylori-related chronic gastritis. Eur J Clin Nutr. 2010 Jul;64(7):714-9.
93. Nirmala DK, Livingston RN, Palanivelu M, Srinivasan B, Rajarathinam D, Kerthi KC. Anti Ulcer Activity of The Leaves of Punica granatum Linn.Res,2011;4(1):116-22.
94. Sandhya S. Comparative Assessment of the Antibacterial activity of Three Tephrosia Species against Helicobacter pylori. Indian J Pharm Sci. 2018;80:460-9.
95. Jawanjal H, Rajput MS, Agrawal P, Dange V. Pharmacological evaluation of fruits of Terminalia belerica Roxb. for antiulcer activity. J Complement Integr Med. 2012 Jun 18;9:9.
96. Mishra V, Agrawal M, Onasanwo SA, Madhur G, Rastogi P, Pandey HP, et al. Anti-secretory and cyto-protective effects of chebulinic acid isolated from the fruits of Terminalia chebula on gastric ulcers. Phytomedicine. 2013 Apr 15;20(6):506-11.
97. Rong Q, Xu M, Dong Q, Zhang Y, Li Y, Ye G, et al. In vitro and in vivo bactericidal activity of Tinospora sagittata (Oliv.) Gagnep. var. craveniana (S.Y.Hu) Lo and its main effective component, palmatine, against porcine Helicobacter pylori. BMC Complement Altern Med. 2016 Aug 30;16(1):331.
98. Malairajan P, Gopalakrishnan G, Narasimhan S, Veni KJ, Kavimani S. Anti-ulcer activity of crude alcoholic extract of Toona ciliata Roemer (heart wood). J Ethnopharmacol. 2007 Mar 21;110(2):348-51.
99. Khushtar M, Kumar V, Javed K, Bhandari U. Protective Effect of Ginger oil on Aspirin and Pylorus Ligation-Induced Gastric Ulcer model in Rats. Indian J Pharm Sci. 2009 Sep;71(5):554-8.
100. Hamedi S, Arian AA, Farzaei MH. Gastroprotective effect of aqueous stem bark extract of Ziziphus jujuba L. against HCl/Ethanol-induced gastric mucosal injury in rats. J Tradit Chin Med. 2015 Dec;35(6):666-70.