Abstract
Rapid, accurate, and comprehensive assessment of hemostasis is of great importance in many clinical settings. Key hematological indices routinely assessed in bleeding patients or patients at risk for bleeding are coagulation function, platelets, and hematocrit. Current clotting assays are limited to a partial analysis of hemostasis (individual faceted clotting elements), thereby providing an incomplete assessment of bleeding and thrombotic risks and status. Recently, we introduced a novel multiparameter approach using a carbon nanotube paper-composite (CPC) based capacitance sensor. We have shown that our technique can provide comprehensive information on coagulation function, platelet function or count, and hematocrit from a single assay within 30 min. Whole blood-based multiparameter hemostasis assessments were conducted using clinically relevant blood samples to demonstrate the clinical utility of the device for various hemostatic conditions including pathological conditions such as hemophilia and thrombocytopenia. Systematic comparisons with a commercial thromboelastography analyzer showed that our sensor was able to provide an accurate and reliable assessment of overall hemostatic function. Overall, this is a new biomedical device for convenient comprehensive evaluation of bleeding risks with attractive advantages, such as whole blood-based non-contact evaluation of multiple key clotting biomarkers, high accuracy, high sensitivity, and low cost.
Keywords
Thrombus, Clinical hematology, Coagulation function, Platelet, Hematocrit, Carbon nanotube-Paper Composite (CPC) capacitance sensor
Background
Hemostasis is a complex physiological process encompassing an ensemble of interactions between the coagulation factors, platelets, and erythrocytes [1]. With the major changes in the healthcare landscape and the desire to move clinical care closer to patients, the need for rapid hemostasis assessment and triage of patients with bleeding or with bleeding risk, as well as ensuring therapeutic compliances are becoming imperative [2,3]. Traditional laboratory-based clotting assays and devices are not only time-consuming and expensive but also provide insufficient information due to a partial analysis of various individual facetted clotting elements, such as coagulation function, fibrinogen, platelet function, or blood cellularity [4]. Evolving whole blood-based viscoelastic assays also lack sensitivity and specificity to platelet counts and platelet dysfunction and can often provide an inaccurate assessment of hemostatic status in abnormal hematocrit conditions [5,6]. As a result, each current assay or testing technology has provided an incomplete assessment of bleeding and thrombosis risks or status. This has also stymied the fields of clinical and experimental hematology given the interdependence among the clotting elements.
Introduction
We recently developed a unique capacitance-based blood permittivity measurement approach using our novel carbon nanotube-paper composite (CPC) capacitance sensor to achieve a comprehensive assessment of hemostasis [7]. It is our working hypothesis that the simultaneous assessments of multiple physiological elements of the hemostasis process such as hematocrit, platelet count, plasma factors, and platelet function may provide better monitoring and compliance that are currently needed to guide diagnosis and therapies in patients. The feasibility, sensitivity, and efficiency of this approach were demonstrated by conducting experiments using clinically relevant human blood samples and comparing the results against a conventional thromboelastography analyzer. In comparison to existing traditional clotting assays, our technology also has attractive advantages such as no need for any sample processing like centrifugation, easy-to-use not requiring to be performed by highly trained laboratory personnel, and non-contact assessment making it reusable and cost-effective, and easy to automate. Additionally, it is also the first reported hemostasis assay that can provide blood hematocrit and platelet count in addition to clotting function assessment.
Methods and Summary of Results
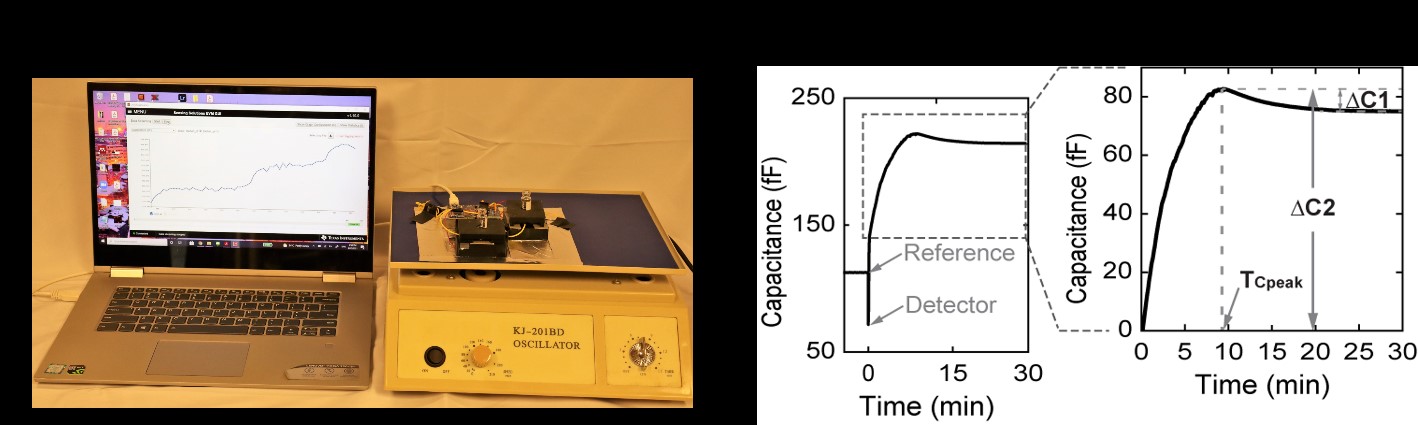
The experimental setup consists of two capacitance sensors in a differential configuration, FDC 2214 capacitance evaluation module (Texas Instruments, TX, U.S.A), glass vials, 3D printed plastic fixtures, and an orbital shaker as shown in Figure 1a. Carbon nanotube-paper composite films were prepared and fabricated as capacitance sensors using previously reported techniques [7,8]. The sensor had a fractured crack region where untangled cellulose fibers exhibited a high fringing field enabling a non-contact hemostasis assessment. A blood sample in glass vials was precisely positioned using plastic fixtures on top of the crack region of the sensor. Orbital shaking minimized the sedimentation of the blood cells from the rouleaux formation.
Figure 1: (a) Bench-top prototype of the hemostasis analyzer (b) Left: A representative overall capacitance signal for a blood sample activated using CaCl2 (12.5 mM). Right: Zoom-in view of the region of interest with the sensing parameters, TCpeak, ΔC1, and ΔC2 for analyses using the presented system.
For a clotting assay, a baseline capacitance was measured for 5 min with the glass vials placed on the sensors. Then, 325 µL of the blood sample was added to the reference sensor. Immediately, 300 µL of the same blood was added to the detector sensor and 80-rpm orbital shaking was applied. The glass vial on the detector sensor was prefilled with 25 µL of 162.5 mM CaCl2 solution (final Ca2+ concentration: 12.5 mM) for recalcification of the blood sample. Differential capacitance was measured for an additional 30 min after activation. From the capacitance signal, three parameters were chosen for analysis (TCpeak, ?C1, and ?C2; Figure 1b). TCpeak was the time point for the maximum capacitance value after activation. ?C1 was evaluated as the magnitude decrease in capacitance from peak to a steady state value. Steady-state capacitance was the averaged value between 28-30 min after activation. ?C2 was the maximum capacitance value after activation. A total of 26 healthy volunteers, 3 hemophilia patients, and 3 thrombocytopenia samples were tested with replicates. Collectively, we demonstrated that this new device provided simultaneous information on coagulation function, platelets, and hematocrit through the readouts namely TCpeak, ?C1, and ?C2, respectively, as summarized in Table 1. Additionally, these readouts showed excellent repeatability and reproducibility. Furthermore, they also demonstrated a Gaussian distribution for all healthy volunteers tested, implying minimal variabilities between samples from the same group.
|
Clotting parameters |
TCpeak |
DC1 |
DC2 |
|
Coagulation function |
ü |
X |
X |
|
Platelet count |
X |
ü |
X |
|
Platelet function |
X |
ü |
X |
|
Hematocrit |
X |
X |
ü |
Potential clinical utility was evaluated by comparing the results with conventional thromboelastography (TEG). There was a strong positive correlation between the two techniques in terms of coagulation function assessments. Results also suggested that our device had a higher sensitivity and specificity to platelet count compared to the TEG assay. We then tested the device for hematologically altered conditions using blood samples with hemophilia and thrombocytopenia conditions. Results from patient samples with absent functional factor VIII or IX showed that TCpeak was sensitive to coagulation factor deficiency and can potentially be used to monitor coagulation function in hemophilia patients. For thrombocytopenia samples, we observed a prolonged TCpeak in addition to the expected reduction in ?C1, which elucidated the multi-faceted role played by platelets in hemostasis. In addition to their key role in primary hemostasis, platelets also play an active role in secondary hemostasis by providing binding sites for coagulation factors in thrombin generation [9,10].
Conclusion
Motivated by the importance of having a rapid, accurate, and convenient hemostasis assessment device, we have developed the novel capacitance-based approach with a unique ability to multiplex the assessments of coagulation function, platelets, and hematocrit in a single measurement. Because the assay is easily automatable, we are working to develop a fully automatic hemostasis analyzer with minimal human interference. Our upcoming scientific studies will be focused on evaluating the effects of biological variabilities such as fibrinogen (level & function), blood types, proteins, vitamins, and ions concentrations, on the sensor performance. In closing, we have made substantial advancements in illustrating an elegant micro-biomedical device that affords a way to test or generate new hypotheses for longstanding questions in the field of hematology. It may be useful for mechanistic studies in clinical and experimental hematology, in addition to serving as a potential diagnostic device.
References
2. Whiting P, Al M, Westwood M, Ramos IC, Ryder S, Armstrong N, et al . Viscoelastic point-of-care testing to assist with the diagnosis, management and monitoring of haemostasis: A Systematic Review And Cost-Effectiveness Analysis..
3. Jackson SP. Arterial thrombosis—insidious, unpredictable and deadly. Nature Medicine. 2011 Nov;17(11):1423-36.
4. Tynngård N, Lindahl TL, Ramström S. Assays of different aspects of haemostasis–what do they measure?. Thrombosis journal. 2015 Dec;13(1):8.
5. Spiezia L, Radu C, Marchioro P, Bertini D, Rossetto V, Castelli M, et al . Peculiar whole blood rotation thromboelastometry (Rotem) profile in 40 sideropenic anaemia patients. Thrombosis and Haemostasis. 2008;100(12):1106-10.
6. Ranucci M, Conti D, Castelvecchio S, Menicanti L, Frigiola A, Ballotta A, et al . Hematocrit on cardiopulmonary bypass and outcome after coronary surgery in nontransfused patients. The Annals of Thoracic Surgery. 2010 Jan 1;89(1):11-7.
7. Sekar PK, Liang XM, Kahng SJ, Shu Z, Dichiara AB, Chung JH, et al . Simultaneous multiparameter whole blood hemostasis assessment using a carbon nanotube-paper composite capacitance sensor. Biosensors and Bioelectronics. 2022 Feb 1;197:113786.
8. Dichiara AB, Song A, Goodman SM, He D, Bai J. Smart papers comprising carbon nanotubes and cellulose microfibers for multifunctional sensing applications. Journal of Materials Chemistry A. 2017;5(38):20161-9.
9. van der Meijden PE, Heemskerk JW. Platelet biology and functions: new concepts and clinical perspectives. Nature Reviews Cardiology. 2019 Mar;16(3):166-79.
10. Michelson AD, Cattaneo M, Frelinger A, Newman P, editors. Platelets. Academic press; 2019 Mar 7.